Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2011, 4, 164-172 JBiSE doi:10.4236/jbise.2011.43023 Published Online March 2011 (http://www.SciRP.org/journal/jbise/). Published Online March 2011 in SciRes. http://www.scirp.org/journal/JBiSE Angiogenesis in rat uterine scar after introduction after autological mesenchymal stem cells of bone marrow origin Igor Maiborodin, Natalia Yakimova, Vera Matveeva, Andrey Shevela, Elena Maiborodina, Ekaterina Pekareva, Olesya Tkachuk Center of New Medical Technologies Institute of Chemical Biology and Fundamental Medicine, Novosibirsk, Russia. Email: imai@mail.ru Received 24 August 2010; revised 10 September 2011; accepted 13 September 2010. ABSTRACT The results of injecting of autologic mesenchymal stem cells of bone marrow origin (AMSCBMO), trans- fected by the GFP gene, into the scar of rat uterine horns were studied by methods of light microscopy. After the in t roduction of AMSCBMO in to the formed scar on the right (2 months after the ligation) large groups of blood vessels with cellular elements inside were present; groups like that were not found in the opposite side. Studying unstained sections under re- flected ultraviolet light the sufficient bright lumines- cence in the endothelium and the external membrane of scar vessels was found in uterine horn only on the side of introduction of AMSCBMO. It was concluded that after the introduction of AMSCBMO into the scar tissue they form blood vessels by differentiation into endotheliocytes and pericytes. GFP gene expres- sion not only in endothelium of vessels, but also in their external membrane indicates that differentia- tion of AMSCMBO is possible in endothelial and in pericytal directions. Keywords: Uterine Scar; Mesenchymal Stem Cells; Angiogenesis; Endo t h eliocy t e s ; Peric ytes 1. INTRODUCTION According to modern concepts, the physiological regen- eration of tissues of the adult organism and their repair in case of damage occurs with direct participation of low-differentiated progenitor cells or stem cells. The main source of stem cells is bone marrow, capable, in addition to its basic - hematopoietic function , to generate precursors of cellular elements of a large number of body tissues. Bone marrow contains two basic types of stem cells: hematopoietic and stromal (mesenchymal) [1]. It is be- lieved that both of these types of cells are precursors with the potential to transdifferentiation into cells of different ph enotypes [2]. In addition to bone marrow, pluripotent stem cells were also found in other tissues of the adult body - the adipose, muscle, brain, as well as in peripheral blood and blood from cord/placenta. Moreover, it is established that depending on a microenvironment the stem cells are able to get through haemopoetic/mesenchymal barrier as possess to high plasticity in case of a differentiation and a transdifferentiation. J. M. Isner [3] isolated supposed endothelial progeni- tor cells from the bone marrow, some surface antigens of these cells were common to hematopoietic stem cells (CD34, CD133, Flk-1, Tie-2). These endothelial pro- genitor cells were injected systemically to immunodefi- ciency rats with pre-performed occlusion of coronary artery. Afterwards was discovered that donor cells were accumulated mainly in the areas of neoangiogenesis within the myocardium. Increasing the number of capil- laries per unit volume associated with a significant im- provement in function and parameters of the left ventri- cle compared with the control group. Similar work with similar results was performed on various models by other researchers [4-7]. H. Kamihata et al. [8] showed that intensive neoan- giogenesis after implantation of bon e marrow cells in the area of myocardial infarction was caused not only by direct participation of these cells, but also by significant expression of angiogenesis factors such as vascular en- dothelial growth factor, angiopoetin and others by these cells. M. Takahashi et al. [9] also supported the idea of paracrine mechanisms of the effect of bone marrow cells on the myocardium by inflammatory cytokines. Since coronary heart disease is characterized by myo- cardial scarring, the expansion of the cavities and dete- rioration of the work of ventricles, which can be pre- vented only by full restoration of the myocardium, the studies were undertaken to assess the possibility of its restoration on the model of ischemic heart disease in  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 165 mice. Cardiac progenitor cells were implanted in scar zone. Also local stimulation by hepatocyte growth factor and insulin-like growth