 International Journal of Organic Chemistry, 2013, 3, 275-279 Published Online December 2013 (http://www.scirp.org/journal/ijoc) http://dx.doi.org/10.4236/ijoc.2013.34039 Open Access IJOC Solvent-Free Synthesis of 5-Alkenyl-2,2-butylidene-1, 3-dioxane-4,6-diones under Ultrasonic Irradiation with o-Phthalimide-N-Sulfonic Acid as Catalyst Chunhua Lin, Zhaohui Xu, Weilin Liao Fine Chemical Key Laboratory of Jiangxi Province, Nanchang, China Email: gotoxzh@163.om, liao1liao2@163.com Received October 20, 2013; revised November 25, 2013; accepted December 11, 2013 Copyright © 2013 Chunhua Lin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT 5-Alkenyl-2,2-butylidene-1,3-dioxane-4,6-diones were synthesized by the Knoevenagel condensation reaction of aro- matic aldehydes with 2,2-butylidene-1,3-dioxane-4,6-dione using o-phthalimide-N-sulfonic acid as catalyst, without solvent under ultrasonic irradiation. The present method has some notable advantages such as mild reaction conditions, short reaction times, less catalyst dosage and high yields with the green aspects by avoiding toxic catalysts and solvents. Further, the catalyst can be reused for five times without any noticeable decrease in the catalytic activity. Keywords: o-Phthalimide-N-Sulfonicacid; 5-Alkenyl-2,2-butylidene-1,3-dioxane-4,6-diones; Aromatic Aldehydes; Knoevenagel Condensation 1. Introduction In recent years, the preparation of 5-alkenyl-1,3-dioxane- 4,6-diones has attracted strong interest owing to the broad spectrum of their properties: these compounds can be employed as versatile intermediates for a series of natural products and heterocyclic compounds with poten- tially biological activity [1-5]. In addition, it is also re- acted with Grignard reagent in conjugate addition [6] or conjugated dienes in Diels-Alder cycloaddition [7]. In particular, 5-alkenyl-2,2-dimethyl-1,3-dioxane-4,6-dione is an important reagent for synthesizing lipid-lowering drugs “Lipitor” [8]. Therefore, the preparation of 5-al- kenyl-1,3-dioxane-4,6-diones is of much current impor- tance. More recently, lots of methods for the synthesis of 5- alkenyl-1,3-dioxane-4,6-diones have been reported in the literature. Generally, they were synthesized by the Kno- evenagel condensation reaction of 1,3-dioxane-4,6- diones and aromatic aldehydes in the presence of bases such as pyridine [9,10], piperidine/glacial acetic acid [11, 12], or hexamethyldisilazane [13] and Lewis acids, such as anhydrous zinc chloride [14], TiCl4/pyridine [15] and CePW12O40 [16]. However, many of these methodologies have not been entirely satisfactory, owing to such draw- backs as low yields, long reaction time, more catalyst dosage, environmentally unfavorable solvents, emerging the problems of tedious