 International Journal of Clinical Medicine, 2013, 4, 32-43 Published Online December 2013 (http://www.scirp.org/journal/ijcm) http://dx.doi.org/10.4236/ijcm.2013.412A1007 Open Access IJCM Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cas es Emeline M. Aviki1*, Sophie M. Cowan2, Laura Young1, Marcela G. Del Carmen1, Whitfield B. Growdon1, Anthony H. Russell3, Annekathryn Goodman1 1Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, USA; 2Department of Radiology, Massachu- setts General Hospital, Boston, USA; 3Department of Radiation Oncology, Massachusetts General Hospital, Boston, USA. Email: *eaviki@partners.org Received October 30th, 2013; revised November 29th, 2013; accepted December 15th, 2013 Copyright © 2013 Emeline M. Aviki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Emeline M. Aviki et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian. ABSTRACT Background: Radiation-induced pelvic insufficiency fracture (PIF) is an important complication associated with pelvic radiation therapy (RT) for patients with gynecologic malignancies. Despite known risk factors and recent reports de- scribing the incidence on the order of 30 percent, there has been a dearth of translational research or consensus state- ments to guide clinical management. Objective: The aim of this study is to describe seven cases of PIF diagnosed and managed at the Massachusetts General Hospital during a 5-year period and to perform a focused review of the literature to inform several clinical questions that remain unanswered. A secondary aim of this study is to highlight the need for additional research related to screening, prophylaxis, diagnostics, and treatment of PIF in patients with gynecologic malignancy. Methods: In the current retrospective review, we report 10 cases of PIF diagnosed over a 5-year period in 7 patients with vulvar (4), vaginal (2), and cervical (1) cancer following chemoradiation therapy at a single institution. Data were collected from the medical records by a single investigator and all diagnostic imaging was reviewed by a single radiologist to confirm the presence or absence of PIF. Results: All 7 patients were post-menopausal and received concurrent chemoradiation, 3 were over the age of 65 years old (42.8%), 3 had BMI < 25 kg/m2 (42.8%), 2 had a his- tory of osteoporosis (28.6%), and 1 had a history of hormone replacement therapy use (14.3%). No patients underwent standard screening for PIF and no patients were started on prophylaxis prior to diagnosis. The plain film was the most common initial imaging performed while MRI was the most common overall study used to diagnose PIF. Median time to the development of fracture was 16 months (range 4 - 114) with femoral neck fracture being the most common (40%) and sacral fractures trailing close behind (30%). 7 of 10 fractures were initially managed expectantly with 1 ultimately failing expectant management and requiring surgical intervention. 4 of 10 fractures required surgical intervention. All patients had resolution of symptoms by 12 months after diagnosis. Conclusion: Radiation-induced PIF remains an im- portant complication associated with pelvic RT. Significant risk factors have been identified and studies have compared various diagnostic imaging modalities. Future studies are needed to compare screening algorithms and evaluate the comparative effectiveness of prophylactic pharmacotherapies. Future studies are also needed to determine the cost-effectiveness of PET/CT versus MRI and compare the morbidity associated with expectant management versus surgical intervention in patients with symptomatic fractures. Keywords: Pelvic Insufficiency Fracture; Vulva Cancer; Cervical Cancer; Radiation; Chemotherapy; Diagnostic Imaging; Fracture Screening; Fracture Prophylaxis 1. Introduction Insufficiency fractures (IFs) are a subtype of stress frac- tures that occur when normal stress is applied to the bone with decreased mineralization and decreased elastic re- sistance. Demineralization and reduced elastic resistance often result from osteoporosis, prior radiation therapy (RT), and prolonged corticosteroid use [1]. These factors, along with advanced age, low body weight, postmeno- pausal status, and others, have been reported as signifi- cant risk factors for developing pelvic insufficiency *Corres ondin autho .  