Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 169-179 doi:10.4236/msa.2011.23021 Published Online March 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Tadashi Uragami1,2, Iusaku Sumida1, Takashi Miyata1,2, Tadashi Shiraiwa1,2, Hiroshi Tamura1,2, Tatsuo Yajima1,2 1Department of Chemistry and Materials Engineering, Faculty of Chemistry, Materials and Bioengineering, Kansai University, Osaka, Japan; 2High Technology Research Center, Kansai University, Osaka, Japan. Email: uragami@kansai-u.ac.jp Received January 15th, 2011; revised February 1st, 2011; accepted February 6th, 2011. ABSTRACT This paper focuses on the effects of the PSt content of polystyrene (PSt)-poly (dimethylsiloxan e) (PDMS) interpenetrat- ing network (IPN) polymer membranes on the pervaporation (PV) characteristics during the removal of benzene from an aqueous solution of dilute benzene. When an aqueous solution of 0.05wt% benzene was permeated through the PSt-PDMS IPN membranes, they showed high benzene/water selectivity. Both the permeability and the benzene/water selectivity of the membranes were enhanced with increasing PSt content in the PSt-PDMS IPN membrane. The phys- icochemical mechanism of permeation and separation through the PSt-PDMS IPN membranes during PV is also dis- cussed. The best normalized permeation rate, separation factor for benzene selectivity, and PV separation index of the PSt-PDMS IPN membrane were 1.27 × 10−6 kgm (m2hr)−1, 3293, and 41821, respectively. These PV characteristics are discussed from the viewpoint of the chemical and physical structure of the PSt-PDMS IPN membranes. Keywords: Interpenetrating Network Polymer, Polystyrene, Poly (Dimethylsiloxane), Pervaporation, Removal of Benzene 1. Introduction Volatile organic compounds (VOCs) such as aromatic hydrocarbons and chlorinated hydrocarbons are used as washing solvents in the chemical industry, and cause soil pollution. Such pollution contaminates the subterranean water and consequently water in rivers and ponds. Drink- ing water sources are often dependent on this contami- nated water. Furthermore, VOCs are known to be che- mical materials that disturb internal secretions (environ- mental hormones), and are thus potentially dangerous to humans. As such, these chemicals have to be removed from contaminated water. An activated carbon method, ozone processing and other methods have been devel- oped to remove VOCs from water [1]. However, these methods do not efficiently remove VOCs and the cost of treatment is high. Pervaporation (PV) is a promising membrane techni- que for the separation of VOCs/water mixtures [2-8], as PV can be used to separate organic liquid mixtures such as azeotropic mixtures or close-boiling point mixtures. Poly(dimethylsiloxane) (PDMS) membranes have been studied as VOC-permselective membranes, because they have both high permselectivity and high permeability for organic chemicals in water [9-12]. The removal of chlo- roform in tap water through a hollow-fiber PDMS mem- brane, which showed high chloroform-permselectivity and permeability was demonstrated [13]. The high VOCs- permselectivity of the PDMS membrane is attributable to its stronger affinity for VOCs than for water. Another advantage of the PDMS membrane is the high diffusivity of VOCs in the membrane due to its low Tg. The PDMS membrane has some disadvantages however, most nota- bly its weak mechanical strength. Nakagawa et al. intro- duced cross-linking into the PDMS membrane to im- prove its mechanical strength. The introduction of cross- linking however, led to a lower diffusivity of the per- meants in the membrane [14]. Polymer blending is a promising technique for im- proving the physical and chemical properties of various  Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 170 materials. However, in general, the blending of general polymers is difficult because there are few combinations of miscible polymers. Since phase separation generally takes place in the blending of immiscible polymers, an improvement of the physical and chemical properties of a material is rarely achieved. One of the methods to blend various polymers is the formation of an interpenetrating polymer network (IPN), which is defined as a combination of two polymers in network form [15,16]. IPNs have attracted our attention, since an improvement in the materials properties can be expected. Therefore, IPNs containing PDMS as a com- ponent were synthesized and their properties have been investigated extensively [17-20]. He et al. [17] obtained PDMS-poly(methyl methacrylate) (PMMA) IPNs by an in situ sequential synthesis and examined the properties of the resulting IPNs. They reported that interpenetrating the PMMA networks in the PDMS networks can improve the poor mechanical properties of the PDMS membranes. The