work-up and effluent pollution. Uncatalyzed reaction was also reported in the literature using DMF or DMSO as solvent, which is toxic, terato- genic and suspected carcinogen. Those methods gave mixtures of unsaturated and Michael addition products [17]. Thus, a mild, efficient, and environmentally friendly method using economical catalyst is desirable. Recently, the solvent-free condition and the use of het- erogeneous catalysts have emerged as an eco-friendly al- ternative of great importance within organic synthesis, as they reduce environmental pollution and bring down han- dling costs due to simplification of work-up technique [18]. Ultrasound has increasingly been used in organic syn- thesis in the last three decades. Compared with tradi- tional methods, the procedure is more convenient and can be carried out in higher yields, shorter reaction time or milder conditions under ultrasound irradiation [19-23]. Our investigations on the application of ultrasound in or- ganic synthesis [16] and our work on Knoevengel con- densations [13,24] are continued. Herein, we would like to report an efficient and practical procedure for the synthe- sis of 5-alkenyl-2,2-butylidene-1,3-dioxane-4,6-diones with 2,2-butylidene-1,3-dioxane-4,6-dione [23,25] and 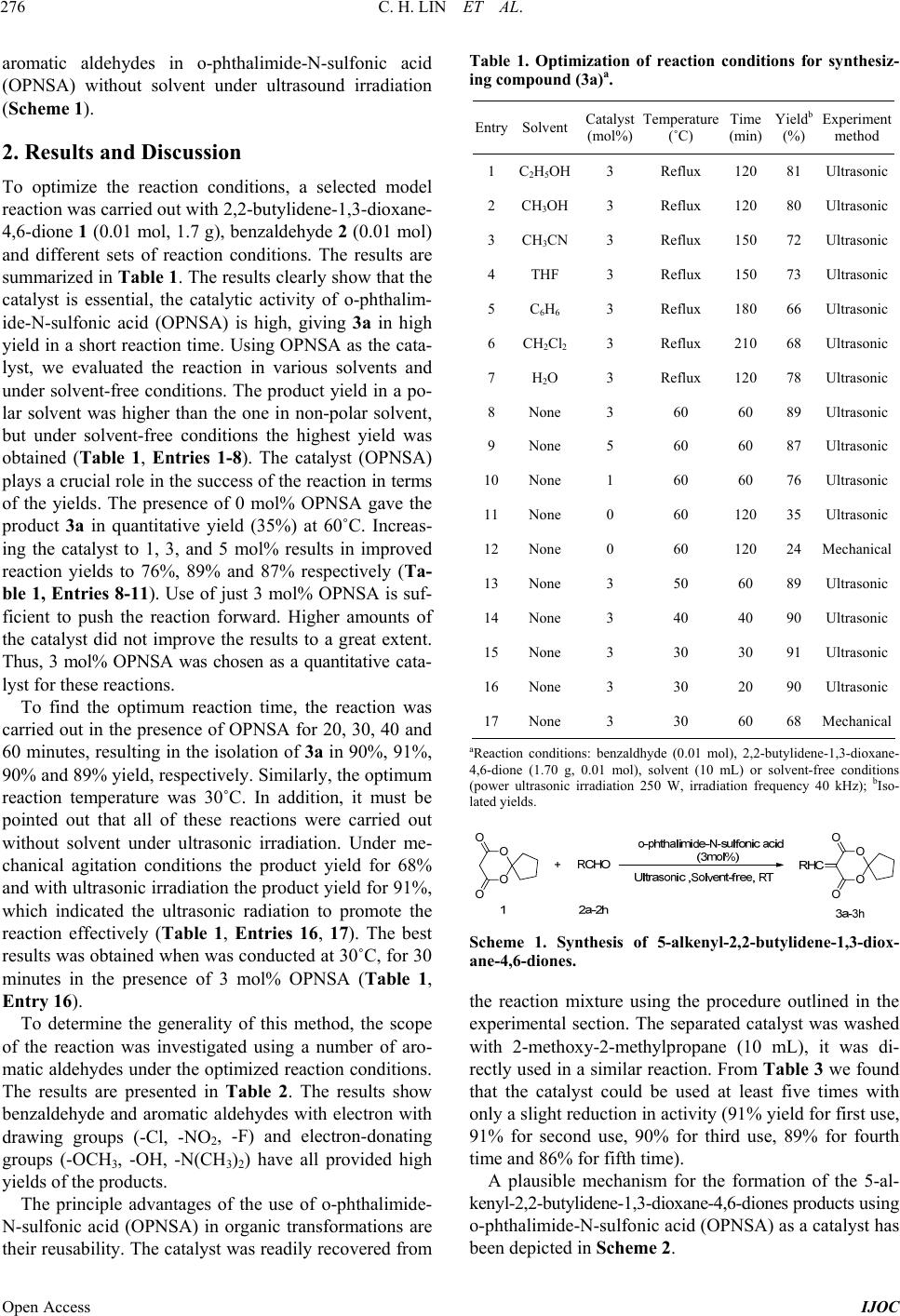 C. H. LIN ET AL. 276 aromatic aldehydes in o-phthalimide-N-sulfonic acid (OPNSA) without solvent under ultrasound irradiation (Scheme 1). 2. Results and Discussion To optimize the reaction conditions, a selected model reaction was carried out with 2,2-butylidene-1,3-dioxane- 4,6-dione 1 (0.01 mol, 1.7 g), benzaldehyde 2 (0.01 mol) and different sets of reaction conditions. The results are summarized in Table 1. The results clearly show that the catalyst is essential, the catalytic activity of o-phthalim- ide-N-sulfonic acid (OPNSA) is high, giving 3a in high yield in a short reaction time. Using OPNSA as the cata- lyst, we evaluated the reaction in various solvents and under solvent-free conditions. The product yield in a po- lar solvent was higher than the one in non-polar solvent, but under solvent-free conditions the highest yield was obtained (Table 1, Entries 1-8). The catalyst (OPNSA) plays a crucial role in the success of the reaction in terms of the yields. The presence of 0 mol% OPNSA gave the product 3a in quantitative yield (35%) at 60˚C. Increas- ing the catalyst to 1, 3, and 5 mol% results in improved reaction yields to 76%, 89% and 87% respectively (Ta- ble 1, Entries 8-11). Use of just 3 mol% OPNSA is suf- ficient to push the reaction forward. Higher amounts of the catalyst did not improve the results to a great extent. Thus, 3 mol% OPNSA was chosen as a quantitative cata- lyst for these reactions. To find the optimum