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 33 fractures (PIF) [2-5]. Although PIF was once thought to be a rare complication of RT, recent studies conducted in gynecologic cancer patients have reported the incidence as high as 11.1% - 36.9% at two years and 8.2% - 19.7% at 5 years after completion of RT [3-9]. More remote studies conducted in the mid-1990’s reported incidences of PIF that range between 2.7% and 89% [2,10]. Despite awareness that PIF is more common than previously ex- pected, there continues to be an absence of standardized guidelines for screening and management of this com- plication. In this case series, we report on the experience at the Massachusetts General Hospital (MGH) and present evi- dence from the literature with a focus on answering the following clinically-relevant questions: What is the optimal method of surveillance for pa- tients after completion of RT? Who is at increased risk of developing PIF and how can fracture risk be reduced in these patients? What diagnostic imaging should be ordered for high risk patients presenting acute pelvic or hip pain? In cases where PIF is diagnosed, what are the differ- ent treatment options? 2. Materials and Methods From November 19, 2007 through November 3, 2012, thirteen women with vulva cancer and forty-two women with cervical cancer received definitive radiotherapy with concurrent chemotherapy at Massachusetts General Hospital. During this time interval, seven patients with pelvic insufficiency fractures following chemoradiation were identified. Medical records were reviewed and pa- tient characteristics such as age, BMI, medical comor- bidities, current medications, smoking status, meno- pausal status, primary cancer and stage were obtained. The patient’s cancer treatment history including surgery, chemotherapy, and RT were also collected. Hospital and clinic records were reviewed and presenting symptoms, date of symptom onset, imaging studies ordered, date of diagnosis, treatment offered, treatment received, and symptom control were recorded. All imaging studies in- cluding plain radiographs (plain film), computed tomo- graphy (CT), magnetic resonance imaging (MRI), and bone scintigraphy (bone scan) were performed at a single institution and reviewed by the same radiologist from the Department of Radiology who documented the presence and location of PIF in each study. 2.1. Follow-Up Patient follow-up information was available for all pa- tients in this series. Patients were evaluated post-RT at 3- to 12-month intervals for assessment of disease status as well as treatment-related toxicities and complications. This evaluation included a review of systems, physical examination, and imaging to assess response to therapy. The range of follow-up for patients was 19 to 124 mon- ths after completion of RT. Following diagnosis of PIF patients were evaluated at 2-week to 3-month intervals for assessment of symptoms and response to treatment. The range of follow-up for patients after diagnosis of PIF was 10 to 65 months. 2.2. Diagnostic Criteria for PIF PIF was defined as evidence of fracture on plain film, CT, MRI or bone scan occurring within the field of irra- diation. On plain films, insufficiency fractures (IF) are generally classified into two broad categories based on appearance: occult or aggressive. Occult IFs usually appear as vertical sclerotic bands, cortical disruptions, or fracture lines and are most commonly seen in the sacrum, the supra-acetabulum or the ilium. Aggressive appearing fractures resemble malignant neoplasms with findings that include areas of sclerosis and periosteal reaction. These are typically found in the parasymphysis and the pubic rami. Whether occult or aggressive in radiographic appearance, further imaging is required for a correct diagnosis of PIFs to be made [11]. On CT, findings su- ggestive of IF include areas of linear sclerosis or fracture lines. On MRI, findings suggestive of IF include both marrow edema and a fracture line. Marrow edema is seen as areas of increased signal intensity on T2-weighted images and as a hypointense linear