factor-1 directly of the scar area was performed. These two factors have important char- acteristics, it is known that the hepatocyte factor is chemoattractant for cardiac progenitor cells. At the same time, insulin-like factor causes a strong cellular prolif- eration and increases the viability of these cells [10]. The results of this research showed decrease of the scar area by 42% with both methods of effects on the myocardium, as well as improved ventricular volume configuration. The reduction of scar area was associated with the formation of new myocardiocytes in which car- diac progenitor cells produce matrix metalloproteinases. In the analysis of chromosomes of myocardiocytes was shown that in the process of recovery of myocardium there is no “merger” of cells. As an alternative to intro- duction of stem cells the authors propose using a com- bination of growth factors [10]. The impoverishment of the population of circulating bone marrow cells with age - an important limiting con- dition for cellular therapy, as a preliminary preparation for the treatment in such cases it is suggested to use small molecules, polymers, growth factors or their com- bination [11]. In experiments on rats were showed that autologic mesenchymal stem cells of bone marrow origin (AM- SCBMO) which hypoxically prepared within 24 hours before the transplantation into peri-infarction zone in- crease the expression of factors of vitality and vascu- logenesis: hypoxia-inducible factor 1, angiopoietin-1, vascular endothelial growth factor, as well as receptors - Flk-1 , erythropoietin, Bcl-2 and Bcl-xL, that eventually leads to improved angiogenesis through increased para- crine mechanisms [12]. We can conclude that in the literature on treatment of scars with the use of cellular technologies, there are 2 main hypotheses about the destiny of implanted AM- SCBMO. The first is that AMSCBMO in the continua- tion of their differentiation in hypoxic tissues are directly involved in angiogenesis and revascularization [4-7]. According to other researchers, the effect of AMSCBMO is mainly due to the expression of various factors of an- giogenesis and other cytokines [8,9,12]. M. Rota et al. [10] believe that there is a combination of direct in- volvement of stem cells with paracrine mechanisms. So, there is no common opinion and until there is remained not clear what happens to AMSCBMO after implanta- tion into the tissues and organs of the body. 2. AIM These days inflammatory diseases of female genitals and, as a result of them, adhesions, lead to reproductive dis- orders in women. The most relevant in this regard is infertility. Studying peculiarities of etiopathogenesis in the development of adhesions and synechiae in the uter- ine cavity allows justifying the search of new directions of their treatment and prevention. Due to the low efficiency of widely used techniques for treating scars of myometrium the attempt to correct the pathology with the use of cellular technologies was made. In addition, cu rrently remains unexplored the des- tiny of stem cells after the introduction one way or an- other into the organism. An attempt to use autologic mesenchymal stem cells of bone marrow origin (AMSCBMO) to accelerate the regeneration of scar of myometrium in the experiment was made due to the numerous reports on the effective- ness of cellular technologies in the treatment of co ronary heart disease [3-10,12]. Cellular-mediated strategies in the treatment of this pathology are based on the implan- tation directly into the ischemic myocardium or the coronary vessels of bone marrow cells. This serves two purposes: myocardial revascularization and eliminating the deficit of functional cellular elements in myocardium. 3. MATERIAL AND METHODS In our research the female Wag-rats weighing 180-200 g at the age of 6 months were used as models. In aseptic conditions lower midline laparotomy was performed. Uterine horns were taken out into the wound and care- fully rounded by sterile gauze. At the end of each horn near the corpus uteri were placed ligatures from catgut and bandaged up in that part. Abdominal cavity was su- tured tightly layer-by-layer. Isolation of mesenchymal stem cells: AMSCBMO iso- lated by washing out the bone marrow from femur epiphysis that taken under ether anesthesia in male rats, inbreeding line Wag. Obtained suspension of cells was placed in plastic bottles (“Nunk”, Denmark), in 48 hours after explantation of bone marrow the cells that did not attach were removed. Attached cells were cultured in α-MEM medium with supplement of 10% fetal calf se- rum (“Biolot”, Russia) at 37˚C in