reaction time, the reaction was carried out in the presence of OPNSA for 20, 30, 40 and 60 minutes, resulting in the isolation of 3a in 90%, 91%, 90% and 89% yield, respectively. Similarly, the optimum reaction temperature was 30˚C. In addition, it must be pointed out that all of these reactions were carried out without solvent under ultrasonic irradiation. Under me- chanical agitation conditions the product yield for 68% and with ultrasonic irradiation the product yield for 91%, which indicated the ultrasonic radiation to promote the reaction effectively (Table 1, Entries 16, 17). The best results was obtained when was conducted at 30˚C, for 30 minutes in the presence of 3 mol% OPNSA (Table 1, Entry 16). To determine the generality of this method, the scope of the reaction was investigated using a number of aro- matic aldehydes under the optimized reaction conditions. The results are presented in Table 2. The results show benzaldehyde and aromatic aldehydes with electron with drawing groups (-Cl, -NO2, -F) and electron-donating groups (-OCH3, -OH, -N(CH3)2) have all provided high yields of the products. The principle advantages of the use of o-phthalimide- N-sulfonic acid (OPNSA) in organic transformations are their reusability. The catalyst was readily recovered from Table 1. Optimization of reaction conditions for synthesiz- ing compound (3a)a. Entry SolventCatalyst (mol%) Temperature (˚C) Time (min) Yieldb (%) Experiment method 1C 2H5OH3 Reflux 120 81 Ultrasonic 2CH 3OH3 Reflux 120 80 Ultrasonic 3CH 3CN3 Reflux 150 72 Ultrasonic 4THF 3 Reflux 150 73 Ultrasonic 5C 6H6 3 Reflux 180 66 Ultrasonic 6CH 2Cl23 Reflux 210 68 Ultrasonic 7H 2O 3 Reflux 120 78 Ultrasonic 8None 3 60 60 89 Ultrasonic 9None 5 60 60 87 Ultrasonic 10None 1 60 60 76 Ultrasonic 11None 0 60 120 35 Ultrasonic 12None 0 60 120 24 Mechanical 13None 3 50 60 89 Ultrasonic 14None 3 40 40 90 Ultrasonic 15None 3 30 30 91 Ultrasonic 16None 3 30 20 90 Ultrasonic 17None 3 30 60 68 Mechanical aReaction conditions: benzaldhyde (0.01 mol), 2,2-butylidene-1,3-dioxane- 4,6-dione (1.70 g, 0.01 mol), solvent (10 mL) or solvent-free conditions (power ultrasonic irradiation 250 W, irradiation frequency 40 kHz); bIso- lated yields. Scheme 1. Synthesis of 5-alkenyl-2,2-butylidene-1,3-diox- ane-4,6-diones. the reaction mixture using the procedure outlined in the experimental section. The separated catalyst was washed with 2-methoxy-2-methylpropane (10 mL), it was di- rectly used in a similar reaction. From Table 3 we found that the catalyst could be used at least five times with only a slight reduction in activity (91% yield for first use, 91% for second use, 90% for third use, 89% for fourth time and 86% for fifth time). A plausible mechanism for the formation of