Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2011, 4, 196-199 JBiSE doi:10.4236/jbise.2011.43027 Published Online March 2011 (http://www.SciRP.org/journal/jbise/). Published Online March 2011 in SciRes. http://www.scirp.org/jour nal/JBiSE Brain activation patterns associated with sexual orientation in homosexual male and female: preliminary study with 3.0T fMRI Gwang-Won Kim1, Jong-Chul Yang2, Gwang-Woo Jeong 3 1Interdisciplinary Program of Biomedical Engineering, Chonnam National University, Gwangju, Republic of Korea; 2Department of Psychiatry, Chonbuk National University Medical School, Jeonju, Republic of Korea; 3Department of Radiology, Chonnam National University Medical School, Chonnam National University Hospital, Gwangju, Repub- lic of Korea. Email: gwjeong@jnu.ac.kr Received 22 November 2010; revised 8 January 2011; accepted 12 January 2011. ABSTRACT This study was performed to clarify the sexual orien- tation in a 19-year-old homosexual male and a 20-year-old homosexual female by using a functional magnetic resonance imaging (fMRI) with viewing male and female erotic nude pictures. The sex hor- mone levels of the homosexual male and female were in the normal range of healthy heterosexual males and females, respectively. In both homosexuals more significant brain activities were observed while view- ing the nude pictures of the same genetic sex than those of the opposite sex in the frontal cortex, parietal cortex, occipital cortex, anterior cingulate gyrus, amygdala, midbrain, hippocampus, orbitofrontal cortex, parahippocampal gyrus, thalamus, globus pallidus, and putamen, which are known to be re- sponsive to sexual arousal. The homosexual male and female showed a tendency toward higher sexual arousal to the same genetic sex as comparison with the opposite sex. This finding may be useful to un- derstand the different neural mechanisms on sexual arousal in homosexuals. Keywords: Sexual Orientation; Homosexual Male; Homosexual Female; Functional Magnetic Resonance Imaging (fMRI) 1. INTRODUCTION The sex is defined by genes and physical characteristics of the body. The decision of sexual orientation is likely to be related with complex correlations between cul- tural, psychological, social-environmental, and bio- logical factors. Sexual orientation involves the expres- sion of sexual arousal as a biological factor rather than the voluntary selection of the opposite sex [1-3]. Ho- mosexual persons are characterized by a persistent dis- comfort with their natal body and a stated desire to be the opposite sex. In addition, they can experience sexual arousal and attraction to the same genetic sex [1]. The most influential biological theory of sexual ori- entation is that hormones influence the development of neural structures that regulate sexual behavior [2]. The sexual hormones show different degrees of variability between males and females. The major male and female hormones can be classified as estrogen and testosterone [4]. Estrogen leads to the growth of the female sex or- gans and feminization, whereas testosterone is response- ble for development of the male reproductive structures and masculinity. Although, the levels of free testosterone and estradiol (E2) in the homosexual male and