СО2-incubator with 5% СО2 in saturated humidity. The culture medium was changed every three days. Performing subculturing the monolayer culture was dispersed with density of 1000- 5000 cells/sm2 (depending on the growth properties of used fetal serum), were used standard solutions of Versene and tripsin. Transfection: Mesenchymal AMSCBMO from second passage, obtained from rats of mentioned line, trans- fected DNA of plasmid pEGFP-N1 (Clontech Laborato- ries), which contained the gene of green fluorescent pro- tein (GFP) under the control of cytomegalovirus pro- moter and neomycin resistance gene under the control of  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 166 the promoter of the virus SV40, that necessary for the subsequent selection using geneticyn G418 (pEGFP-N1; Clontech Laboratories). Transfection was performed in the presence of the reagent for transfection TurboFect (Fermentas) as recommended by the manufacturer; the protocol for transfection of cellular suspension was used. Transfection was perf ormed using 1x106 cells in 1 ml of the suspension, 4 mg plasmid DNA and 10 ml of reagent for transfection (TurboFect). Cells after transfection were cultured in α-MEM medium with supplement of 10% fetal calf serum (Biolot, Russia), using a selection of G-418 (Sigma) at a concentration of 400 ng/ml. As specific unique markers for AMSCBMO are un- known, for identification of these cells were used physi- cal, morphological and functional properties. Untrans- fected and transfected cells (for demonstration that of AMSCBMO properties after transfection remain without changes) were characterized by the following physical, morphological and functional properties which are pecu- liar for mesenchymal stem cells cultured in vitro: at- tachment to the surface of the cultivation, morphology, proliferation, formation of colonies of fibroblast-like cells and the ability to ind uced differentiation in bone tissue. Using methods of light and fluorescent microscopy and cytological staining methods was showed that the cultured untransfected and transfected rat bone marrow cells in vitro: were attached to the surface of cultivation, had fibroblast-like morphology continued at all times of cultivation, maintained in culture fo r several subcultures, formed colonies of fibroblast-like cells when were dis- persed at low density, in th e presence of a linear-specific factors differentiated into cells of the bone tissue. For research the untransfected cells of 0-3 passages and transfected cells of 0-2 passage were used. However, the physical, morphological and phenotypi- cal characteristics are not unique criteria that can be used for specific identification of AMSCBMO. AMSCBMO ability for induced differentiation in vitro into bone, adipose tissue and cartilage is the only critical requirement for determining of prospective populations of stem cells. Induction of osteogenic differentiation of mesenchy- mal stem cells in vitro: For induction of osteogenic dif- ferentiation the 0.1 μM desoximethasone, 50 μM ascor- bic acid and 10 mM β-glycerophosphate (Sigma) was used. As AMSCBMO have properties to differentiation into cells of bone tissue, the cond itions of its implemen- tation are most reproducible. Such differentiation is typically used to characterize the cultures of stem cells in vitro and is typical way by default for the majority of AMSCBMO in culture. Osteogenic differentiation was determined by two markers: the alkaline phosphatase activity and miner- alization of extracellular matrix by calcium ions: Cyto- chemical detection of alkaline phosphatase was per- formed by using nitrotetrazolium blue in the presence of substrate for alkaline phosphatase of 5-bromo-4-chloro- 3-indolyl. Accumulation of calcium in the extracellular matrix was recorded by alizarin red staining. In 4 hours after the transfection the cells were diluted with untransfected cells at a ratio of 1:2.5, respectively, and 0.1 ml of the mixture was injected during procedure of relaparotomy and after removal of residual unlysed suture material into the region of formed scar within 2 months after ligation of right uterine horns of female rats Wag. Remaining cells after transplantation cultured for 10 days to evaluate the efficiency of transfection and stabil- ity of gene expression. Expression of introduced gene GFP by rat mesenchymal AMSCBMO was assessed visually under a fluorescent microscope, directly looking at culture in 48 hours after the transfection. The effi- ciency of transfection was assessed as a percentage of luminescent cells relative to all cells. The percentage of transfected cells in the