the 5-al- kenyl-2,2-butylidene-1,3-dioxane-4,6-diones products using o-phthalimide-N-sulfonic acid (OPNSA) as a catalyst has been depicted in Scheme 2. Open Access IJOC 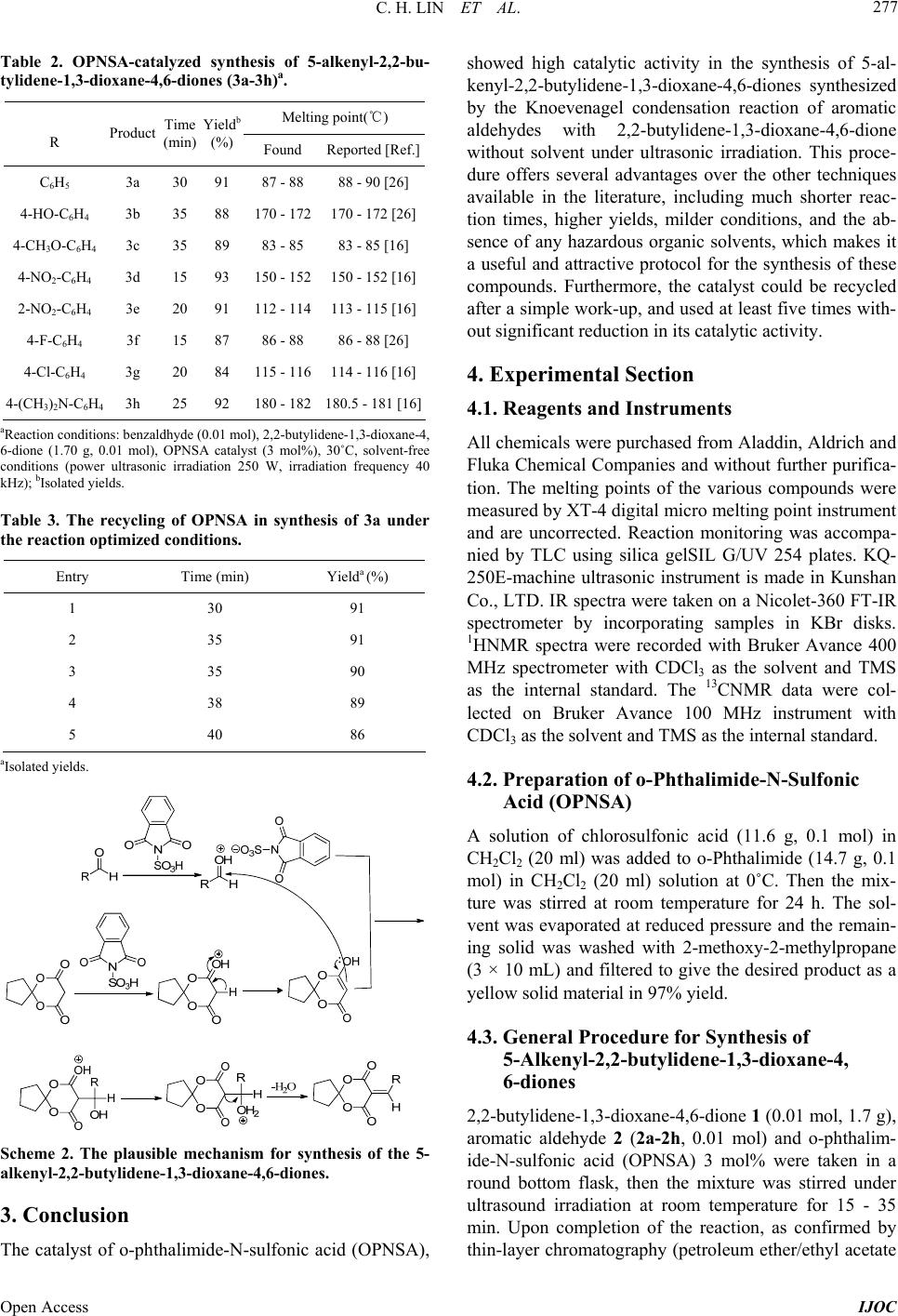 C. H. LIN ET AL. 277 Table 2. OPNSA-catalyzed synthesis of 5-alkenyl-2,2-bu- tylidene-1,3-dioxane-4,6-diones (3a-3h)a. Melting point()℃ R Product Time (min) Yieldb (%) Found Reported [Ref.] C6H5 3a 30 91 87 - 88 88 - 90 [26] 4-HO-C6H4 3b 35 88 170 - 172 170 - 172 [26] 4-CH3O-C6H4 3c 35 89 83 - 85 83 - 85 [16] 4-NO2-C6H4 3d 15 93 150 - 152 150 - 152 [16] 2-NO2-C6H4 3e 20 91 112 - 114 113 - 115 [16] 4-F-C6H4 3f 15 87 86 - 88 86 - 88 [26] 4-Cl-C6H4 3g 20 84 115 - 116 114 - 116 [16] 4-(CH3)2N-C6H4 3h 25 92 180 - 182 180.5 - 181 [16] aReaction conditions: benzaldhyde (0.01 mol), 2,2-butylidene-1,3-dioxane-4, 6-dione (1.70 g, 0.01 mol), OPNSA catalyst (3 mol%), 30˚C, solvent-free conditions (power ultrasonic irradiation 250 W, irradiation frequency 40 kHz); bIsolated yields. Table 3. The recycling of OPNSA in synthesis of 3a under the reaction optimized conditions. Entry Time (min) Yielda (%) 1 30 91 2 35 91 3 35 90 4 38 89 5 40 86 aIsolated yields. Scheme 2. The plausible mechanism for synthesis of the 5- alkenyl-2,2-butylidene-1,3-dioxane-4,6-diones. 3. Conclusion The catalyst of o-phthalimide-N-sulfonic acid (OPNSA), showed high catalytic activity in the synthesis of 5-al- kenyl-2,2-butylidene-1,3-dioxane-4,6-diones synthesized by the Knoevenagel condensation reaction of aromatic aldehydes with 2,2-butylidene-1,3-dioxane-4,6-dione without solvent under ultrasonic irradiation. This proce- dure offers several advantages over the other techniques available in the literature, including much shorter reac- tion times, higher yields, milder conditions, and the ab- sence of any hazardous organic solvents, which makes it a useful and attractive protocol for the synthesis of these compounds. Furthermore, the catalyst could be recycled after a simple work-up, and used at least five times with- out significant reduction in its catalytic activity. 4. Experimental Section 4.1. Reagents and Instruments All chemicals were purchased from Aladdin, Aldrich and Fluka Chemical Companies and without further purifica- tion. The melting points of the various compounds were measured by XT-4 digital micro melting point instrument and are uncorrected. Reaction monitoring was accompa- nied by TLC using silica gelSIL G/UV 254 plates. KQ- 250E-machine ultrasonic instrument is made in Kunshan Co., LTD. IR spectra were taken on a Nicolet-360 FT-IR spectrometer by incorporating samples in KBr disks. 1HNMR spectra were recorded with Bruker Avance 400 MHz spectrometer with CDCl3 as the solvent and TMS as the internal standard. The 13CNMR data were col- lected on Bruker Avance 100 MHz instrument with CDCl3 as the solvent and TMS as the internal standard. 