female in our study were in the normal range of healthy hetero- sexual males and females, respectively, they are sexually interested in members of their genetic sex. In this study, the differential brain activation patterns in response to visual sexual stimuli with male and fe- male erotic nude pictures in both homosexual male and female subjects were compared to discriminate the sex- ual orientation for homosexual persons by using 3.0 Tesla functional magnetic resonance imaging (fMRI). 2. CASE REPORT AND RESULTS The first case deals with a homosexual male whose name is YS. He is 19 years old and is sexually interested in male. He has received any hormonal and/or surgical therapy. At present he lives with his parents and is a freshman in a university with a major tied of the early childhood education. YS has had a difficult time adjust- ing to school life since primary school because of sexual identity. Growing up, YS did not feel socially isolated, 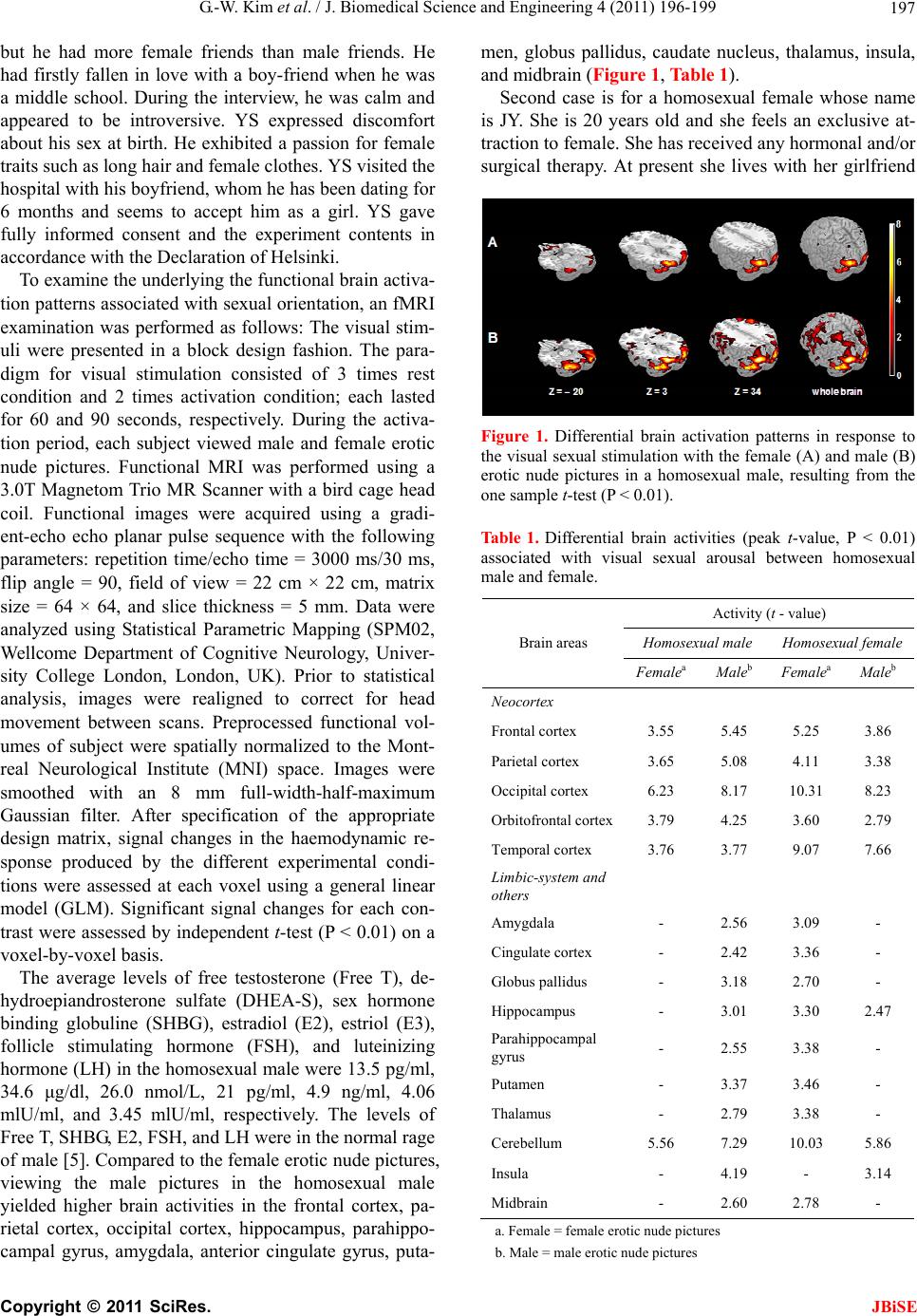 G.-W. Kim et al. / J. Biomedical Science and Engineering 4 (2011) 196-199 Copyright © 2011 SciRes. JBiSE 197 but he had more female friends than male friends. He had firstly fallen in love with a boy-friend when he was a middle school. During the interview, he was calm and appeared to be introversive. YS expressed discomfort about his sex at birth. He exhibited a passion for female traits such as long hair and female clothes. YS visited the hospital with his boyfriend, whom he has been dating for 6 months and seems to accept him as a girl. YS gave fully informed consent and the experiment contents in accordance with the Declaration of Helsinki. To examine the underlying the functional brain activa- tion patterns associated with sexual orientation, an fMRI examination was performed as follows: The visual stim- uli were presented in a block design fashion. The para- digm for visual stimulation consisted of 3 times rest condition and 2 times activation condition; each lasted for 60 and 90 seconds, respectively. During the activa- tion period, each subject viewed male and female erotic nude pictures. Functional MRI was performed using a 3.0T Magnetom Trio MR Scanner with a bird cage head coil. Functional images were acquired using a gradi- ent-echo echo planar pulse sequence with the following parameters: repetition time/echo time = 3000 ms/30 ms, flip angle = 90, field of view = 22 cm × 22 cm, matrix size = 64 × 64, and slice thickness = 5 mm. Data were analyzed using Statistical Parametric Mapping (SPM02, Wellcome Department of Cognitive Neurology, Univer- sity College London, London, UK). Prior to statistical analysis, images were realigned to correct for head movement between scans. Preprocessed functional vol- umes of subject were spatially normalized to the Mont- real Neurological Institute (MNI) space. Images were smoothed with an 8 mm full-width-half-maximum Gaussian filter. After specification of the appropriate design matrix, signal changes in the haemodynamic re- sponse produced by the different experimental condi- tions were assessed at each voxel using a general linear model (GLM). Significant signal changes for each con- trast were assessed by independent t-test (P < 0.01) on a voxel-by-voxel basis. The average