diluted culture was about 3%. As in most cases, using technology based on the in- troduction of plasmid DNA was observed only a tempo- rary expression of the introduced GFP gene in rat AM- SCBMO. Cultivation of cells, transfected by plasmid pEGFP-N1, without selection (not using geneticyn (G418, Sigma)) showed a decrease in the number of cells synthesizing a green fluorescent protein, as a result of their replacement by untransfected cells. Nevertheless, after 1 week in culture of transfected cells of first pas- sage, seeded with density of 5000 cells per 1 cm2, were observed cells that synthesized green fluorescent protein. Thus, as in most cases, using technology based on the introduction of plasmid DNA with cationic lipids, with- out subsequent selection for creation of stable clone, was obtained culture of rat AMSCBMO temporarily ex- pressin g gr e en fluore s cent protein. Fragments of the uterine horns with scar tissues and synechiae, biopsied after 1, 2, 3 and 4 weeks after the introduction of AMSCBMO, were preserved in a 4% paraformaldehyde on biphosphate buffer (pH 7.4) for at least 24 hour, dehydrated in a gradien of ethanol, light- ened in xylene and embedded in paraffin. Unpainted microscopic sections 5-7 microns thick were studied under a light microscope Axioimager M1 (Carl Zeiss, Germany) with a magnification of up to 1500 times in the luminescence mode with a filter Alexa 488. The scar of left uterine horn in a corresponding time after the in- troduction of a suspension AMSCBMO and myometrium scar of animals 2 months after ligation of uterine horns with no subsequent use AMSCBMO were used as the controls. 6 animals were examined at each point of the experiment. 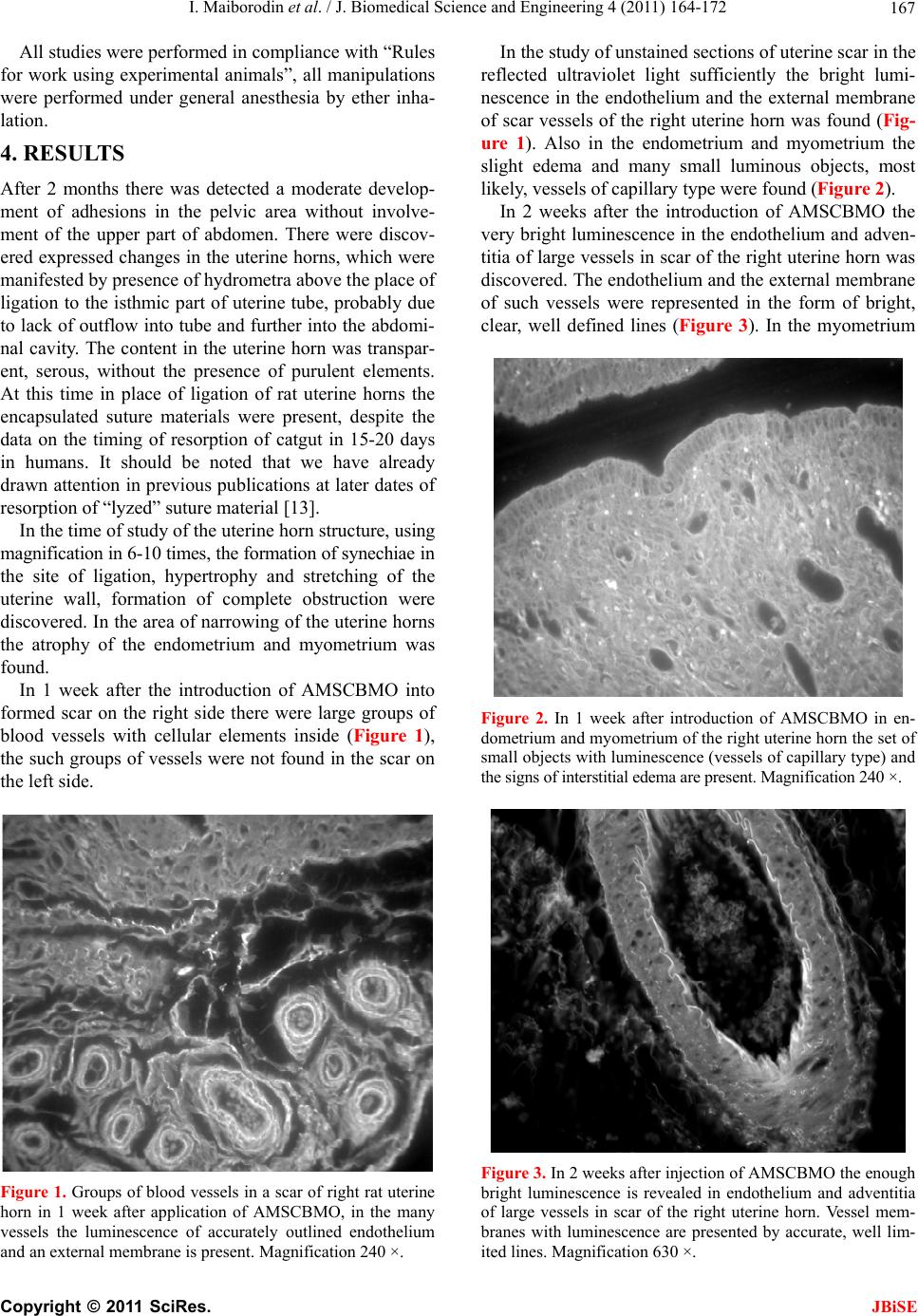 I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 167 All studies were performed in compliance with “Rules for work using experimental animals”, all manipulations were performed under general anesthesia by ether inha- lation. 4. RESULTS After 2 months there was detected a moderate develop- ment of adhesions in the pelvic area without involve- ment of the upper part of abdomen. There were discov- ered ex pressed changes in the uterine horn s, which were manifeste d by presence of hydrometra above the place of ligation to the isthmic part of uterine tube, probably due to lack of outflow into tube and further into the ab domi- nal cavity. The content in the uterine horn was transpar- ent, serous, without the presence of purulent elements. At this time in place of ligation of rat uterine horns the encapsulated suture materials were present, despite the data on the timing of resorption of catgut in 15-20 days in humans. It should be noted that we have already drawn attention in previous pub lications at later dates of resorption of “lyzed” suture material [13]. In the time of study of the uterine horn structure, using magnification in 6-10 times, the formation of synechiae in the site of ligation, hypertrophy and stretching of the uterine wall, formation of complete obstruction were discovered. In the area of narrowing of the uterine horns the atrophy of the endometrium and myometrium was found. In 1 week after the introduction of AMSCBMO into formed scar on the right side there were large groups of blood vessels with cellular elements inside (Figure 1), the such groups of vessels were no t found in the scar on the left side. Figure 1. Groups of blood vessels in a scar of right rat uterine horn in 1 week after application of AMSCBMO, in the many vessels the luminescence of accurately outlined endothelium and an external membrane is present. Magnification 240 ×. In the study of unstained sections of uterine scar in the reflected ultraviolet light sufficiently the bright lumi- nescence in the endothelium and the external membrane of scar vessels of the right uterine horn was found (Fig- ure 1). Also in the endometrium and myometrium the slight edema and many small luminous objects, most likely, vessels of capillary type were found (Figure 2). In 2 weeks after the introduction of AMSCBMO the very bright luminescence in the endothelium and adven- titia of large vessels in scar of the right uterine horn was discovered. The endothelium and the external membrane of such vessels were represented in the form of bright, clear, well defined lines (Figure 3). In the myometrium Figure 2. In 1 week after introduction of AMSCBMO in en- dometrium and myometrium of the right uterine horn the set of small objects with luminescence (vessels of capillary type) and the signs of interstitial edema are present. Magnification 240 ×. Figure 3. In 2 weeks after injection of AMSCBMO the enough bright luminescence is revealed in endothelium and adventitia of large vessels in scar of the right uterine horn. Vessel mem- branes with luminescence are presented by accurate, well lim- ited lines. Magnification 630 ×.  