bands on T1- weighted images. A hypointense fracture line is usually evident within the area of edema on both T1 and T2 weighted images, though it is not seen in around 7% of cases. MRI can usually differentiate marrow edema secondary to IFs from malignancy, with fat-saturation and postgadolinium imaging being particularly useful in this regard [12]. On bone scan, findings suggestive of insufficiency fracture include various patterns of increa- sed radiotracer uptake on delayed images and positive blood pool in acute cases. In the sacrum, increased up- take is seen in a vertical pattern through the sacral ala with or without a horizontal component through the sa- crum. The “Honda” sign or H-pattern is considered diag- nostic of sacral IFs in the correct clinical setting [12]. In vertebral bodies, findings include linear uptake in the superior endplate progressing to complete vertebral body involvement. In the proximal femur, findings include increased linear transverse uptake, with the femoral neck being the most common site. All imaging studies ob- tained on the seven cases were reviewed by the same radiologist from the Department of Radiology who de- termined whether PIF was evident based on the de- scribed, well-established, modality-specific criteria for diagnosis. Open Access IJCM  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 34 2.3. Endpoints and Analysis The time of PIF diagnosis was defined as the date of de- tection on imaging study. In patients with multiple frac- tures, the date of diagnosis for each fracture was re- corded separately. Time from completion of RT to diag- nosis of PIF was defined as the number of months from completion of radiation therapy to the diagnosis of PIF on imaging. Time from symptom onset to diagnosis of PIF on imaging was defined as the number of days be- tween documentation of initial symptom in the medical record to the date of diagnosis for each PIF. Institutional Review Board approval was obtained un- der protocol number 2010P002350, approved on October 15, 2012. 3. Results 3.1. Patient Characteristics Table 1 provides a summary of the patient characteristics for the 7 patients in this series. Three different gyneco- logic malignancies were treated: vulvar (4), vaginal (2), cervical (1). The patients ranged in age from 46 to 86 years old and in BMI from 20 to 31.7 kg/m2. All seven women were post-menopausal. Two had a history of to- bacco abuse, although only one remained a smoker throughout treatment. Three of the patients had a history of hypertension; only one was actively on antihyperten- sive medications. One patient had diabetes and hypothy- roidism, both of which were well controlled. One patient had a pre-existing diagnosis of severe osteoporosis diag- nosed in her 40’s with two prior known fractures in the left upper extremity; she already completed five years of estrogen replacement therapy prior to initiating RT. 3.2. Cancer Treatment Table 2 includes a summary of the radiation and chemo- therapy received by the 7 women in this series. All 7 wo- men received some form of both chemotherapy and ra- diation therapy. Five of the 7 women received cisplatin; 3 received cisplatin with 5-FU and 1 with Xeloda. One of the 7 received Xeloda alone and one received 5-FU with Mitomycin. The women in the series received a median of 57.6 Gy of pelvic radiation. Radiation was adminis- tered with a pelvic boost for 4 of 7 women with a median boost of 13 Gy. All patients received 40 - 45 Gy of radio- therapy to future sites of PIF. Six of the seven women underwent surgery for their cancer and 3 of the 6 under- went a pelvic lymph node dissection at the time of sur- gery. 3.3. Pelvic Insufficiency Fractures Table 3 provides a summary of information including presentation, diagnostics, treatment, and outcome associ- Table 1. Summary of patient characteristics (N = 7). Variable Value Range or Percent Age at XRT (years) Median 64 46 - 86 BMI (kg/m2) Median 26.6 21.5 - 31.7 Post-menopausal 7/7 100% Past Medical History Hypertension 3/7 42.8% Diabetes mellitus 1/7 14.3% Osteoporosis 2/7 28.6% Tobacco abuse 2/7 28.6% Primary Cancer Vulva 4/7 57.1% Vaginal 2/7 28.6% Cervical 1/7 14.3% FIGO Stage II 2/7 28.6% III 4/7 57.1% IV 