structure of the PDMS component in the PDMS/ PMMA IPN which is the first-formed network, and con- stitutes a continuous phase. As such, the morphology of the membrane significantly affects the membrane its performance, and thus the morphology must be con- trolled by the volume fraction of each component, the preparation conditions, etc. This paper focuses on the effects of the structure of IPN membranes composed of polystyrene (PSt), which is one of hydrophobic polymers, and PDMS on the removal of benzene from an aqueous solution of dilute benzene through their IPN membranes during PV. The relation- ship between the removal characteristics for the ben- zene/water selectivity and the structure of IPN membrane is discussed in detail. 2. Experimental 2.1. Materials Hydroxyl-terminated PDMS base polymer (weight-ave- rage molecular weight was 20 000), was supplied by To- ray Dow Corning Silicons Co. Ltd. Tetraethoxysilane (TEOS) and stannous dibutyldilaurate were employed as a cross-linker and a catalyst in the polycondensation re- action of the PDMS base polymer, respectively. Benzoyl peroxide (BPO) as an initiator in the bulk-polymerization of styrene (St) was purified by reprecipitation from chloroform into methanol. Divinylbenzene (DVB) was used as a cross-linker in the bulk-polymerization of sty- rene. All other solvents and reagents were of analytical grade from commercial sources, and were used without further purification. 2.2. Preparation of PSt-PDMS IPN Membranes PSt-PDMS IPN membranes were prepared by the poly- condensation of the PDMS base polymer and the bulk- polymerization of St as follows: a mixture of the PDMS base polymer, TEOS (excess to the PDMS base polymer), and stannous dibutyldilauraete (4.0 wt% relative to the PDMS base polymer) for the formation of the PDMS network was mixed with a mixture of the St monomer, DVB, and BPO (0.3 wt% relative to the St monomer) for the formation of the PSt network. Two glass plates with a silicon sheet were separated by a 100 μm of spacer to form a mold. The above mixture was then transferred into the mold. At first, the PDMS network was formed at 25˚C for 8 h. Then, the temperature was raised to 80˚C, and the St monomer was bulk-polymerized for 8 h under a nitrogen atomosphere in situ. The resulting PSt-PDMS IPN membranes were sufficiently washed in n-hexane to completely remove the unreacted St monomer and PDMS base polymer which had not interpenetrated, the St ho- mopolymer and other reagents. The PSt-PDMS mem- branes were dried sufficiently in vacuo, and their compo- sitions determined by elementary analysis. 2.3. Glass Transition Temperature (Tg) Measurements The glass transition temperatures (Tgs) of the dry PSt- PDMS IPN membranes were determined by differential scanning calorimetry (DSC) (Rigaku, TAS-200). The specimens were heated from about −150˚C to +159˚C at a heating rate of 20˚C/min. 2.4. Transmission Electron Microscope (TEM) The PSt-PDMS IPN membranes were vapor-stained with an aqueous solution of 0.5 wt % RuO4 in glass-covered dishes [21]. The stained membranes were embedded in epoxy resin and cross-sectioned into thin films using a microtome (Leica; Reichert Ultracut E). The resin mor- phological features of the stained membranes were ob- served with a transmission electron microscope (TEM) (JEOL JEM-1210) at an accelerating voltage of 80 kV. 2.5. Contact Angle Measurements The contact angles of water on the surface of the PSt- PDMS IPN membranes were measured using a contact angle meter (Erma model G-1) at 25˚C. The contact an- gle, θ, was determined from the advancing contact angle, θa, and the receding contact angle, θr, by Equation (1): 1cos cos cos 2 ar (1) where θa and θr are the advancing contact angle and the 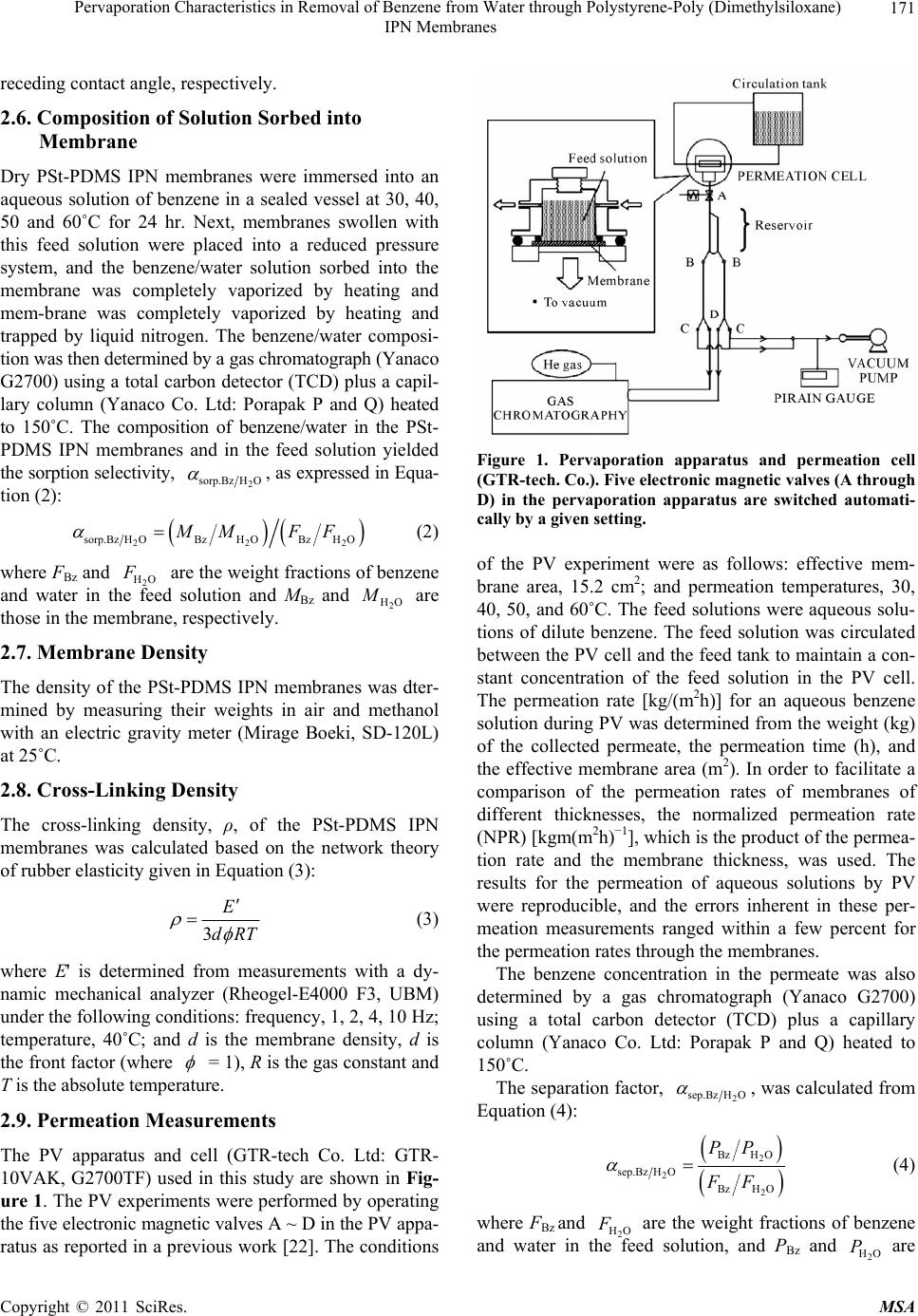 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 171 receding contact angle, respectively. 2.6. Composition of Solution Sorbed into Membrane Dry PSt-PDMS IPN membranes were immersed into an aqueous solution of benzene in a sealed vessel at 30, 40, 50 and 60˚C for 24 hr. Next, membranes swollen with this feed solution were placed into a reduced pressure system, and the benzene/water solution sorbed into the membrane was completely vaporized by heating and mem-brane was completely vaporized by heating and trapped by liquid nitrogen. The benzene/water composi- tion was then determined by a gas chromatograph (Yanaco G2700) using a total carbon detector (TCD) plus a capil- lary column (Yanaco Co. Ltd: Porapak P and Q) heated to 150˚C. The composition of benzene/water in the PSt- PDMS IPN membranes and in the feed solution yielded the sorption selectivity, 2 sorp.Bz H O , as expressed in Equa- tion (2): 222 sorp.BzH OBzH OBzH O MM FF (2) where FBz and 2 HO F are the weight fractions of benzene and water in the feed solution and MBz and 2 HO M are those in the membrane, respectively. 