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 168 and endometrium of the left uterine horn at this time the luminescent structures were completely absent (Figure 4). After 3 weeks only in the wall of some blood vessels on the right was noted weak luminescence, almost at the level of background (Figure 5). By week 4 the luminescence intensity of the vascular walls and the number of vessels in the structures, in which was marked luminescence, was even more re- duced (Figure 6). On the opposite side for all periods of study the lumi- nescence of the vascular walls were at the level of back- ground, is necessary to repeat that large groups of ves- sels were absent. In the myometrium and endometrium small luminous objects were absent, but at the same time Figure 4. Absence of structures with luminescence and signs of edema in endometrium and myometrium of right uterine horn in 2 weeks after use of AMSCBMO. Magnification 240 ×. Figure 5. The weak luminescence, practically at background level, is present in a wall of some blood vessels in 3 weeks after introduction of AMSCBMO. Magnification 240 ×. there were no signs of edema (Figures 7-11). In the scar of the uterine horn of animals without the AMSCBMO introduction (control) the luminescence objects were absent in vessels, in the structure of the scar and in sur- rounding tissues (Figure 12). Unfortunately, after using AMSCBMO the more rapid resorption of the scar and reduction of hydrometra were not found. But, this pr eliminary study was conducted on a small number of observations. For accurate conclu- sions about the effectiveness of this procedure reg arding the status of the scar there is necessary to repeat this experiment with larger number of animals and to ob serve longer periods after the introduction of AMSCBMO. Figure 6. In 4 weeks after application of AMSCBMO the weak luminescence remains in a wall of only individual vessels. Magnification 240 ×. Figure 7. In 1 week after introduction of AMSCBMO in a scar of rat left uterine horn the number of vessels is much less, a luminescence of their structures is practically at background level. Magnification 320 ×.  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 169 Figure 8. In left uterine horn the objects with luminescence and signs of edema in 1 week after injection of AMSCBMO are absent. Magnification 240 ×. Figure 9. The absence of signs of edema and structures with luminescence in vessels of a scar of left uterine horn in 2 weeks after use of AMSCBMO. Magnification 240 ×. 5. DISCUSSIONS Rat uterine horn is a thin tube, one end of which con- nects with the uterine cavity, the other - with the fallo- pian tube, opening into the peritoneal cavity near the ovarian surface. Normally in mammals there is outflow of content from fallopian tubes into the peritoneal cavity with subsequent resorption by peritoneum. After ligation of uterine horns in this section takes place ischemia fol- lowed by necrosis, scar and hydrometra formation. These changes are clearly seen in macropreparation with a slight magnification. Concerning the results of use of AMSCBMO it is nec- essary to pay attention to the development of the vascu- lar net at the injection site (Figures 1 and 3-5). The proof, that these groups of vessels were formed precisely as a result of AMSCBMO and from them, is the lumi- nescence of walls of these vessels (Figures 1 and 3-5). GFP gene, introduced in the DNA of AMSCBMO, transfers without changes to daughter cells and cells of next generations. These cells and structures formed from them are also shone in the reflected ultraviolet light. Until recently by prevailing dogma was that the an- giogenic response, which develops in postnatal life, oc- curs because of the growth of existing capillaries. How- ever, now it is convincingly proven that a small, but biologically significant portion of endothelial cells in- volved in the formation of new capillaries, have bone marrow ori gin [1 4- 1 6] . Figure 10. In 3 weeks after injection of AMSCBMO the ob- jects with luminescence are absent in structures of left uterine horn and surrounding tissues. Magnification 180 ×. Figure 11. Absence of a luminescence in vessels of scar of left uterine horn in 4 weeks after introduction of AMSCBMO. Magnification 480 ×.  