1/7 14.3% Table 2. Cancer treatment history (N = 7). Variable Value Range or Percent Radiation Therapy Median EBRT (Gy) 57.6 44.8 - 68.4 Pelvic Boost 4/7 57.1% Median Boost (Gy) 13 9-23 Concurrent Chemotherapy 7/7 100% Ciaplatin alone 1/7 14.3% Cisplatin + 5FU 3/7 42.8% Cisplatin + Xeloda 1/7 14.3% 5FU + Mitomycin 1/7 14.3% Xeloda alone 1/7 14.3% Surgical Resection 6/7 85.7% Surgery with lymphadenectomy 3/7 42.8% Surgery without lymphadenectomy 3/7 42.8% ated with the ten fractures identified in this case series. Hip pain was the most common presenting symptom associated with PIF; two patients experienced pain only with ambulation, while one experienced pain that was suprapubic in location. Fractures were identified in five different areas of the pelvis; the most common location for PIF was on the femoral neck (40%) with the sacrum trailing close behind (30%). The distribution of fractures on the pelvis can be seen in Figure 1. The median time from completion of RT to diagnosis of PIF was 16 months (range 4 - 114 months), with most patients developing fractures less than six months following completion of RT. When time to fracture development was broken down by fracture location, the median time for development of acetabular, sacral, and femoral neck fractures appears to be less than Open Access IJCM 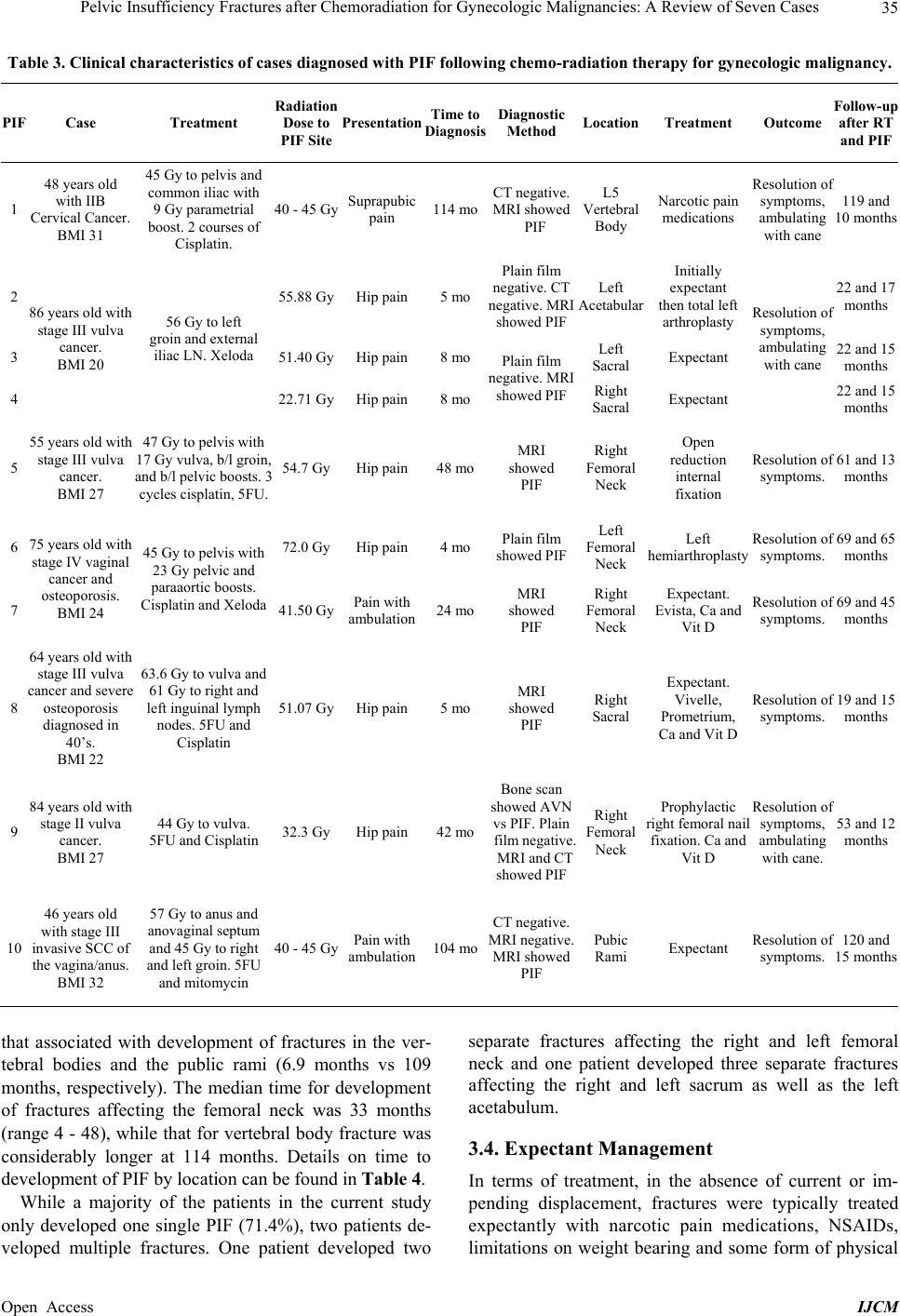 Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases Open Access IJCM 35 Table 3. Clinical characteristics of cases diagnosed with PIF following chemo-radiation therapy for gynecologic malignancy. PIF Case Treatment Radiation Dose to PIF Site Presentation Time to Diagnosis Diagnostic Method LocationTreatment OutcomeFollow-up after RT and PIF 1 48 years old with IIB Cervical Cancer. BMI 31 45 Gy to pelvis and common iliac with 9 Gy parametrial boost. 2 courses of Cisplatin. 40 - 45 Gy Suprapubic pain 114 mo CT negative. MRI showed PIF L5 Vertebral Body Narcotic pain medications Resolution o symptoms, ambulating with cane 119 and 10 months 2 55.88 Gy Hip pain 5 mo Plain film negative. CT negative. MRI showed PIF Left Acetabular Initially expectant then total left arthroplasty 22 and 17 months 3 51.40 Gy Hip pain 8 mo Left Sacral Expectant 22 and 15 months 4 86 years old with stage III vulva cancer. BMI 20 56 Gy to left groin and external iliac LN. Xeloda 22.71 Gy Hip pain 8 mo Plain film negative. MRI showed PIFRight Sacral Expectant Resolution o symptoms, ambulating with cane 22 and 15 months 5 55 years old with stage III vulva cancer. BMI 27 47 Gy to pelvis with 17 Gy vulva, b/l groin, and b/l pelvic boosts. 3 cycles cisplatin, 5FU. 54.7 Gy Hip pain 48 mo MRI showed PIF Right Femoral Neck Open reduction internal fixation Resolution o symptoms. 61 and 13 months 6 72.0 Gy Hip pain 4 mo Plain film showed PIF Left Femoral Neck Left hemiarthroplasty Resolution o symptoms. 69 and 65 months 7 75 years old with stage IV vaginal cancer and osteoporosis. BMI 24 45 Gy to pelvis with 23 Gy pelvic and paraaortic boosts. Cisplatin and Xeloda 41.50 Gy Pain with ambulation 24 mo MRI showed PIF Right Femoral Neck Expectant. Evista, Ca and Vit D Resolution o symptoms. 69 and 45 months 8 64 years old with stage III vulva cancer and severe osteoporosis diagnosed in 40’s. BMI 22 63.6 Gy to vulva and 61 Gy to right and left inguinal lymph nodes. 5FU and Cisplatin 51.07 Gy Hip pain 5 mo MRI showed PIF Right Sacral Expectant. Vivelle, Prometrium, Ca and Vit D Resolution o symptoms. 19 and 15 months 9 84 years old with stage II vulva cancer. BMI 27 44 Gy to vulva. 5FU and Cisplatin 32.3 Gy Hip pain 42 mo Bone scan showed AVN vs PIF. Plain film negative. MRI and CT showed PIF Right Femoral Neck Prophylactic right femoral nail fixation. Ca and Vit D Resolution o symptoms, ambulating with cane. 53 and 12 months 10 46 years old with stage III invasive SCC of the vagina/anus. BMI 32 57 Gy to anus and anovaginal septum and 45 Gy to right and left groin. 5FU and mitomycin 40 - 45 Gy Pain with ambulation 104 mo CT negative. MRI negative. MRI showed PIF Pubic Rami Expectant Resolution o symptoms. 