levels of free testosterone (Free T), de- hydroepiandrosterone sulfate (DHEA-S), sex hormone binding globuline (SHBG), estradiol (E2), estriol (E3), follicle stimulating hormone (FSH), and luteinizing hormone (LH) in the homosexual male were 13.5 pg/ml, 34.6 μg/dl, 26.0 nmol/L, 21 pg/ml, 4.9 ng/ml, 4.06 mlU/ml, and 3.45 mlU/ml, respectively. The levels of Free T, SHBG, E2, FSH, and LH were in the normal rage of male [5]. Compared to the female erotic nude pictures, viewing the male pictures in the homosexual male yielded higher brain activities in the frontal cortex, pa- rietal cortex, occipital cortex, hippocampus, parahippo- campal gyrus, amygdala, anterior cingulate gyrus, puta- men, globus pallidus, caudate nucleus, thalamus, insula, and midbrain (Figure 1, Table 1). Second case is for a homosexual female whose name is JY. She is 20 years old and she feels an exclusive at- traction to female. She has received any hormonal and/or surgical therapy. At present she lives with her girlfriend Figure 1. Differential brain activation patterns in response to the visual sexual stimulation with the female (A) and male (B) erotic nude pictures in a homosexual male, resulting from the one sample t-test (P < 0.01). Table 1. Differential brain activities (peak t-value, P < 0.01) associated with visual sexual arousal between homosexual male and female. Activity (t - value) Homosexual male Homosexual female Brain areas FemaleaMaleb FemaleaMaleb Neocortex Frontal cortex 3.55 5.45 5.25 3.86 Parietal cortex 3.65 5.08 4.11 3.38 Occipital cortex 6.23 8.17 10.31 8.23 Orbitofrontal cortex3.79 4.25 3.60 2.79 Temporal cortex 3.76 3.77 9.07 7.66 Limbic-system and others Amygdala - 2.56 3.09 - Cingulate cortex - 2.42 3.36 - Globus pallidus - 3.18 2.70 - Hippocampus - 3.01 3.30 2.47 Parahippocampal gyrus - 2.55 3.38 - Putamen - 3.37 3.46 - Thalamus - 2.79 3.38 - Cerebellum 5.56 7.29 10.03 5.86 Insula - 4.19 - 3.14 Midbrain - 2.60 2.78 - a. Female = female erotic nude pictures b. Male = male erotic nude pictures  G.-W. Kim et al. / J. Biomedical Science and Engineering 4 (2011) 196-199 Copyright © 2011 SciRes. JBiSE 198 who she met one year ago. She reported that her father abused her physically during her childhood and her pa- rents got divorced. Her behavior has become more mas- culine and often aggressive. She wishes she were a male and has applied a bandage to her breast to appear more masculine. She has not had the surgery to change her sex because of the high cost, although she is currently working at a transgender bar to earn money for a sex reassignment surgery. JY stated that she was displeased with her parents during the interview. Furthermore, she stated that if she is upset, she cannot control herself and she showed signs of impulsive behavior. She also com- plained about things often and was easily angered by her girlfriend during the interview. She expressed discomfort about her sex at birth. JY gave fully informed consent and the experiment contents in accordance with the Declaration of Helsinki. The average levels of Free T, DHEA-S, SHBG, E2, E3, FSH, and LH in the homosexual female were 0.98 pg/ml, 247 μg/dl, 34.6 nmol/L, 87 pg/ml, 4.7 ng/ml, 2.74 mlU/ml, and 4.53 mlU/ml, respectively. The levels of Free T, DHEA-S, SHBG, E2, FSH, and LH were in the normal rage of female [5]. The brain areas showing higher ac- tivities in viewing the female erotic nude pictures over the male pictures included the frontal cortex, parietal cortex, occipital cortex, hippocampus, parahip-pocampal gyrus, amygdala, anterior cingulate gyrus, putamen, globus pallidus, caudate nucleus, thalamus, and midbrain (Figure 2, Table 1). 