2.7. Membrane Density The density of the PSt-PDMS IPN membranes was dter- mined by measuring their weights in air and methanol with an electric gravity meter (Mirage Boeki, SD-120L) at 25˚C. 2.8. Cross-Linking Density The cross-linking density, ρ, of the PSt-PDMS IPN membranes was calculated based on the network theory of rubber elasticity given in Equation (3): 3 E dRT (3) where E' is determined from measurements with a dy- namic mechanical analyzer (Rheogel-E4000 F3, UBM) under the following conditions: frequency, 1, 2, 4, 10 Hz; temperature, 40˚C; and d is the membrane density, d is the front factor (where = 1), R is the gas constant and T is the absolute temperature. 2.9. Permeation Measurements The PV apparatus and cell (GTR-tech Co. Ltd: GTR- 10VAK, G2700TF) used in this study are shown in Fig- ure 1. The PV experiments were performed by operating the five electronic magnetic valves A ~ D in the PV appa- ratus as reported in a previous work [22]. The conditions Figure 1. Pervaporation apparatus and permeation cell (GTR-tech. Co.). Five electronic magnetic valves (A through D) in the pervaporation apparatus are switched automati- cally by a given setting. of the PV experiment were as follows: effective mem- brane area, 15.2 cm2; and permeation temperatures, 30, 40, 50, and 60˚C. The feed solutions were aqueous solu- tions of dilute benzene. The feed solution was circulated between the PV cell and the feed tank to maintain a con- stant concentration of the feed solution in the PV cell. The permeation rate [kg/(m2h)] for an aqueous benzene solution during PV was determined from the weight (kg) of the collected permeate, the permeation time (h), and the effective membrane area (m2). In order to facilitate a comparison of the permeation rates of membranes of different thicknesses, the normalized permeation rate (NPR) [kgm(m2h)−1], which is the product of the permea- tion rate and the membrane thickness, was used. The results for the permeation of aqueous solutions by PV were reproducible, and the errors inherent in these per- meation measurements ranged within a few percent for the permeation rates through the membranes. The benzene concentration in the permeate was also determined by a gas chromatograph (Yanaco G2700) using a total carbon detector (TCD) plus a capillary column (Yanaco Co. Ltd: Porapak P and Q) heated to 150˚C. The separation factor, 2 sep.Bz H O , was calculated from Equation (4): 2 2 2 BzH O sep.Bz H O BzH O PP FF (4) where FBz and 2 HO F are the weight fractions of benzene and water in the feed solution, and PBz and 2 HO P are 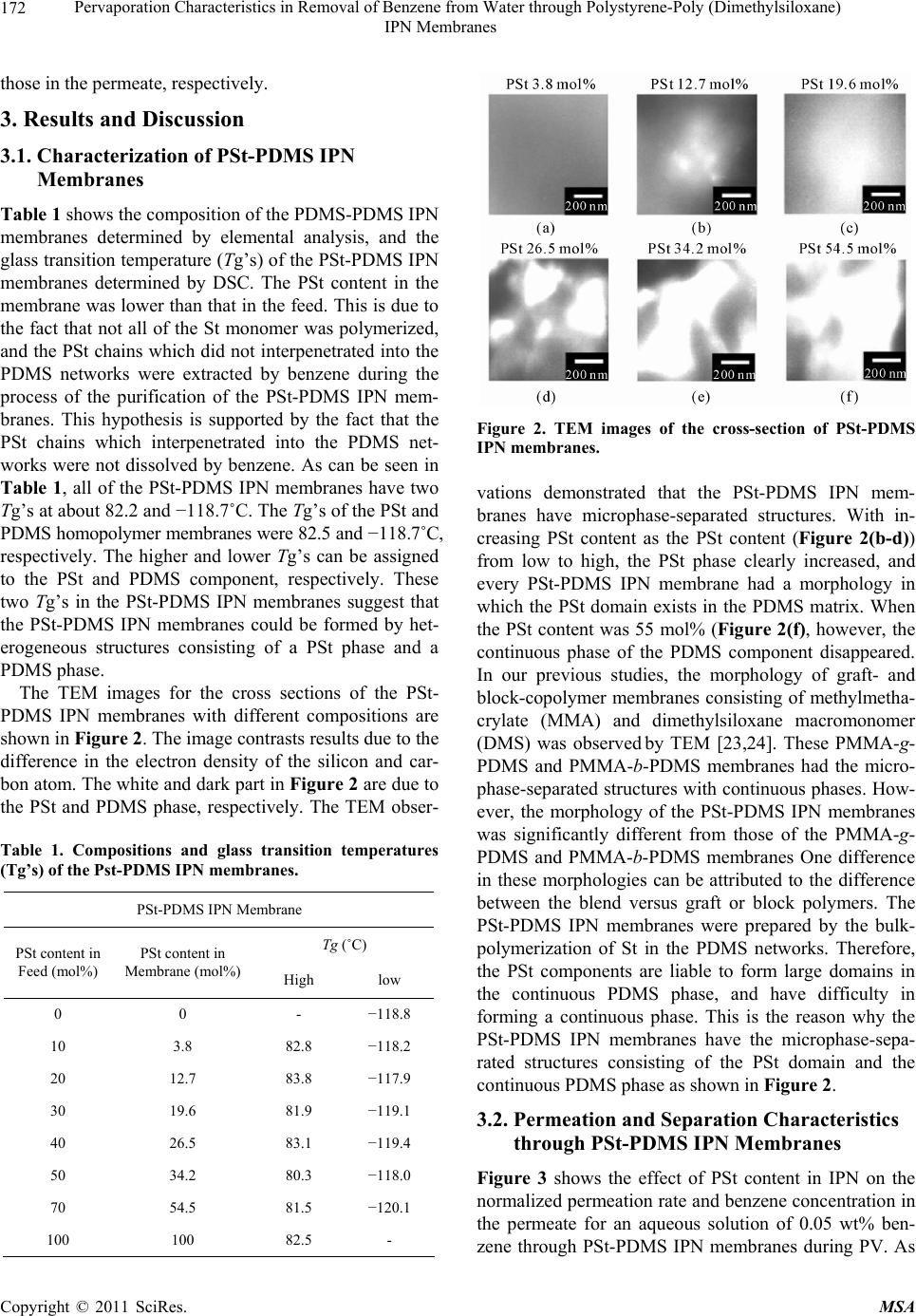 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 172 those in the permeate, respectively. 3. Results and Discussion 3.1. Characterization of PSt-PDMS IPN Membranes Table 1 shows the composition of the PDMS-PDMS IPN membranes determined by elemental analysis, and the glass transition temperature (Tg’s) of the PSt-PDMS IPN membranes determined by DSC. The PSt content in the membrane was lower than that in the feed. This is due to the fact that not all of the St monomer was polymerized, and the PSt chains which did not interpenetrated into the PDMS networks were extracted by benzene during the process of the purification of the PSt-PDMS IPN mem- branes. This hypothesis is supported by the fact that the PSt chains which interpenetrated into the PDMS net- works were not dissolved by benzene. As can be seen in Table 1, all of the PSt-PDMS IPN membranes have two Tg’s at about 82.2 and −118.7˚C. The Tg’s of the PSt and PDMS homopolymer membranes were 82.5 and −118.7˚C, respectively. The higher and lower Tg’s can be assigned to the PSt and PDMS component, respectively. These two Tg’s in the PSt-PDMS IPN membranes suggest that the PSt-PDMS IPN membranes could be formed by het- erogeneous structures consisting of a PSt phase and a PDMS phase. The TEM images for the cross sections of the PSt- PDMS IPN membranes with different compositions are shown in Figure 2. The image contrasts results due to the difference in the electron density of the silicon and car- bon atom. The white and dark part in Figure 2 are due to the PSt and PDMS phase, respectively. The TEM obser- Table 1. Compositions and glass transition temperatures (Tg’s) of the Pst-PDMS IPN membranes. PSt-PDMS IPN Membrane Tg (˚C) PSt content in Feed (mol%) PSt content in Membrane (mol%)High low 0 0 - −118.8 10 3.8 82.8 −118.2 20 12.7 83.8 −117.9 30 19.6 81.9 −119.1 40 26.5 83.1 −119.4 50 34.2 80.3 −118.0 70 54.5 81.5 −120.1 100 100 82.5 - Figure 2. TEM images of the cross-section of PSt-PDMS IPN membranes. vations demonstrated that the PSt-PDMS IPN mem- branes have microphase-separated structures. With in- creasing PSt content