120 and 15 months that associated with development of fractures in the ver- tebral bodies and the public rami (6.9 months vs 109 months, respectively). The median time for development of fractures affecting the femoral neck was 33 months (range 4 - 48), while that for vertebral body fracture was considerably longer at 114 months. Details on time to development of PIF by location can be found in Table 4. While a majority of the patients in the current study only developed one single PIF (71.4%), two patients de- veloped multiple fractures. One patient developed two separate fractures affecting the right and left femoral neck and one patient developed three separate fractures affecting the right and left sacrum as well as the left acetabulum. 3.4. Expectant Management In terms of treatment, in the absence of current or im- pending displacement, fractures were typically treated expectantly with narcotic pain medications, NSAIDs, limitations on weight bearing and some form of physical  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 36 Figure 1. Distribution of ten pelvic insufficiency fractures found in seven patients (N = 10). Table 4. Pelvic insufficiency fracture summary. Location (N) Median Time to Fracture (months) Range (months) All Fractures (10) 16 4 - 114 Femoral Neck (4) 33 4 - 48 Sacrum (3) 5 5 - 8 Acetabulum (1) 5 N/A Pubic Rami (1) 104 N/A L5 Vertebrae (1) 114 N/A therapy. There were a total of seven fractures in four different patients that were initially treated with expectant man- agement. One of the seven fractures ultimately failed expectant management and required surgical treatment. This case was that of a fracture located in the left ace- tabulum which continued to cause pain and progressed despite expectant measures. It ultimately developed a protrusion that required complex total hip arthroplasty. In the remaining six fractures in four different patients, ex- pectant management resulted in resolution of symptoms and improved functional capacity. In the series of cases that underwent expectant man- agement, the median follow-up after diagnosis of PIF was 15 months (range 10 - 45 months). At time of last patient follow-up, symptoms had resolved for all 6 ex- pectantly managed fractures. 3.5. Surgical Management Four out of seven patients in this series underwent surgi- cal management for four different fractures. Treatments included open reduction with internal fixation, prophy- lactic internal fixation, hemiarthroplasty, and total com- plex arthroplasty. One patient was diagnosed with a right femoral neck fracture 42 months following completion of radiation therapy and received an elective prophylactic right femoral nail fixation in the setting of advanced age of 88 and severe osteopenia throughout the pelvis. At 4 month post-op follow-up, the patient’s symptoms persisted and she continued to require narcotic medications for pain control; however, by 12 months, her symptoms had com- pletely resolved and she was able to ambulate without assistance. Among the three non-elective surgical cases, there was only one case where expectant management was attem- pted for three co-existing fractures, yet failed due to per- sistence of symptoms and progression of the fracture in left acetabulum. In this case, the left acetabular fracture progressed to the point of protrusion which ultimately required a complex left total hip arthroplasty. One patient developed a left femoral neck fracture 4 months following completion of RT which was treated with a left hemiarthroplasty; this patient then went on to develop a right femoral neck fracture 24 months follow- ing completion of RT, which was managed successfully with expectant management including initiation of Evista along with calcium and vitamin D supplementation. The final surgical case experienced an acute onset of hip pain one day after a normal routine bone scan and on subsequent imaging was found to have a right femoral neck fracture with high suspicion for hematoma forma- tion which was treated with an open reduction with in- ternal fixation two days later. Median follow-up after diagnosis of PIF for patients undergoing surgical management was 15 months (range 12 - 65 months). Non-elective surgical management in these cases resulted in resolution of symptoms and im- proved functional capacity. The one case managed with elective prophylactic femoral