4.2. Preparation of o-Phthalimide-N-Sulfonic Acid (OPNSA) A solution of chlorosulfonic acid (11.6 g, 0.1 mol) in CH2Cl2 (20 ml) was added to o-Phthalimide (14.7 g, 0.1 mol) in CH2Cl2 (20 ml) solution at 0˚C. Then the mix- ture was stirred at room temperature for 24 h. The sol- vent was evaporated at reduced pressure and the remain- ing solid was washed with 2-methoxy-2-methylpropane (3 × 10 mL) and filtered to give the desired product as a yellow solid material in 97% yield. 4.3. General Procedure for Synthesis of 5-Alkenyl-2,2-butylidene-1,3-dioxane-4, 6-diones 2,2-butylidene-1,3-dioxane-4,6-dione 1 (0.01 mol, 1.7 g), aromatic aldehyde 2 (2a-2h, 0.01 mol) and o-phthalim- ide-N-sulfonic acid (OPNSA) 3 mol% were taken in a round bottom flask, then the mixture was stirred under ultrasound irradiation at room temperature for 15 - 35 min. Upon completion of the reaction, as confirmed by thin-layer chromatography (petroleum ether/ethyl acetate Open Access IJOC  C. H. LIN ET AL. 278 5:1), the reaction mixture was diluted with 20 mL etha- nol. The catalyst was collected from the filtrate, which was concentrated on reduced pressure. After the remain- ing catalyst was washed with 2-methoxy-2-methylpro- pane (10 mL), it was directly used for the next reaction. The crude solid product was filtered and then purified by recrytallization from ethanol to afford the pure product 3 (3a-3h). The physical data (mp, IR, NMR) of known compounds were identical to the corresponding literature data. 4.4. The Data for Representative Compounds 5-Benzylidene-2 ,2-butyliden e-1,3-dioxane-4,6-dione (Co- mpound 3a, Table 1): White solid (yield: 91%), M.p. 87˚C - 88˚C; IR max (KBr): 2951, 1763, 1734, 1626, 1571, 741,693 cm−1; 1H NMR (CDCl3, 400 MHz): 8.37 (s, 1H), 8.02 (d, J = 7.14 Hz, 2H), 7.56 - 7.47 (m, 3H), 2.22 (s, 4H), 1.89 (s, 4H); 13CNMR (CDCl3, 100 MHz): δ163.86, 160.48, 157.65, 133.65, 133.49, 131.68, 128.76, 115.71, 113.75, 38.54, 23.28. 5-(4-Hydroxybenzylidene)-2,2- butylidene-1,3- dioxane- 4,6-dione (Compound 3b, Table 1): White solid (yield: 88%), M.p. 170˚C - 172˚C; IR max (KBr): 2965, 2957, 1750, 1700, 1638, 1537, 1400, 1170, 800 cm−1; 1H NMR (CDCl3, 400 MHz): 8.35 (s, 1H), 8.12 (d, J = 8.82 Hz, 2H), 6.94 (d, J = 8.82 Hz, 2H), 2.24 - 2.19 (m, 4H), 1.90 - 1.85 (m, 4H); 13CNMR (CDCl3, 100 MHz,): δ 164.93, 162.17, 158.37, 137.88, 124.43, 116.21, 113.67, 110.94, 38.41, 23.26. 5-(4-Methoxybenzylidene)-2,2- butylidene-1,3- dioxane- 4,6-dione (Compound 3c, Table 1): White solid (yield: 89%), M.p. 83˚C - 85˚C; IR max (KBr): 2972, 2901, 1756, 1718, 1624, 1573, 1455, 1381, 1183, 800 cm−1; 1H NMR (CDCl3, 400 MHz): 8.32 (s, 1H),8.19 (d, J = 8.97 Hz, 2H), 6.98 (d, J = 8.97 Hz, 2H), 3.90 (s, 3H), 2.23 - 2.18 (m, 4H), 1.90 - 1.85 (m, 4H); 13CNMR (CDCl3, 100 MHz,): δ 164.62, 161.19, 157.56, 137.48, 124.71, 114.38, 113.37, 111.65, 55.68, 38.41, 23.26. 