as the PSt content (Figure 2(b-d)) from low to high, the PSt phase clearly increased, and every PSt-PDMS IPN membrane had a morphology in which the PSt domain exists in the PDMS matrix. When the PSt content was 55 mol% (Figure 2(f), however, the continuous phase of the PDMS component disappeared. In our previous studies, the morphology of graft- and block-copolymer membranes consisting of methylmetha- crylate (MMA) and dimethylsiloxane macromonomer (DMS) was observed by TEM [23,24]. These PMMA-g- PDMS and PMMA-b-PDMS membranes had the micro- phase-separated structures with continuous phases. How- ever, the morphology of the PSt-PDMS IPN membranes was significantly different from those of the PMMA-g- PDMS and PMMA-b-PDMS membranes One difference in these morphologies can be attributed to the difference between the blend versus graft or block polymers. The PSt-PDMS IPN membranes were prepared by the bulk- polymerization of St in the PDMS networks. Therefore, the PSt components are liable to form large domains in the continuous PDMS phase, and have difficulty in forming a continuous phase. This is the reason why the PSt-PDMS IPN membranes have the microphase-sepa- rated structures consisting of the PSt domain and the continuous PDMS phase as shown in Figure 2. 3.2. Permeation and Separation Characteristics through PSt-PDMS IPN Membranes Figure 3 shows the effect of PSt content in IPN on the normalized permeation rate and benzene concentration in the permeate for an aqueous solution of 0.05 wt% ben- zene through PSt-PDMS IPN membranes during PV. As 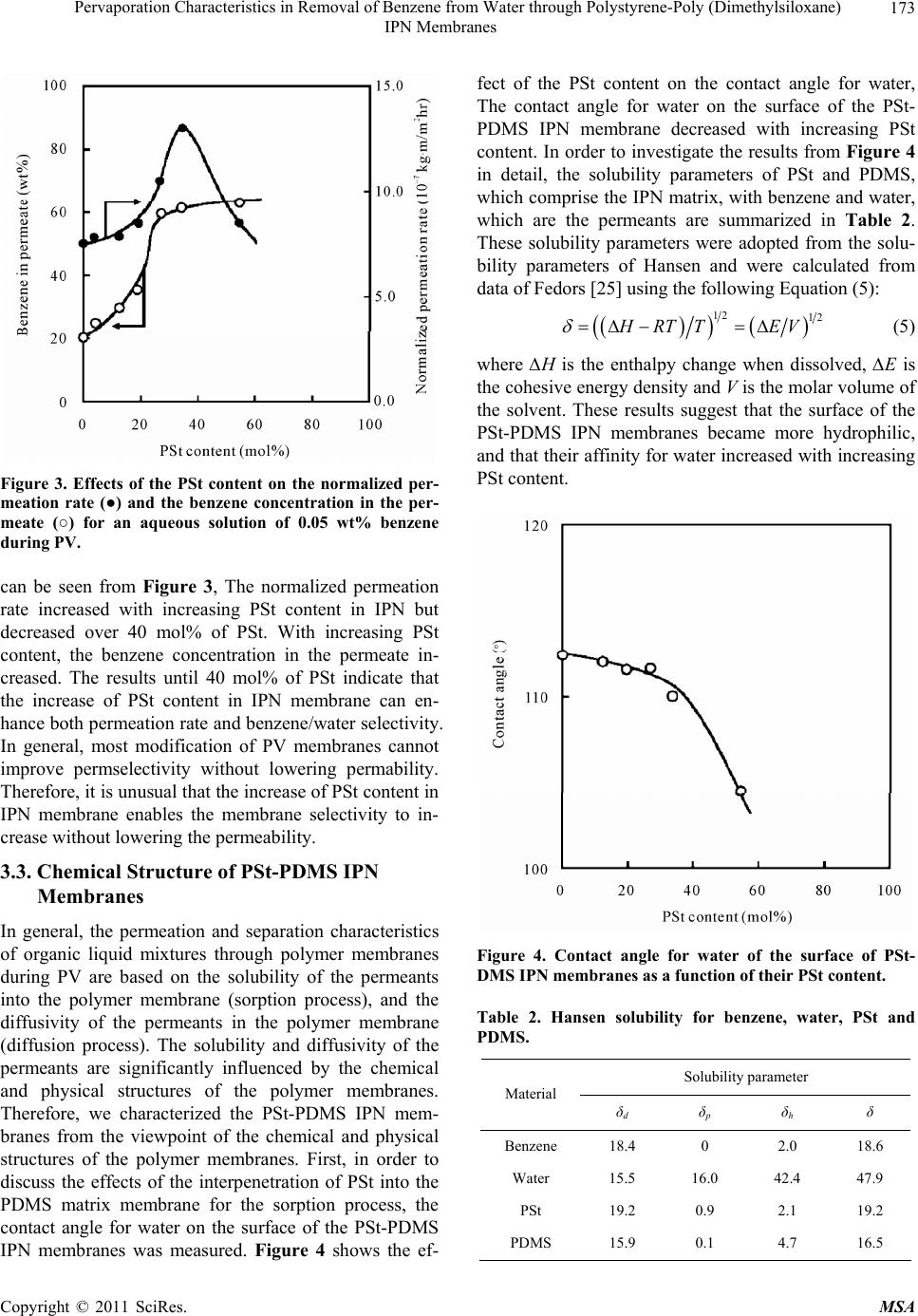 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 173 Figure 3. Effects of the PSt content on the normalized per- meation rate (●) and the benzene concentration in the per- meate (○) for an aqueous solution of 0.05 wt% benzene during PV. can be seen from Figure 3, The normalized permeation rate increased with increasing PSt content in IPN but decreased over 40 mol% of PSt. With increasing PSt content, the benzene concentration in the permeate in- creased. The results until 40 mol% of PSt indicate that the increase of PSt content in IPN membrane can en- hance both permeation rate and benzene/water selectivity. In general, most modification of PV membranes cannot improve permselectivity without lowering permability. Therefore, it is unusual that the increase of PSt content in IPN membrane enables the membrane selectivity to in- crease without lowering the permeability. 3.3. Chemical Structure of PSt-PDMS IPN Membranes In general, the permeation and separation characteristics of organic liquid mixtures through polymer membranes during PV are based on the solubility of the permeants into the polymer membrane (sorption process), and the diffusivity of the permeants in the polymer membrane (diffusion process). The solubility and diffusivity of the permeants are significantly influenced by the chemical and physical structures of the polymer membranes. Therefore, we characterized the PSt-PDMS IPN mem- branes from the viewpoint of the chemical and physical structures of the polymer membranes. First, in order to discuss the effects of the interpenetration of PSt into the PDMS matrix membrane for the sorption process, the contact angle for water on the surface of the PSt-PDMS IPN membranes was measured. Figure 4 shows the ef- fect of the PSt content on the contact angle for water, The contact angle for water on the surface of the PSt- PDMS IPN membrane decreased with increasing PSt content. In order to investigate the results from Figure 4 in detail, the solubility parameters of PSt and PDMS, which comprise the IPN matrix, with benzene and water, which are the permeants are summarized in Table 2. These solubility parameters were adopted from the solu- bility parameters of Hansen and were calculated from data of Fedors [25] using the following Equation (5): 12 12 HRTT EV (5) where ∆H is the enthalpy change when dissolved, ∆E is the cohesive energy density and V is the molar volume of the solvent. These results suggest that the surface of the PSt-PDMS IPN membranes became more hydrophilic, and that their affinity for water increased with increasing PSt content. Figure 4. Contact angle for water of the surface of PSt- DMS IPN membranes as a function of their PSt content. Table 2. Hansen solubility for benzene, water, PSt and PDMS. Solubility parameter Material δd δp δh δ Benzene 18.4 0 2.0 18.6 Water 15.5 16.0 42.4 47.9 PSt 19.2 0.9 2.1 19.2 PDMS 15.9 0.1 4.7 16.5 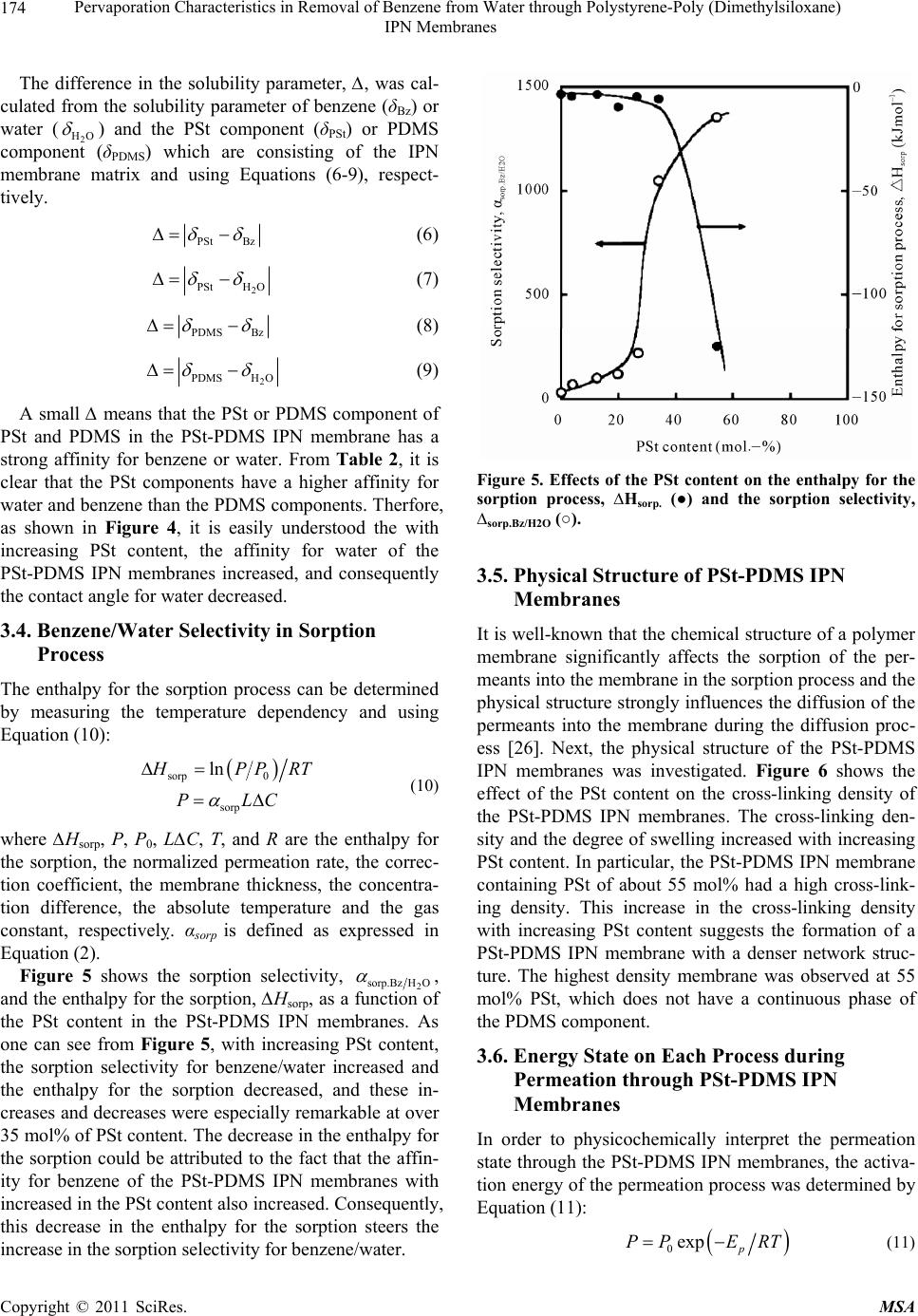 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 174 The difference in the solubility parameter, ∆, was cal- culated from the solubility parameter of benzene (δBz) or water (2 HO ) and the PSt component (δPSt) or PDMS component (δPDMS) which are consisting of the IPN membrane matrix and using Equations (6-9), respect- tively. PSt Bz (6) 2 PStH O (7) PDMS Bz (8) 2 PDMSHO (9) A small ∆ means that the PSt or PDMS component of PSt and PDMS in the PSt-PDMS IPN membrane has a strong affinity for benzene or water. From Table 2, it is clear that the PSt components have a higher affinity for water and benzene than the PDMS components. Therfore, as shown in Figure 4, it is easily understood the with increasing PSt content, the affinity for water of the PSt-PDMS IPN membranes increased, and consequently the contact angle for water decreased. 