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 170 Figure 12. A control animal without introduction of AM- SCBMO. The objects with luminescence in scar structures of uterine horn completely are absent. Magnification 480 ×. Endothelial cells divide every three years, but in the case of retinal vessels - every 14 years. However endo- theliocytes can be induced to replication as response to physiological or pathological stimulation. In some cases, bone marrow populations of endothelial progenitor cells can be completely replaced every five days. This is a real advantage when the physiological circumstances require increased blood supply, both in healing wounds, and in preparation of a fertilized egg for th e implantation in the richly vascularized endometrium [17]. In 1962 was first demonstrated the possibility of exis- tence of circulating endothelial cells. It was shown that on Dacron patch, which was isolated from contact with the surrounding tissues and was located in the wall of th e current vascular prosthesis of the aorta (experimental prosthetics of the pig thoracic aorta), con nective tissue is forming. After 14 days and later after implantation of Dacron, there was determined a layer of endothelial and other cells, including mononuclear cells, fibroblasts, as well as giant cells of foreign bodies. Detected cells could have only one source—the circulating blood [18,19]. In experiments on dogs was shown that in a few days on the surfaces of endothelial impermeable vascular prostheses the isolated islands of endothelial cells are appeared, as when Dacron grafts was installed in inferior vena cava and as in thoracic aorta. This also confirms that the source of endothelial cells - circulating blood [15]. These results were confirmed when after total irra- diation of dogs the bone marrow transplantation from animals of another kind was made, in which the donor DNA of circulating endothelial cells were clearly differ- ent from the DNA of recipient cells [16]. Blood vessels are forming by two interacting cellular types. They are endothelial cells lining the inner surface of the bloodstream and perivascular cells, named peri- cytes, enveloping the outer surface of the vascular tubes [20]. Pericytes are not only included in the hemody- namic process, but also play an active role in the forma- tion of blood vessels. Some researchers hypothesize that endothelial cells and pericytes have common precursor with hematopoietic cells. This hypoth esis is based on the fact that developing hematopoietic and endothelial cells have common surface markers and that hematopoietic cells can be developed from embryonic cells of major blood vessels [21-23]. Most commonly origin of pericytes associate with mesenchymal stem cell [24]. After the initiation of dif- ferentiation the progenitor of pericytes chemotactically adhere to endothelial cells in the capillary plexus in the time of beginning of angiogenesis [25]. In addition, there are reports that pericytes can be generated from endothe- lial cells during their transdifferentiation. This was dis- covered in the posterior aorta [26], as well as in heart valves [27]. The luminescence of the endothelium and the external membrane of the vessels, discovered in our studies (Figures 1 and 3-5), indicates that AMSCBMO directly rather than indirectly (through cytokines or other cellular signals), were involved in the formation of blood vessels, possibly because of the presence of pluripotent cells which already were stimulated to differentiate into en- dothelial or pericytal directions. It is also possible that tissue hypoxia, as a result of ligation of structures to- gether with the vessels, stimulates the differentiation of introduced AMSCBMO into endotheliocytes [12]. Additional evidence of angiogenesis, as a result of ap- plication of AMSCBMO, is the groups of vessels in the scar that were found only on the right – the side of AMSCBMO introduction (Figures 1-6), but not in the other side (Figures 7-11) and not in the control (Figure 12). It is necessary to attract attention to another aspect of the findings. Expression of introduced GFP gene not only in the endothelium of blood vessels, but also in the external membrane (Figures 1 and 3), is most likely a sign that endotheliocytes and pericytes have the same progenitor cells, or that differentiation of AMSCBMO is possible as well as in endothelial and in pericytal direc- tions. We have already pointed out that, in the opinion of many researchers, endothelial cells and pericytes have common precursor with hematopoietic cells [21-23]. Besides that, the unilateral development of blood ves- sels and the presence of luminescence objects in walls (Figures 1 and 3-5) indicate that in this case introduced AMSCBMO