5-(4-Nitrobenzylidene)-2,2-butylidene-1,3-dioxane-4,6- dione (Compound 3d, Table 1): Pale yellow solid (yield: 93%), M.p. 150˚C - 152˚C; IR max (KBr): 2976, 2901, 1763, 1731, 1626, 1605, 1524, 1350, 800 cm−1; 1H NMR (CDCl3, 400 MHz): 8.41 (s, 1H), 8.30 (d, J = 8.82 Hz, 2H), 8.06 (d, J = 8.70 Hz, 2H), 2.27 - 2.23 (m, 4H), 1.95 - 1.90 (m, 4H); 13CNMR (CDCl3, 100 MHz): δ 162.36, 159.36, 153.67, 149.26, 137.11, 132.73, 123.30, 119.17, 114.08, 36.43, 23.00. 5-(2-Nitrobenzylidene)-2,2-butylidene-1,3-dioxane-4,6- dione (Compound 3e, Ta ble 1): Pale yellow solid (yield: 91%), M.p. 112˚C - 114˚C; IR max (KBr): 2963, 2908, 1765, 1736, 1625, 1604, 1524, 1363, 800 cm−1; 1H NMR (CDCl3, 400 MHz): 8.75 (s, 1H), 8.28 (d, J = 8.22 Hz, 1H), 7.78 - 7.62 (m, 2H), 7.49 (d, J = 7.56 Hz, 1H), 2.24 - 2.21 (m, 4H), 1.91 - 1.89 (m, 4H); 13CNMR (CDCl3, 100 MHz): δ 161.69, 159.22, 155.09, 145.99, 133.46, 130.57, 129.99, 129.39, 124.44, 118.19, 114.14, 38.23, 22.87. 5-(4-Furylmethylene)-2,2-butylidene-1,3-dioxane-4,6- dione (Compound 3e, Tabl e 1 ): White solid (yield: 87%), M.p. 86˚C - 88˚C; IR max (KBr): 2961, 2943, 1757, 1726, 1598, 1604, 1508, 1364, 1081, 840, 804 cm−1; 1H NMR (CDCl3, 400 MHz): 8.33 (s, 1H), 8.14 (d, J = 5.58, 8.64 Hz, 2H), 7.16 (t, J = 8.61 Hz, 1H), 2.24 - 2.19 (m, 4H), 1.91 - 1.86 (m, 4H); 13CNMR (CDCl3, 100 MHz,): δ 167.44, 164.01, 163.81, 160.54, 156.21, 136.73, 136.60, 128.07, 128.03, 116.27, 115.98, 114.99, 114.96, 113.74, 38.49, 23.24. 5. Acknowledgements The authors thank the financial support from the National Science and Technology Project (No. 2001BA323C) and the Graduate Innovation Foundation of Jiangxi Province (No.YC10A051). REFERENCES [1] R. T. Acobs, A. D. Wright and F. X. Smith, “Condensa- tion of Monosubstituted Isopropylidene Malonates with Mannich Bases,” Journal of Organic Chemistry, Vol. 47, No. 19, 1982, pp. 3769-3772. http://dx.doi.org/10.1021/jo00140a038 [2] R. Bruns, A. Wernicke and P. Koll, “Application of the Reaction of D-Glucose with Meldrum’s Acid: Total Syn- thesis of the Styryl Lactones (+)-Goniofufurone and (+)- 7-epi-goniofufurone,” Tetrahedron, Vol. 55, No. 32, 1999, pp. 9793-9800. [3] I. N. Nestrova, A. K. Shanazarov and A. M. Poznyak, “Improved Method of Synthesis 2,2-Dimethyl-4,6-dioxo- 1,3-dioxane (Meldrum’s Acid),” Pharmaceutical Chemistry Journal, Vol. 28, No. 8, 1994, pp. 583-585. http://dx.doi.org/10.1007/BF02219035 [4] E. M. Abdelaziz, A. C. Jhonny Azuaje, C. Ernesto, Y. C. Matilde, Y. C. Vicente and S. Eddy, “Discovery and Pre- liminary SAR of 