3.4. Benzene/Water Selectivity in Sorption Process The enthalpy for the sorption process can be determined by measuring the temperature dependency and using Equation (10): sorp 0 sorp ln H PP RT PLC (10) where ∆Hsorp, P, P0, L∆C, T, and R are the enthalpy for the sorption, the normalized permeation rate, the correc- tion coefficient, the membrane thickness, the concentra- tion difference, the absolute temperature and the gas constant, respectively. αsorp is defined as expressed in Equation (2). Figure 5 shows the sorption selectivity, 2 sorp.Bz H O , and the enthalpy for the sorption, ∆Hsorp, as a function of the PSt content in the PSt-PDMS IPN membranes. As one can see from Figure 5, with increasing PSt content, the sorption selectivity for benzene/water increased and the enthalpy for the sorption decreased, and these in- creases and decreases were especially remarkable at over 35 mol% of PSt content. The decrease in the enthalpy for the sorption could be attributed to the fact that the affin- ity for benzene of the PSt-PDMS IPN membranes with increased in the PSt content also increased. Consequently, this decrease in the enthalpy for the sorption steers the increase in the sorption selectivity for benzene/water. Figure 5. Effects of the PSt content on the enthalpy for the sorption process, ∆Hsorp. (●) and the sorption selectivity, ∆sorp.Bz/H2O (○). 3.5. Physical Structure of PSt-PDMS IPN Membranes It is well-known that the chemical structure of a polymer membrane significantly affects the sorption of the per- meants into the membrane in the sorption process and the physical structure strongly influences the diffusion of the permeants into the membrane during the diffusion proc- ess [26]. Next, the physical structure of the PSt-PDMS IPN membranes was investigated. Figure 6 shows the effect of the PSt content on the cross-linking density of the PSt-PDMS IPN membranes. The cross-linking den- sity and the degree of swelling increased with increasing PSt content. In particular, the PSt-PDMS IPN membrane containing PSt of about 55 mol% had a high cross-link- ing density. This increase in the cross-linking density with increasing PSt content suggests the formation of a PSt-PDMS IPN membrane with a denser network struc- ture. The highest density membrane was observed at 55 mol% PSt, which does not have a continuous phase of the PDMS component. 3.6. Energy State on Each Process during Permeation through PSt-PDMS IPN Membranes In order to physicochemically interpret the permeation state through the PSt-PDMS IPN membranes, the activa- tion energy of the permeation process was determined by Equation (11): 0exp p P PERT (11) 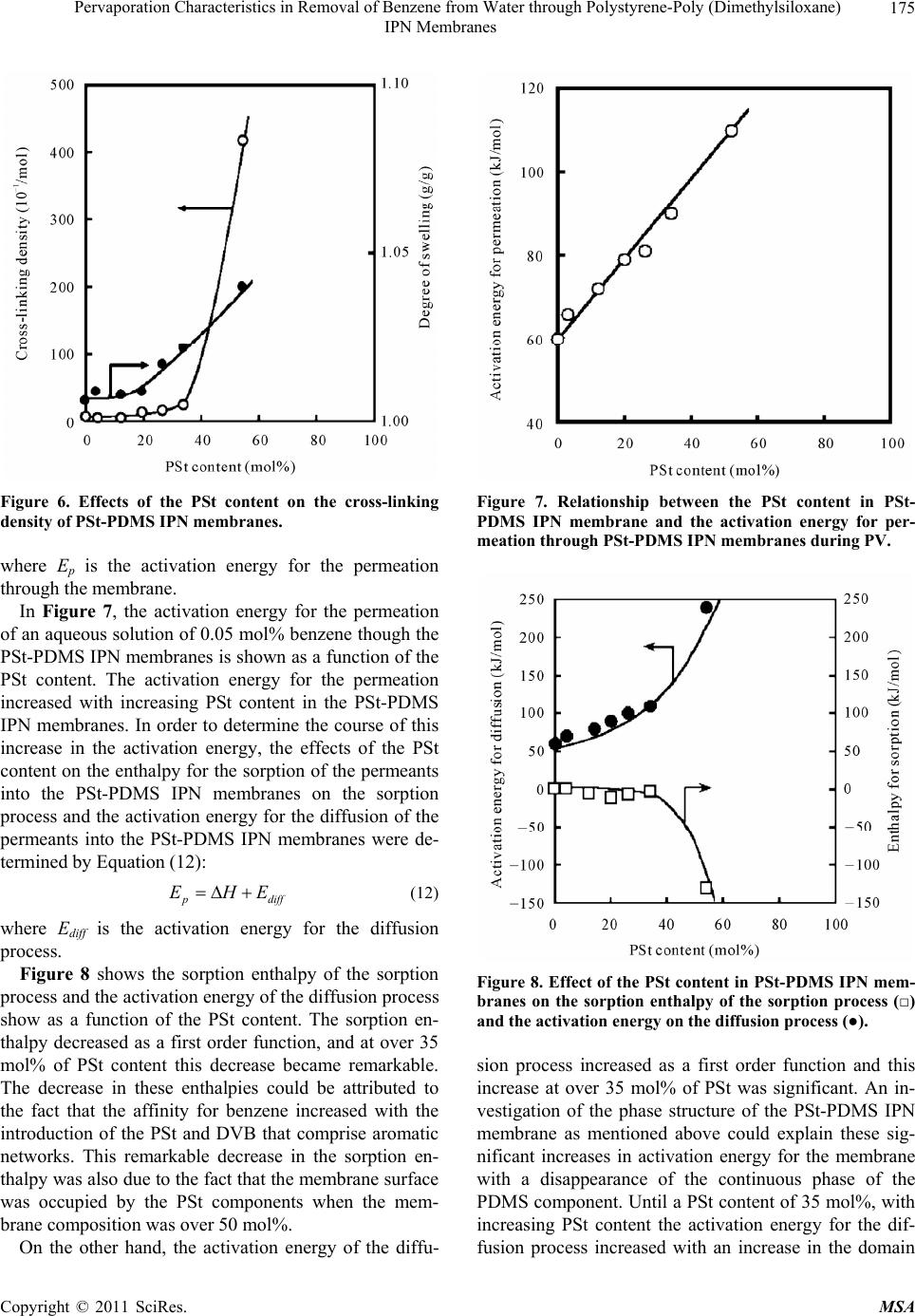 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 175 Figure 6. Effects of the PSt content on the cross-linking density of PSt-PDMS IPN membranes. where Ep is the activation energy for the permeation through the membrane. In Figure 7, the activation energy for the permeation of an aqueous solution of 0.05 mol% benzene though the PSt-PDMS IPN membranes is shown as a function of the PSt content. The activation energy for the permeation increased with increasing PSt content in the PSt-PDMS IPN membranes. In order to determine the course of this increase in the activation energy, the effects of the PSt content on the enthalpy for the sorption of the permeants into the PSt-PDMS IPN membranes on the sorption process and the activation energy for the diffusion of the permeants into the PSt-PDMS IPN membranes were de- termined by Equation (12): p diff EHE (12) where Ediff is the activation energy for the diffusion process. Figure 8 shows the sorption enthalpy of the sorption process and the activation energy of the diffusion process show as a function of the PSt content. The sorption en- thalpy decreased as a first order function, and at over 35 mol% of PSt content this decrease became remarkable. The decrease in these enthalpies could be attributed to the fact that the affinity for benzene increased with the introduction of the PSt and DVB that comprise aromatic networks. This remarkable decrease in the sorption en- thalpy was also due to the fact that the membrane surface was occupied by the PSt components