nail fixation also resulted in resolution of symptoms and improved functional ca- pacity by 12 month follow-up. 3.6. Diagnostic Imaging The most common initial test ordered when a patient in the current study presented with new onset hip pain was the plain film. Though not the most common initial test, MRI was the most common study ordered during the work-up of new onset hip or pelvic pain. Furthermore, MRI was required for diagnosis of PIF in all but one case, where plain film alone was sufficient to diagnose a com- plete left femoral neck fracture. In this series, when plain film was the initial imaging study, an average of 2 studies (range 1 - 3) were required to reach a diagnosis of PIF. When MRI was the initial imaging study (n = 3), no additional imaging was needed to reach a diagnosis of PIF. In the one case where bone scan was the initial imaging study ordered, an abnormal- Open Access IJCM  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 37 ity was confirmed on bone scan; however, additional imaging was required to determine what the abnormality represented. In this case the bone scan could not differ- entiate between AVN versus PIF so a plain film was or- dered and returned negative, followed by CT and MRI which both confirmed that a PIF was present. Details regarding the sequence of imaging studies ordered in each case can be found in Table 5. Select cases of insuf- ficiency fracture diagnosed using multiple imaging mo- dalities are illustrated in Figures 2-4. 4. Discussion Over the five-year study period, 7 cases of pelvic insuf- ficiency fracture were diagnosed in women who had un- dergone radiation therapy for gynecologic malignancies at Massachusetts General Hospital. All 7 patients were post-menopausal and received concurrent chemoradia- tion, 3 were over the age of 65 years old (42.8%), 3 had BMI less than 25 kg/m= (42.8%), 2 had a history of os- teoporosis (28.6%), and 1 had a history of hormone re- placement therapy use (14.3%). All fractures occurred within the irradiated field and were at locations that re- ceived significant doses of radiation (40 - 45 Gy). No pa- tients underwent standard screening for PIF and no pa- tients were started on prophylaxis prior to diagnosis. Plain film was the most common initial imaging per- formed while MRI was the most common overall study used to diagnose PIF. Median time to development of fracture was 16 months (range 4 - 114) with femoral neck fracture being the most common (40%) and sacral fractures trailing close behind (30%). 7 of 10 fractures were initially managed expectantly with 1 ultimately fail- ing expectant management and requiring surgical inter- vention. 4 of 10 fractures required surgical intervention. All patients had resolution of symptoms by 12 months after diagnosis. The MGH experience exposes a signifi- cant need for future research to inform evidence based clinical management of PIF in patients with gynecologic malignancies undergoing pelvic radiation therapy. 4.1. What Is the Optimal Method of Surveillance for Patients after Completion of RT? In women with gynecologic malignancy who have com- pleted pelvic RT, no formal or suggested screening algo- rithms have been published to guide clinicians on PIF surveillance. On the topic of surveillance, there are three specific questions that will be important to address: 1) When should surveillance begin and how long should it last? 2) What should surveillance involve? 3) How long after completion of RT should surveil- lance continue? At MGH, after completion of RT, patients are seen at 3 - 4 months intervals for the first 2 years and every six months thereafter, with routine imaging every 4 - 6 months. The focus of these visits is to assess for chemo- radiation related toxicity and cancer progression. There is no formal surveillance in place to screen for PIF in these patients. Since there are no published studies evaluating the topic of surveillance in these patients, findings in the existing literature can be used to guide our approach to surveillance. Evidence from retrospective studies suggest that the median time from completion of RT to development of PIF can range anywhere from 6 to 20 months [4-6,8,9, 13,14]. This is consistent