3. DISCUSSION This study identified whether the sexual orientation in- fluenced the neural activation in response to viewing the male or female erotic nude pictures in homosexual male and female. In our study, distinct brain activation with higher signal intensities in both homosexual male and female was induced by sexual stimuli with the same ge- netic sex pictures especially in the occipital cortex, fron- tal cortex, and parietal cortex. These results are pre- sumably related to the subject’s attention and concentra- tion on the preferable nude pictures [2,6,7]. It is well known that the vision-related cortices play important roles in the perception of visual information. The predominantly activated brain areas, others inter- esting areas in our study, observed in viewing the erotic nude pictures of the same genetic sex over those of the opposite sex in both subjects included the anterior cin- gulate gyrus, amygdala, parahippocampal gyrus, thalamus, hippocampus, globus pallidus, putamen, and midbrain. Safron et al. [2] reported that the brain areas associated with sexual arousal were organized a priori into a hy- pothesized network supporting sexual arousal, which included the anterior cingulate gyrus, amygdala, hippo- Figure 2. Differential brain activation patterns in response to the visual sexual stimulation with the female (A) and male (B) erotic nude pictures in a homosexual female, resulting from the one sample t-test (P < 0.01). campus, hypothalamus, midbrain, orbitofrontal cortex, and visual areas. Based on the previously published pa- pers [6-10], visual sexual stimulation affects the thala- mus along the optic nerve transmits to the parahippo- campal gyrus through the visual cortex and a object is recognized. Subsequently, the visual information affects the thalamus, hippocampus and amgydala, the auto- nomic nervous system which adjusts sexual behavior in the hypothalamus was responded. At this time, increased sexual arousal affects the frontal lobe through the cingu- late gyrus, and sexual arousal and pleasure are consid- ered to be felt. In addition, Ponseti et al. [6] reported that the preferred sexual stimuli induced activation of the anterior cingulate gyrus, orbitofrontal cortex, thalamus and ventral striatum relative to the non-preferred sexual stimuli. Note that the homosexual male and female in our study showed a tendency toward higher sexual arousal to the same genetic sex than those of the oppo- site sex. Although the homosexual subjects maintain the sex hormone levels of their born sex in the normal ranges, the neural activation associated with sexual arousal re- flected their sexual orientation, giving higher activities being observed in response to viewing the erotic nude pictures of their genetic sex. Consequently, the brain activation patterns in response to visual sexual stimuli were