when the mem- brane composition was over 50 mol%. On the other hand, the activation energy of the diffu- Figure 7. Relationship between the PSt content in PSt- PDMS IPN membrane and the activation energy for per- meation through PSt-PDMS IPN membranes during PV. Figure 8. Effect of the PSt content in PSt-PDMS IPN mem- branes on the sorption enthalpy of the sorption process (□) and the activation energy on the diffusion process (●). sion process increased as a first order function and this increase at over 35 mol% of PSt was significant. An in- vestigation of the phase structure of the PSt-PDMS IPN membrane as mentioned above could explain these sig- nificant increases in activation energy for the membrane with a disappearance of the continuous phase of the PDMS component. Until a PSt content of 35 mol%, with increasing PSt content the activation energy for the dif- fusion process increased with an increase in the domain 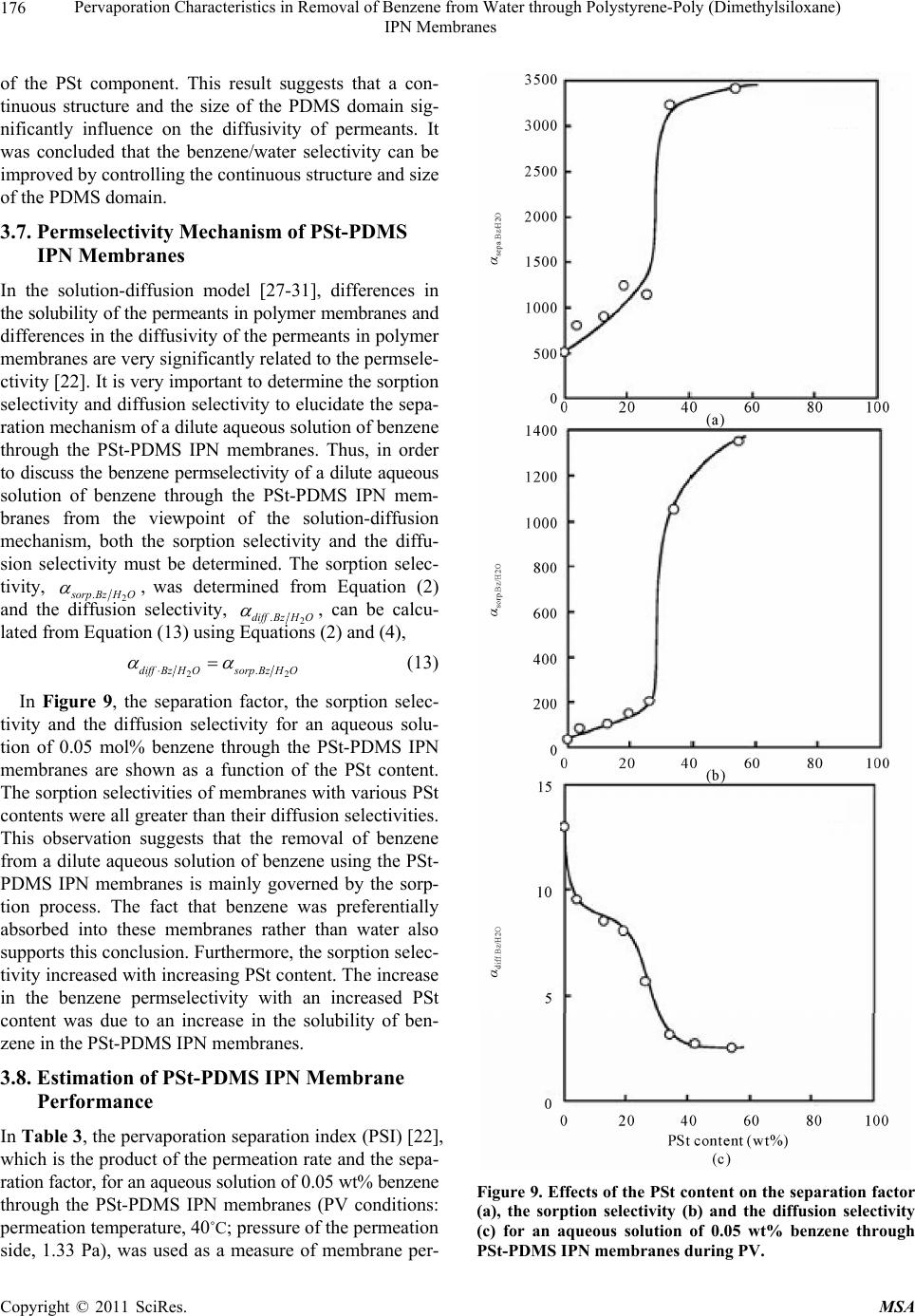 Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 176 of the PSt component. This result suggests that a con- tinuous structure and the size of the PDMS domain sig- nificantly influence on the diffusivity of permeants. It was concluded that the benzene/water selectivity can be improved by controlling the continuous structure and size of the PDMS domain. 3.7. Permselectivity Mechanism of PSt-PDMS IPN Membranes In the solution-diffusion model [27-31], differences in the solubility of the permeants in polymer membranes and differences in the diffusivity of the permeants in polymer membranes are very significantly related to the permsele- ctivity [22]. It is very important to determine the sorption selectivity and diffusion selectivity to elucidate the sepa- ration mechanism of a dilute aqueous solution of benzene through the PSt-PDMS IPN membranes. Thus, in order to discuss the benzene permselectivity of a dilute aqueous solution of benzene through the PSt-PDMS IPN mem- branes from the viewpoint of the solution-diffusion mechanism, both the sorption selectivity and the diffu- sion selectivity must be determined. The sorption selec- tivity, 2 . s orp BzHO , was determined from Equation (2) and the diffusion selectivity, 2 .diff Bz H O , can be calcu- lated from Equation (13) using Equations (2) and (4), 22 .diffBz HOsorpBz H O (13) In Figure 9, the separation factor, the sorption selec- tivity and the diffusion selectivity for an aqueous solu- tion of 0.05 mol% benzene through the PSt-PDMS IPN membranes are shown as a function of the PSt content. The sorption selectivities of membranes with various PSt contents were all greater than their diffusion selectivities. This observation suggests that the removal of benzene from a dilute aqueous solution of benzene using the PSt- PDMS IPN membranes is mainly governed by the sorp- tion process. The fact that benzene was preferentially absorbed into these membranes rather than water also supports this conclusion. Furthermore, the sorption selec- tivity increased with increasing PSt content. The increase in the benzene permselectivity with an increased PSt content was due to an increase in the solubility of ben- zene in the PSt-PDMS IPN membranes. 3.8. Estimation of PSt-PDMS IPN Membrane Performance In Table 3, the pervaporation separation index (PSI) [22], which is the product of the permeation rate and the sepa- ration factor, for an aqueous solution of 0.05 wt% benzene through the PSt-PDMS IPN membranes (PV conditions: permeation temperature, 40˚C; pressure of the permeation side, 1.33 Pa), was used as a measure of membrane per- Figure 9. Effects of the PSt content on the separation factor (a), the sorption selectivity (b) and the diffusion selectivity (c) for an aqueous solution of 0.05 wt% benzene through PSt-PDMS IPN membranes during PV.  Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 177 Table 3. Membrane perfomance of PSt-PDMS IPN mem- branes. PSt Content in Membrane (mol%) Separation Factor Normalized Permeation Rate (10−7 kgm (m2h)−1) PSI 0 479 6.99 3348 3.8 751 7.53 5655 12.7 1750 8.19 14332 19.6 1996 9.17 18303 26.5 2926 10.1 29552 34.2 3293 12.7 41821 54.5 3563 8.45 30107 formance during PV. As can be seen in Table 3, both the normalized permeation rate and the separation factor for the benzene/water selectivity of PSt-PDMS IPN mem- branes were improved with increasing PSt content. Based on this result, we discovered that the introduction of the PSt component into the IPN membrane matrix using a suitable component with a high affinity for a permeant is a very effective method to give both a high permeation rate and high permselectivity. 