do not migrate from the site of injection, do not destroy, and they are not only “building material” or  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 171 “signal” to the beginning of angiogenesis for own cells of the donor organism . In all these cases, of course, it is possible that luminescence protein and transfected GFP gene leave the destroyed AMSCBMO and then are ab- sorbed by neighbor cells. However, proteins and DNA fragments that got into cells by way of phagocytosis or pinocytosis, degraded with the loss of ability to lumi- nescence and insertion into the genome. So, when AM- SCBMO are destroyed, intensity o f luminescence should be minimal (the protein GFP leaves AMSCBMO) and very quickly should entirely disappear; more so in this case should not be clearly defined and limited luminous structures. The decrease of light intensity in the vessels of the scar in the uterine horn after introduction of AMSCBMO with time, probably due to the gradual restoration of genome of transfected cells or the displacement of AM- SCBMO by own cells. Attention should b e paid, that the reduction of number of objects, which can synthesize green fluorescent pro- tein, was showed during cultivation of cells, transfected by plasmid pEGFP-N1 without selection, as a result of their replacement by untransfected cells. In conclusion, it should be noted that after increasing the number of vessels, especially “young” with thin walls, metabolic processes in tissues with scar improve. As a result of optimizing the conditions of life and func- tioning of fibroblasts the exchange of components of extracellular matrix of scar connective tissue may inten- sify, rejuvenation of collagen and elastin fibers may oc- cur, that will further manifest by appearance of thinner structures, ordering of their location [28] and passable- ness of uterine horns with formed adhesions can be re- stored. 6. CONCLUSIONS 1) After introduction into uterine scar of AMSCBMO the increase of number of vessels due to the processes of neoangiogenesis was detected. In this case the AM- SCBMO do not migrate and were not destroyed in the site of introduction, but form the blood vessels by dif- ferentiation into endotheliocytes and pericytes. In 1 week in rats after introduction of AMSCBMO the ves- sels formed from these cells are already fully functional, consist of all the structural walls and contain blood cells. 2) Expression of GFP gene not only in the endothe- lium of blood vessels, but also in their outer membranes indicates that differentiation of AMSCBMO in endothe- lial and in pericytal directions is possible. REFERENCES [1] Campagnoli, C., Roberts, I.A., Kumar, S., Bennett, P.R., Bellantuono, I. and Fisk, N.M. (2001) Identification of mesenchymal stem/progenitor cells in human first-tri- mester fetal blood, liver, and bone marrow. Blood, 98, 2396-2402. doi:10.1182/blood.V98.8.2396 [2] Huss, R. (2000) Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells, 18, 1-9. doi:10.1634/stemcells.18-1-1 [3] Isner, J.M. (2000) Tissue responses to ischemia: Local and remote responses for preserving perfusion of ischemic muscle. The Journal of Clinical Investigation, 106, 615-619. doi:10.1172/JCI10961 [4] Fukushima, S., Varela-Carver, A., Coppen, S.R., Yama- hara, K., Felkin, L.E., Lee, J., Barton, P.J., Terracciano, C.M., Yacoub, M.H. and Suzuki, K. (2007) Direct intra- myocardial but not intracoronary injection o f bone marr ow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation, 115, 2254-2261. doi:10.1161/CIRCULATIONAHA.106.662577 [5] Grauss, R.W., Winter, E.M., van Tuyn, J., Pijnappels, D.A., Steijn, R.V., Hogers, B., van der Geest, R.J., de Vries, A.A., Steendijk, P., van der Laarse, A., Gittenber- ger de Groot, A.C., Schalij, M.J. and Atsma, D.E. (2007) Mesenchymal stem cells from ischemic heart disease pa- tients improve left ventricular function after acute myo- cardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 293, H2438-H2447. doi:10.1152/ajpheart.00365.2007 [6] Jackson, K.A., Majka, S.M., Wang, H., Pocius, J., Hart- ley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation, 107, 1395-1402. doi:10.1172/JCI12150 [7] Kocher, A.A., Schuster, M.D., Szabolcs, M.J., Takuma, S., Burkhoff, D., Wang, J., Homma, S., Edwards, N.M. and Itescu, S. (2001) Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine, 7, 430-436. doi:10.1038/86498 [8] Kamihata, H., Matsubara, H., Nishiue, T., Fujiyama, S., Tsutsumi, Y., Ozono, R., Masaki, H., Mori, Y., Iba, O., Tate ishi, E., Kosaki, A., Shintani, S., Murohara, T., Imai- zumi, T. and Iwasaka, T. (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cyto- kines. Circulation, 