not considered as the sex hormones, and the neural mechanisms on sexual arousal correlate with their sexual preference. This study provided in valuable information that the homosexual subjects showed a tendency towards higher brain activation in response to the visual stimuli with the erotic nude pictures of the same genetic sex over those of the opposite sex. Future studies are needed to clarify how the brain activation patterns associated with sexual arousal in homosexual persons is changed with the levels of the sex hormones, and how the brain activation pat- terns between homosexual persons and transgender are different to each other.  G.-W. Kim et al. / J. Biomedical Science and Engineering 4 (2011) 196-199 Copyright © 2011 SciRes. JBiSE 199 4. ACKNOWLEDGEMENTS This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2009-0077677). REFERENCES [1] Vocks, S., Stahn, C., Loenser, K. and Legenbauer, T. (2009) Eating and body image disturbances in male-to- female and female-to-male transsexuals. Archives of Sexual Behavior, 38, 364-377. doi:10.1007/s10508-008-9424-z [2] Safron, A., Barch, B., Bailey, J.M., Gitelman, D.R., Par- rish, T.B. and Reber, P.J. (2007) Neural correlates of sexual arousal in homosexual and heterosexual men. Be- havioral Neuroscience, 121, 237-248. doi:10.1037/0735-7044.121.2.237 [3] Swaab, D.F. (2004) Sexual differentiation of the human brain: Relevance for gender identity, transsexualism and sexual orientation. Gynecological Endocrinology, 19, 301-312. doi:10.1080/09513590400018231 [4] Negri-Cesi, P., Colciago, A., Pravettoni, A., Casati, L., Conti, L. and Celotti, F. (2008) Sexual differentiation of the rodent hypothalamus: Hormonal and environmental influences. The Journal of Steroid Biochemistry and Mo- lecular Biology, 109, 294-299. doi:10.1016/j.jsbmb.2008.03.003 [5] Green Cross Reference Lab (2007) Sex Hormones in Green cross reference lab guidebook, Yongin, Republic of Korea, 201-224. [6] Ponseti, J., Bosinski, H.A., Wolff, S., Peller, M., Jansen, O., Mehdorn, H.M., Büchel, C. and Siebner, H.R. (2006) A functional endophenotype for sexual orientation in humans. Neuroimage, 33, 825-833. doi:10.1016/j.neuroimage.2006.08.002 [7] Paul, T., Schiffer, B., Zwarg, T., Krüger, T.H., Karama, S., Schedlowski, M., Forsting, M. and Gizewski, E.R. (2008) Brain response to visual sexual stimuli in hetero-sexual and homosexual males. Human Brain Mapping, 29, 726-735. doi:10.1002/hbm.20435 [8] Jeong, G.W., Park, K., Youn, G., Kang, H.K., Kim, H.J., Seo, J.J. and Ryu, S.B. (2005) Assessment of cerebro- cortical regions associated with sexual arousal in premeno- pausal and menopausal women by using BOLD-based functional MRI. The Journal of Sexual Medicine, 2, 645-651. doi:10.1111/j.1743-6109.2005.00134.x [9] Mouras, H., Stoléru, S., Bittoun, J., Glutron, D., Pé- légrini-Issac, M., Paradis, A.L. and Burnod, Y. (2003) Brain processing of visual sexual stimuli in healthy men: A functional magnetic resonance imaging study. Neuroi- mage, 20, 855-869. doi:10.1016/S1053-8119(03)00408-7 [10] Karama, S., Lecours, A.R., Leroux, J.M., Bourgouin, P., Beaudoin, G., Joubert, S. and Beauregard, M. (2002) Ar- eas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping, 16, 1-13. doi:10.1002/hbm.10014 |