4. Conclusions The mechanism of permeation and separation for an aqueous solution of dilute benzene through PSt-PDMS IPN membranes could be explained by the model illus- tration shown in Figure 10. For the separation characte- ristics for an aqueous solution of dilute benzene through the PSt-PDMS IPN membranes, with an increasing PSt content in the PSt-PDMS IPN membrane, the benzene molecule was predominantly incorporated into the PSt- PDMS IPN membranes with a decrease in the sorption enthalpy during the sorption process. Consequently the PSt-PDMS membranes showed an excellent benzene/ water selectivity. A PSt-PDMS IPN membrane containing 55 mol% of PSt showed the highest benzene selectivity. On the other hand, in the term of permeation characteris- tics, the permeability was increased by an increase in the degree of swelling of the PSt-PDMS IPN membrane and the formation of a continuous phase of the PDMS com- ponent until 35 mol% PSt content. A PSt-PDMS IPN membrane with a PSt content of 55 mol% had the highest degree of swelling but the permeability was decreased by a decrease in the diffusivity of the permeants, because the continuous phase of the PDMS component disap- peared. It is noted that the permeation and separation behavior of the PST-PDMS IPN membranes was de- pendent on the continuity of the PDMS phase in the PSt-PDMS IPN membranes, and significantly influenced the permeability. On the basis of the above results, it is suggested that an excellent benzene permeable mem- Figure 10. Tentative mechanism of the permeation and separation of an aqueous solution of dilute benzene though PSt-PDMS IPN membranes during PV.  Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 178 brane can be prepared by controlling both the continuous phase and the domain size of the PDMS component. 5. Acknowledgements This work was financially supported by the Kansai Uni- versity Special Research Fund, 2008 and by the ‘High- Tech Research Center’’ Project in Matching Fund Subsidy for Private Universities, 2005-2009. REFERENCES [1] R. W. Baker, “Pervaporation Process,” In: R. W. Baker, E. L. Cussler, W. Eykamp, W. J. Koros and H. Strathmann, Eds.; Membrane Separation System, Recent developments and Future Directions, Noyes Data Corp, Park Ridge, 1991, p. 151. [2] W. W. Lau, J. Finlayson, J. M. Dickson, J. Jian and M. A. Brook, “Pervaporation Performance of Oligosilylstyrene- Polydimethylsiloxane Membrane for Separation of Orga- nics from Water,” Journal of Membrane Science, Vol. 134, No. 2, 1997, pp. 209-217. doi:10.1016/S0376-7388(97)00131-2 [3] T. A. C. Oliveria, J. T. Scarpello and A. G. Livinhston, “Pervaporation-Biological Oxidationhybrid Process for Removal of Volatile Organic Compounds from Waste- water,” Journal of Membrane Science, Vol. 195, No. 1, 2002, pp. 75-88. doi:10.1016/S0376-7388(01)00555-5 [4] Q. L. Liu and H. Xiao, “Silicalite-Filled Poly (Siloxane Imide) Membranes for Removal of VOCs from Ground- water,” Journal of Membrane Science, Vol. 230, 2004, pp. 121-129. doi:10.1016/j.memsci.2003.11.004 [5] F. Vilaplana, M. Osorio-Galindo, A. Iborra-Clar, M. Alcaina-Miranda and A. Ribes-Greus, “Swelling Behavior of PDMS-PMHS Pervaporation Membranes in Ethyl Acetate-Water Mixtures,” Journal of Applied Polymer Science, Vol. 93, 2004, pp. 1384-1393. doi:10.1002/app.20596 [6] F. Heymes, P. M. Demoustier, F. Carbit, J. L. Fanlo and P. Moulin, “Recovery of Toluene from High Temperature Boiling Adsorbents by Pervspoprstion,” Journal of Mem- brane Science, Vol. 284, 2006, pp. 145-154. doi:10.1016/j.memsci.2006.07.029 [7] H. F. Zhen, S. M. L. Jang W. K. Teo and K. Li, “Modi- fied Silicone-PVDF Composite Hollow-Fiber Membrane Preparation and Its Application in VOC Separation,” Journal of Applied Polymer Science, Vol. 99, No. 5, 2006, pp. 2497-2503. doi:10.1002/app.22860 [8] G. O. Yahara, “Separation of Volatile Organic Compounds (BTEX) from Aqueous Solutions by a Composite Orga- nophilic Hollow Fiber Membrane-Based Pervaporation Process,” Journal of Membrane Science, Vol. 319, No. 1-2, 2008, pp. 82-89. doi:10.1016/j.memsci.2008.03.024 [9] T. Miyata, J. Higuchi, H. Okuno and T. Uragami, “Preparation of Poly(Dimethylsiloxane)/Polystyrene Inter- penetrating Polymer Network Membranes and Permea- tion of Aqueous Ethanol Solutions through The Mem- branes by Pervaporation,” Journal of Applied Polymer Science, Vol. 61, 1996, pp. 1315-1324. doi:10.1002/(SICI)1097-4628(19960822)61:8<1315::AID -APP11>3.0.CO;2-Y [10] T. Miyata, Y. Nakanishi and T. Uragami. “Ethanol Per- mselectivity of Poly(Dimethylsiloxane) Membranes Con- trolled by Simple Surface Modifications using Polymer Additives,” Macromolecures, Vol. 30, No. 18, 1997, pp. 5563- 5565. doi:10.1021/ma9618843 [11] T. Uragami, T. Tsukamoto, K. Inui and T. Miyata, “Perva- poration Characteristics of a Benzoylchiotsan Membrane for Benzene/Cychlohexane Mixtures,” Macromolecular Chemistry & Physics, Vol. 199, 1998, pp. 49-54. doi:10.1002/(SICI)1521-3935(19980101)199:1<49::AID- MACP49>3.0.CO;2-B [12] K. Inui, H. Okumura, T. Miyata and T. Uragami, “Per- meation and Separation of Benzene/Cyclohexane Mix- tures through Cross-Linked Poly(Alkyl Methacrylate) Membranes” Journal of Membrane Science, Vol. 132, No. 2, 1997, pp. 193-202. doi:10.1016/S0376-7388(97)00069-0 [13] P. R. Brooks and A. G. Livingstone, “Aqueous-Aqueous Extraction of Organic Pollutants through Tubular Silicone Rubber Membranes,” Journal of Membrane Science, Vol. 104, No. 1-2, 1995, pp. 119-139. doi:10.1016/0376-7388(95)00020-D [14] S. Mishima and T. Nakagawa, “Permselectivity of Vo- latile Organic Chlorinated Compounds through Modified Poly(Dimethylsiloxane) Membranes,” Kobunnshi Ron- bunshu, Vol. 54, 1997, pp. 375-383. [15] D. Klempner and K. C. Frisch, “Polymer Alloys,” Plenum Press, New York, 1977. [16] L. H. Sperling, “Interpenetrating Polymeric Networks and Related Materials,” Plenum Press, New York, 1981. [17] X. W. He, J. M. Widmaier, J. E. Herz and G. C. Meyer, “Polydimethylsiloxane/Poly(Methylmethacrylate) Inter- penetrating Polymer Networks 2. Synthesis and Properties,” Polymer, Vol. 33, No. 4, 1992, pp. 866-871. doi:10.1016/0032-3861(92)90351-V [18] J. S. Turner and Y. L. Cheng, “pH Dependence of PDMS- PMMA IPN Morphology and Transport Properties,” Journal of Membrane Science, Vol. 240, 2004, No. 1-2, pp. 19-24. doi:10.1016/j.memsci.2004.04.008 [19] A. Hillerstrom, M. Andersson, J. Pedersen, A. Altskar, M. Langton and J. van Stam, “Transparency and Wetability of PVP/PDMS-IPN Synthesized in Different Organic Solvents,” Journalof Applied Polymer Science, Vol. 114, No. 3, 2009, pp. 1828-1839. doi:10.1002/app.30673 [20] R. Marques, T. MacLeod, I. Yoshida, V. Mno and M. Shiavon, “Synthesis and Characterization of Semi-Inter- penetrating Networks Based on Poly(Dimethysiloxane) and Poly(Vinyl Alcohol),” Journal of Applied Polymer Science, Vol. 115, 2010, pp. 158-166. doi:10.1002/app.31006 [21] J. S. Trent, J. I. Scheinbeim, and P. R. Couchman, “Ru- thenium Tetraoxide Staining of Polymers for Electron  Pervaporation Characteristics in Removal of Benzene from Water through Polystyrene-Poly (Dimethylsiloxane) IPN Membranes Copyright © 2011 SciRes. MSA 179 Microscopy,” Macromolecures, Vol. 16, 1983, 1983, pp. 589-598. [22] T. Uragami, S. Yamamoto and T. Miyata, (2003), “De- hydration from Alcohols by Polyioncomplex Cross-Linked Chitosan Composite Membranes during Evapomeation,” Biomacromolecules, Vol. 4, No. 1, 2003, pp. 137-144. doi:10.1021/bm025642o [23] T. Uragami, H. Yamada and T. Miyata, “Removal of Dilute Volatile Organic Compounds (VOCs) in Water through Graft Copolymer Membranes Consisting of Poly (Alkylmethacrylate) and Poly(Dimethylsiloxane) by Perva- poration and Their Membrane Morphology,” Journal of Membrane Science, Vol. 187, No. 1-2, 2001, pp. 255-269. doi:10.1016/S0376-7388(01)00355-6 [24] T. Uragami, T. Meotoiwa and T. Miyata, “Effects of the Addition of Calixarene Microphase-Separated Membranes for the Removal of Volatile Organic Compounds from Dilute Aqueous Solutions,” Macromolecures, Vol. 34, No. 19, 2001, pp. 6806-6811. [25] W. S. John, “Polymer Handbook,” 3rd Edition, Elsevier, Amsterdam, 1989. [26] T. Ohshima, M. Matsumoto, T. Miyata and T. Uragami, “Organic-Inorganic Hybrid Membranes for Removal of Benzene from an Aqueous Solution by Pervaporation,”. Macromolecular Chemistry & Physics, Vol. 206, No. 4, 2005, pp. 473-483. doi:10.1002/macp.200400401 [27] R. C. Binning, R. J. Lee, J. F. Jennings and E. C. Martin, “Separation of Liquid Mixtures by Permeation,” Indus- trial Engineering Chemistry, Vol. 53, 1961, pp. 45-54. doi:10.1021/ie50613a030 [28] P. Aptel, J. Cuny, J. Jozefonvicz, G. Morel and J. Neel, “Liquid Transport through Membranes Prepared by Grafting of Polar Monomers onto Poly(Tetrafluoroe- thylene) Films. III. Steady Sate Distribution in Membrane during Pervaporation,” Journal of Applied Polymer Science, Vol. 18, No. 2, 1974, pp. 365-398. doi:10.1002/app.1974.070180205 [29] E. E. Lee and A. L. Bunge, “Chemical Transport in Sili- cone Rubber Membranes from Pure Powders and Satu- lated Aqueous Solutions,” Journal of Membrane Science, Vol. 292, 2007, pp. 35-44. doi:10.1016/j.memsci.2007.01.007 [30] S. Y. Nam and J. R. Dorgan, “Non-Equuilibrium Nano- bleds via Forced Assembly for Pervaporation Separation of Benzene from Cyclohexane: UNIFAQ-FV Group Con- tribution Caluculations,” Journal of Memrane Science, Vol. 306, No. 1-2, 2007, pp. 186-195. doi:10.1016/j.memsci.2007.08.047 [31] S. J. Leu, J. S. Ou, C. H. Kuo, H. Y. Chen and T. H. Yang, “Pervaporation Separation of Azeotropic Mixtures in Polyurethane-Poly(Dimethylsiloxane) (PU-PDMS) Blend Membranes: Correlation with Sorption and Diffusion Behaviors in a Binary Solution System,” Journal of Mem- brane Science, Vol. 347, 2010, pp. 108-115. doi:10.1016/j.memsci.2009.10.012 |