104, 1046-1052. doi:10.1161/hc3501.093817 [9] Taka hashi, M., Li , T. S., Suzuki, R., Kobay ashi, T., Ito, H., Ikeda, Y., Matsuzaki, M. and Hamano, K. (2006) Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by pro- tecting cardiomyocytes from ischemic injury. American Journal of Physiology. Heart and Circulatory Physiology, 291, H886-H893. doi:10.1152/ajpheart.00142.2006 [10] Rota, M., Padin-Irue gas, M.E., Misa o, Y., de Ange lis, A., Maestroni, S., Ferreira-Martins, J., Fiumana, E., Rastaldo, R., Arcarese, M.L., Mitchell, T.S., Boni, A., Bolli, R., Urbanek, K., Hosoda, T., Anversa, P., Leri, A. and Kajstura, J. (2008) Local activation or implantation of  I. Maiborodin et al. / J. Biomedical Science and Engineering 4 (2011) 164-172 Copyright © 2011 SciRes. JBiSE 172 cardiac progenitor cells rescues scarred infarcted myo- cardium improving cardiac function. Circulation Re- search, 103, 107-116. doi:10.1161/CIRCRESAHA.108.178525 [11] Dimmelerm, S. and Leri, A. (2008) Aging and disease as modifiers of efficacy of cell therapy. Circulation Re- search, 102, 1319-1330. doi:10.1161/CIRCRESAHA.108.175943 [12] Hu, X., Yu, S.P., Fraser, J.L., Lu, Z., Ogle, M.E., Wang, J.A. and Wei, L. (2008) Transplantation of hypoxia-pre- conditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. The Journal of Thoracic and Cardio- vascular Surgery, 135, 799-808. doi:10.1016/j.jtcvs.2007.07.071 [13] Maiborodin, I.V., Maiborodina, E.I., Iakimova, N.V., Motorina, I.P. and Pekarev, O.G. (2008) Absorbable su- ture material in the body. Ark hiv Patologii, 70, 51-53. [14] Carmeliet, P. and Luttun, A. (2001) The emerging role of the bone marrow-derived stem cells in (therapeutic) an- giogenesis. Thrombosis and Haemostasis, 86, 289-297. [15] Shi, Q., Wu, M.H., Hayashida, N., Wechezak, A.R., Clowes, A.W. and Sauvage, L.R. (1994) Proof of fallout endothelialization of impervious dacron grafts in the aorta and inferior vena cava of the dog. Journal of Vas- cular Surgery, 20, 546-557. [16] Shi, Q., Rafii, S., Wu, M.H., Wijelath, E.S., Yu, C., Ishida, A., Fujita, Y., Kothari, S., Mohle, R., Sauvage, L.R., Moore, M.A., Storb, R.F. and Hammond, W.P. (1998) Evidence for circulating bone marrow-derived endothe- lial cells. Blood, 92, 362-367. [17] Carmeliet, P. and Jain, R.K. (2000) Angiogenesis in can- cer and other diseases. Nature, 407, 249-257. doi:10.1038/35025220 [18] Poole, J.C., Sabiston Jr, D.C., Florey, H.W. and Allison, P.R. (1962) Growth of endothelium in arterial prosthetic grafts and following endarterectomy. Surgical Forum, 13, 225-227. [19] Stump, M.M., Jordan Jr, G.L., Debakey M.E. and Halpert, B. (1963) Endothelium grown from circulating blood on isolated intravascular dacron hub. The American Journal of Pathology, 43, 361-367. [20] Bergers, G. and Song, S. (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-On- cology, 7, 452-464. doi:10.1215/S1152851705000232 [21] Carmeliet, P. (2004) Manipulating angiogenesis in medi- cine. Journal of Internal Medicine, 255, 538-561. doi:10.1111/j.1365-2796.2003.01297.x [22] Cho, H., Kozasa, T., Bondjers, C., Betsholtz, C. and Kehrl, J.H. (2003) Pericyte-specific expression of rgs5: implica- tions for PDGF and EDG receptor signaling during vas- cular maturation. The FASEB Journal, 17, 440-442. [23] Ribatti, D., Vacca, A., Nico, B., Ria, R. and Dammacco, F. (2002) Cross-talk between hematopoiesis and angio- genesis signaling pathways. Current Molecular Medicine, 2, 537-543. doi:10.2174/1566524023362195 [24] Creazzo, T.L., Godt, R.E., Leatherbury, L., Conway, S.J. and Kirby, M.L. (1998) Role of cardiac neural crest cells in cardiovascular development. Annual Review of Physi- ology, 60, 267-286. doi:10.1146/annurev.physiol.60.1.267 [25] Hellström, M., Kalén, M., Lindahl, P., Abramsson, A. and Betsholtz, C. (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and peri- cytes during embryonic blood vessel formation in the mouse. Development, 126, 3047- 3055. [26] Gittenberger-de Groot, A.C., DeRuiter, M.C., Bergwerff, M. and Poelmann, R.E. (1999) Smooth muscle cell origin and its relation to heterogeneity in development and dis- ease. Arteriosclerosis, Thrombosis, and Vascular Biolog y, 19, 1589-1594. [27] Nakajima, Y., Mironov, V., Yamagishi, T., Nakamura, H. and Markwald, R.R. (1997) Expression of smooth mus- cle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Developmental Dynamics, 209, 296-309. doi:10.1002/(SICI)1097-0177(199707)209:3<296::AID- AJA5>3.0.CO;2-D [28] Tsuji, T. and Sawabe, M. (1988) Elastic fibers in striae distensae. Journal of Cutaneous Pathology, 15, 215-222. doi:10.1111/j.1600-0560.1988.tb00547.x |