with the institutional experi- ence at MGH where the data shows a median of 16 months for all fractures and 6.5 months if the observa- tional period is limited to 5 years, which was the maxi- mum period observed in the reviewed studies. There are additional insights offered in two prospec- tive studies where routine surveillance was conducted using imaging studies. In 2012 Tokomaru et al. pub- lished a prospective multi-institutional study on 59 cer- vical cancer patients who underwent definitive pelvic RT. In this study, patients were evaluated by both pelvic CT and MRI at 3, 6, 12, 18, and 24 months after completion of RT [3]. The cumulative incidence of IF was 36.9% at 2 years in all patients and 16.1% in symptomatic patients [3]. In 1996, Blomie et al. published a prospective study conducted on 18 women who underwent definitive RT for advanced cervical cancer [2]. These patients were assessed using MRI before, during, and after RT. After RT, MRI was performed at 3-month intervals for the first year then 6-month intervals until 30 months. The cumu- lative incidence of PIF was 89% during the 30 month study period. Focal lesions were not seen until 7 weeks post-RT with the greatest number of lesions developing between 3 and 18 months following completion of RT. Signal changes on MRI could be seen in the pelvic bones Table 5. Diagnostic imaging used to diagnose PIF. PIFImaging #1 Imaging #2 Imaging #3 Time to diagnosis 1CT (-) MRI (+) 4 days 2Plain film (-)CT (-) MRI (+) 5 days 3Plain film (-)MRI (+) 11 days 4Plain flim (-)MRI (+) 11 days 5MRI (+) 2 days 6Plain flim (+) 4 days 7MRI (+) 5 days 8MRI (+) 1 day 9Bone scan (?AVN vs PIF)Plain film (-) MRI (+) and CT (+) N/A 10CT (-) MRI (-) MRI (+) 21 days Open Access IJCM 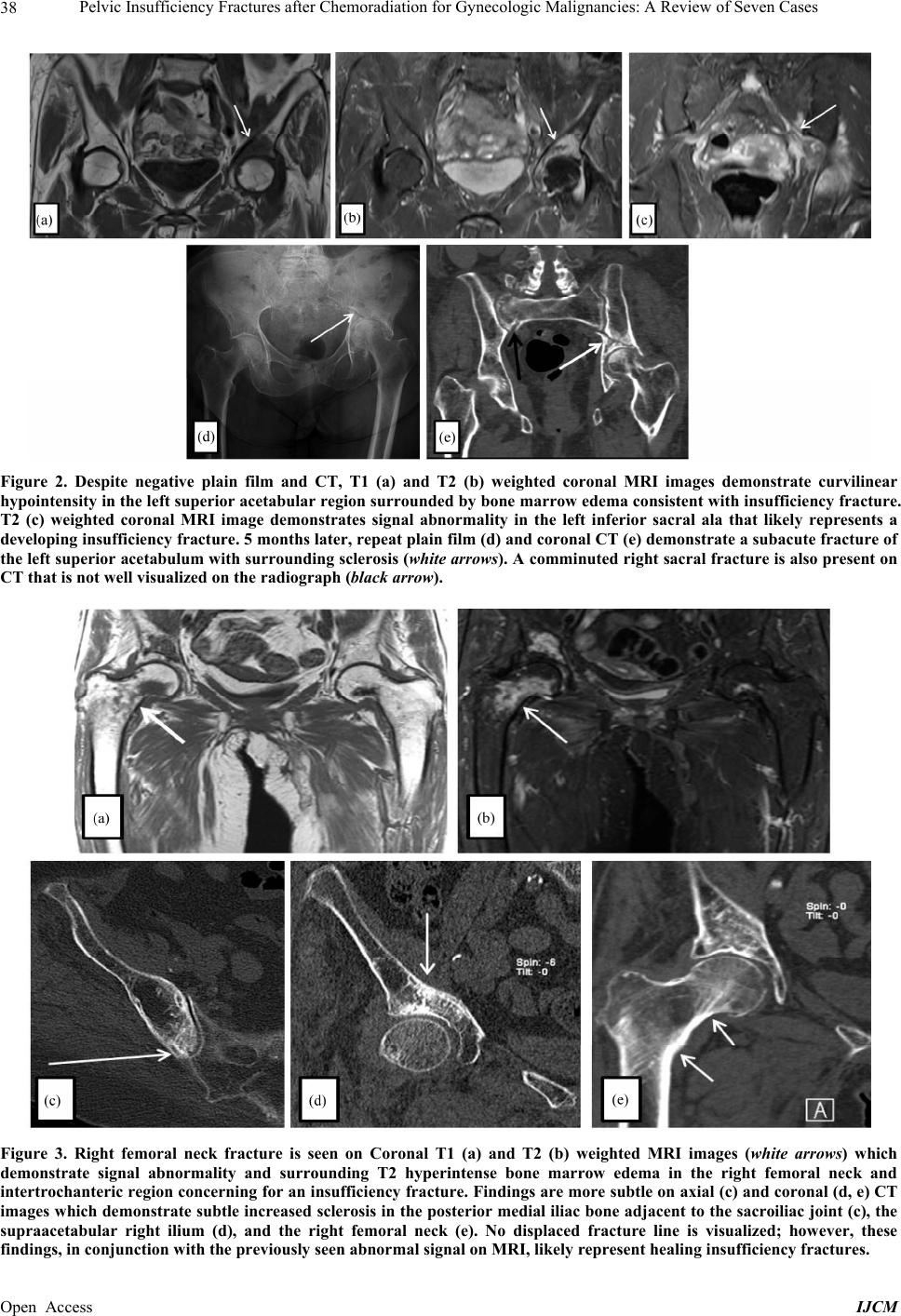 Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases Open Access IJCM 38 Figure 2. Despite negative plain film and CT, T1 (a) and T2 (b) weighted coronal MRI images demonstrate curvilinear hypointensity in the left superior acetabular region surrounded by bone marrow edema consistent with insufficiency fracture. T2 (c) weighted coronal MRI image demonstrates signal abnormality in the left inferior sacral ala that likely represents a developing insufficiency fracture. 5 months later, repeat plain film (d) and coronal CT (e) demonstrate a subacute fracture of the left superior acetabulum with surrounding sclerosis (white arrows). A comminuted right sacral fracture is also present on CT that is not well visualized on the radiograph (black arrow). Figure 3. Right femoral neck fracture is seen on Coronal T1 (a) and T2 (b) weighted MRI images (white arrows) which demonstrate signal abnormality and surrounding T2 hyperintense bone marrow edema in the right femoral neck and intertrochanteric region concerning for an insufficiency fracture. Findings are more subtle on axial (c) and coronal (d, e) CT images which demonstrate subtle increased sclerosis in the posterior medial iliac bone adjacent to the sacroiliac joint (c), the supraacetabular right ilium (d), and the right femoral neck (e). No displaced fracture line is visualized; however, these findings, in conjunction with the previously seen abnormal signal on MRI, likely represent healing insufficiency fractures. 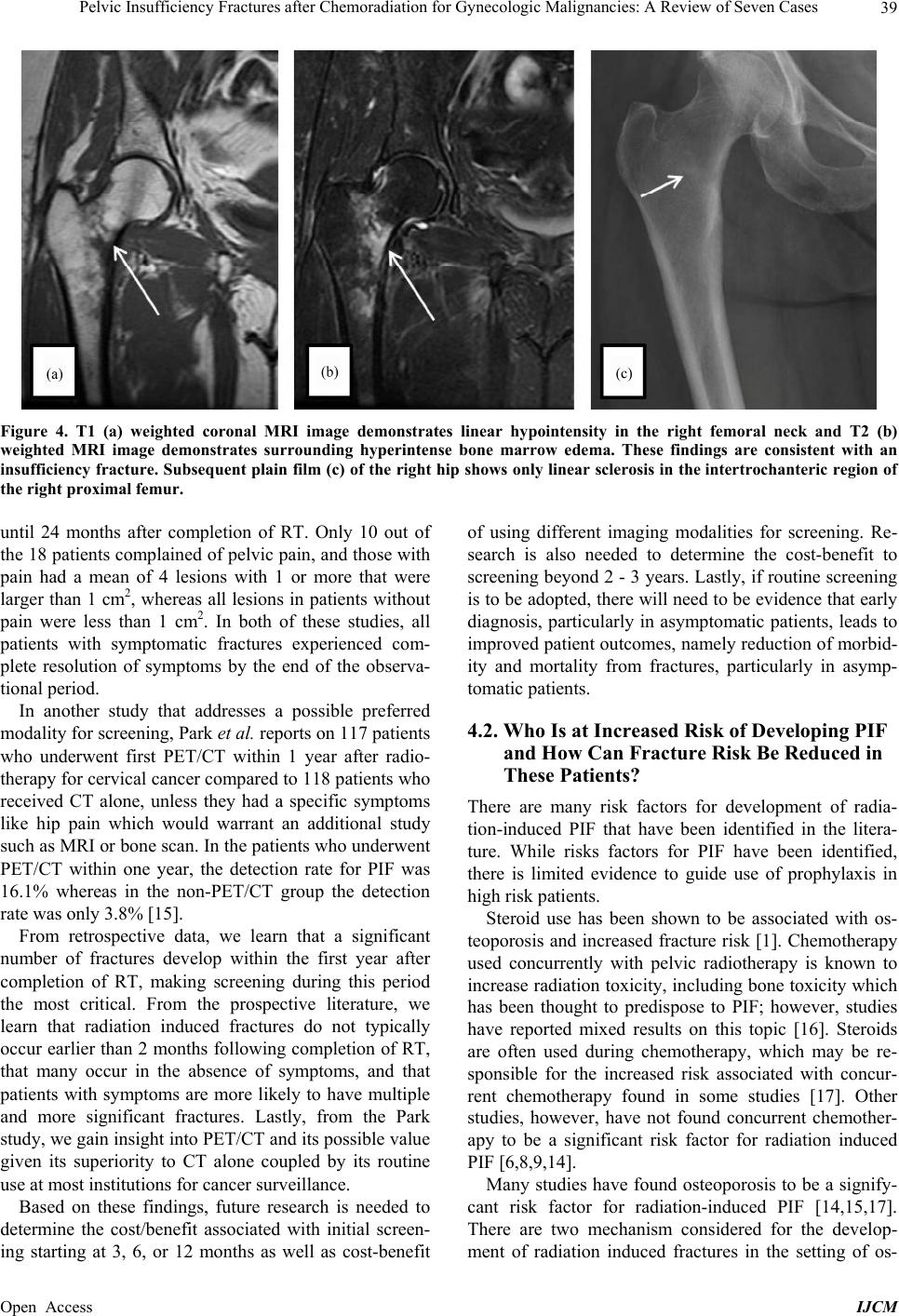 Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 39 Figure 4. T1 (a) weighted coronal MRI image demonstrates linear hypointensity in the right femoral neck and T2 (b) weighted MRI image demonstrates surrounding hyperintense bone marrow edema. These findings are consistent with an insufficiency fracture . Subsequent plain film (c) of the right hip shows only linear sclerosis in the intertrochanteric region of the right proximal femur. until 24 months after completion of RT. Only 10 out of the 18 patients complained of pelvic pain, and those with pain had a mean of 4 lesions with 1 or more that were larger than 1 cm2, whereas all lesions in patients without pain were less than 1 cm2. In both of these studies, all patients with symptomatic fractures experienced com- plete resolution of symptoms by the end of the observa- tional period. In another study that addresses a possible preferred modality for