5-Arylidene-2,2-dimethyl-1,3-dioxane-4, 6-diones as Platelet Aggregation Inhibitors,” Combinato- rial Chemistry & High Throughput Screening, Vol. 15, No. 7, 2012, pp. 551-554. http://dx.doi.org/10.2174/138620712801619122 [5] W. G. Rajecwarna, R. B. Labroo and L. A. Cohen, “Syn- thesis of 5-[(Indol-2-on-3-yl)methyl]-2,2-dimethyl-1,3-di- oxane-4,6-diones and Spirocyclopropyloxindole Deriva- tives. Potential Aldose Reductase Inhibitors,” Journal of Organic Chemistry, Vol. 64, No. 4, 1999, pp. 1369-1371. [6] T. Watanabe, T. F. Knpfel and E. M. Carreira, “Asym- metric Conjugate Addition Reactions of Meldrum’s Acid Derived Acceptors Employing Chiral Phosphoramidite Ligands,” Organic Letters, Vol. 5, No. 25, 2003, pp. 4557- 4558. http://dx.doi.org/10.1021/ol035575q [7] E. Fillion, A. M. Dumas and S. A. Hogg, “Modular, Open Access IJOC  C. H. LIN ET AL. Open Access IJOC 279 Modular Synthesis of Tetrahydrofluorenones from 5-Al- kylidene Meldrum’s Acids,” The Journal of Organic Chem- istry, Vol. 71, No. 26, 2006, pp. 9899-9902. http://dx.doi.org/10.1021/jo0618876 [8] S. Rádl, J. Stach and J. Hajicek, “An Improved Synthesis of 1,1-Dimethylethyl6-cyanomethyl-2,2-dimethyl-1,3-diox- ane-4-acetate, a Key Intermediate for Atorvastatin Syn- thesis,” Tetrahedron Letters, Vol. 43, No. 11, 2002, pp. 2087-2090. [9] D. Davidson and S. A. Bernhard, “The Structure of Mel- drum’s Supposed β-Lactonic Acid,” Journal of the Ameri- can Chemical Society, Vol. 70, No. 10, 1948, pp. 3426- 3428. http://dx.doi.org/10.1021/ja01190a060 [10] E. J. Corey, “The Mechanism of the Decarboxylation of α,β- and β,γ-Unsaturated Malonic Acid Derivatives and the Course of Decarboxylative Condensation Reactions in Pyridine,” Journal of the American Chemical Society, Vol. 74, 1952, pp. 5897-5905. http://dx.doi.org/10.1021/ja01143a021 [11] G. A. Kraus and M. E. Krolski, “Synthesis of a Precursor to Quassimarin,” Journal of Organic Chemistry, Vol. 51, No. 17, 1986, pp. 3347-3450. http://dx.doi.org/10.1021/jo00367a017 [12] P. Scuster, O. E. Polansky and F. Wessely, “Zur Kenntnis cyclischer Acylale, 6. Mitt.,” Monatshefte für Chemie, Vol. 95, 1964, pp. 53-58. http://dx.doi.org/10.1007/BF00909251 [13] Z. H. Xu, N. Yan and W. L. Liao, “Knoevenagel Conden- sation Reaction of 2,2-Dimethyl-1,3-dioxane-4,6-dione and Aromatic Aldehydes by Hexamethyldisilazane,” Chi- nese Journal of Jiangxi Normal University (Natural Sci- ence), Vol. 36, No. 5, 2012, pp. 524-526. [14] P. S. Rao and R. V. Venkataratnam, “Zinc Chloride as a New Catalyst for Knoevenagel Condensation,” Tetrahe- dron Letters, Vol. 32, No. 41, 1991, pp. 5821-5822. http://dx.doi.org/10.1016/S0040-4039(00)93564-0 [15] A. M. Dumas and E. Fillion, “Meldrum’s Acids and 5- Alkylidene Meldrum’s Acids in Catalytic Carbon-Carbon Bond-Forming Processes,” Accounts of Chemical Research, Vol. 43, No. 3, 2009, pp. 440-454. http://dx.doi.org/10.1021/ar900229z [16] Z. H. Xu and C. H. L, “Solvent-Free Synthesis of 5-Al- kenyl-2,2-pentamethylene-1,3-dioxane-4,6-diones under Ul- trasonic Irradiation with CePW12O40 as Catalyst,” Chi- nese Journal of Organic Chemistry, Vol. 33, No. 7, 2013, pp. 1540-1544. [17] A. M. Dumas, A. Seed, A. K. Zorzitto and E. Fillion, “A General and Practical Preparation of Alkylidene Mel- drum’s Acids,” Tetrahedron Letters, Vol. 48, No. 40, 2007, pp. 7072-7074. http://dx.doi.org/10.1016/j.tetlet.2007.08.012 [18] B. Janardhan, G. Rajitha, V. Ravibabu, B. Rajitha and A. C. Peter, “An Eco-Friendly Improved Protocol for the Synthesis of Bis(3-indolyl)methanes Using Poly(4-vi- nylpyridinium)hydrogen Sulfate as Efficient, Heteroge- neous, and Recyclable Solid Acid Catalyst,” ISRN Or- ganic Chemistry, Vol. 2013, 2013, pp. 1-5. [19] Z. H. Jun, J. T. Li and Y. J. Bian, “Pinacolization of Aromatic Aldehydes Using Zn/Montmorillonite K10-ZnCl2 in Aqueous THF under Ultrasound,” Chemical Journal on Internet, Vol. 5, No. 1, 2003, pp. 8-9. [20] J. T. Li, X. H. Zhang and Z. P. Lin, “An Improved Synthe- sis of 1,3,5-Triaryl-2-pyrazolines in Acetic Acid Aqueous Solution under Ultrasound Irradiation,” Beilstein Journal of Organic Chemistry, Vol. 13, No. 3, 2007, pp. 1-4. [21] T. P. Caulier and J. Reisse, “On Sonochemical Effects on the Diels-Alder Reaction,” Journal of Organic Chemistry, Vol. 60, No. 7, 1996, pp. 2547-2548. http://dx.doi.org/10.1021/jo9520203 [22] M. M. Mojtahedi, M. S. Abaee, M. Samianifard, A. Sham- loo, M. Padyab, A. W. Mesbah and K. Harms, “Ultra- sound Mediation for Efficient Synthesis of Monoarylidene Derivatives of Homo- and Heterocyclic Ketones,” Ultra- sonics Sonochemistry, Vol. 20, No. 3, 2013, pp. 924-930. http://dx.doi.org/10.1016/j.ultsonch.2012.11.004 [23] A. Lahyani, M. Chtourou, M. H. Frikha and M. Trabels, “Amberlyst-15 and Amberlite-200C: Efficient Catalysts for Aldol and Cross-Aldol Condensation under Ultrasound Irradiation,” Ultrasonics Sonochemistry, Vol. 20, No. 5, 2013, pp. 1296-1303. http://dx.doi.org/10.1016/j.ultsonch.2013.01.017 [24] N. Yan, B. Xiong, W. L. Liao and Z. H. Xu, “Catalytic Synthesis of 1,3-Dioxane-4,6-dione by La(OTf)3,” Chi- nese Journal of Organic Chemistry, Vol. 30, No. 30, 2010, pp. 1391-1394. [25] Z. H. Xu, C. H. Lin and J. H. Xia, “An Efficient Synthe- sis of 1,3-Dioxane-4,6-diones by Boric Acid,” Heterocyc- lic Letters, Vol. 3, No. 3, 2013, pp. 319-323. [26] F. P. Xu, W. F. Ding and B. L. Da, “Reactions of 2,2- Pentamethylene-1,3-dioxan-4,6-dione with Triethyl Ortho- formate and Aromatic Amines,” Chinese Journal of Or- ganic Chemistry, Vol. 11, No. 3, 1991, pp. 318-321.
|