screening, Park et al. reports on 117 patients who underwent first PET/CT within 1 year after radio- therapy for cervical cancer compared to 118 patients who received CT alone, unless they had a specific symptoms like hip pain which would warrant an additional study such as MRI or bone scan. In the patients who underwent PET/CT within one year, the detection rate for PIF was 16.1% whereas in the non-PET/CT group the detection rate was only 3.8% [15]. From retrospective data, we learn that a significant number of fractures develop within the first year after completion of RT, making screening during this period the most critical. From the prospective literature, we learn that radiation induced fractures do not typically occur earlier than 2 months following completion of RT, that many occur in the absence of symptoms, and that patients with symptoms are more likely to have multiple and more significant fractures. Lastly, from the Park study, we gain insight into PET/CT and its possible value given its superiority to CT alone coupled by its routine use at most institutions for cancer surveillance. Based on these findings, future research is needed to determine the cost/benefit associated with initial screen- ing starting at 3, 6, or 12 months as well as cost-benefit of using different imaging modalities for screening. Re- search is also needed to determine the cost-benefit to screening beyond 2 - 3 years. Lastly, if routine screening is to be adopted, there will need to be evidence that early diagnosis, particularly in asymptomatic patients, leads to improved patient outcomes, namely reduction of morbid- ity and mortality from fractures, particularly in asymp- tomatic patients. 4.2. Who Is at Increased Risk of Developing PIF and How Can Fracture Risk Be Reduced in These Patients? There are many risk factors for development of radia- tion-induced PIF that have been identified in the litera- ture. While risks factors for PIF have been identified, there is limited evidence to guide use of prophylaxis in high risk patients. Steroid use has been shown to be associated with os- teoporosis and increased fracture risk [1]. Chemotherapy used concurrently with pelvic radiotherapy is known to increase radiation toxicity, including bone toxicity which has been thought to predispose to PIF; however, studies have reported mixed results on this topic [16]. Steroids are often used during chemotherapy, which may be re- sponsible for the increased risk associated with concur- rent chemotherapy found in some studies [17]. Other studies, however, have not found concurrent chemother- apy to be a significant risk factor for radiation induced PIF [6,8,9,14]. Many studies have found osteoporosis to be a signify- cant risk factor for radiation-induced PIF [14,15,17]. There are two mechanism considered for the develop- ment of radiation induced fractures in the setting of os- Open Access IJCM 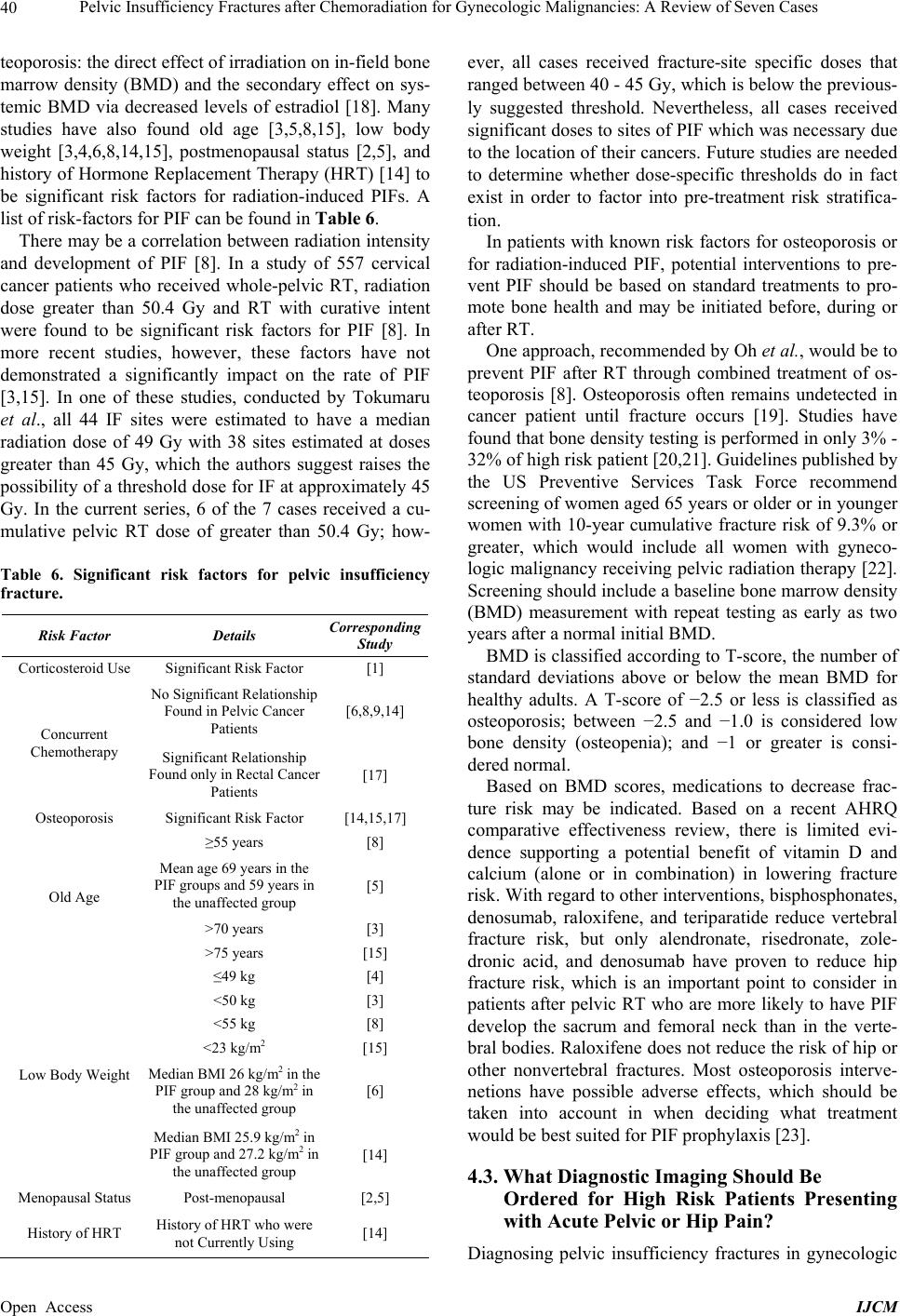 Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 40 teoporosis: the direct effect of irradiation on in-field bone marrow density (BMD) and the secondary effect on sys- temic BMD via decreased levels of estradiol [18]. Many studies have also found old age [3,5,8,15], low body weight [3,4,6,8,14,15], postmenopausal status [2,5], and history of Hormone Replacement Therapy (HRT) [14] to be significant risk factors for radiation-induced PIFs. A list of risk-factors for PIF can be found in Table 6. There may be a correlation between radiation intensity and development of PIF [8]. In a study of 557 cervical cancer patients who received whole-pelvic RT, radiation dose greater than 50.4 Gy and RT with curative intent were found to be significant risk factors for PIF [8]. In more recent studies, however, these factors have not demonstrated a significantly impact on the rate of PIF [3,15]. In one of these studies, conducted by Tokumaru et al., all 44 IF sites were estimated to have a median radiation dose of 49 Gy with 38 sites estimated at doses greater than 45 Gy, which the authors suggest raises the possibility of a threshold dose for IF at approximately 45 Gy. In the current series, 6 of the 7 cases received a cu- mulative pelvic RT dose of greater than 50.4 Gy; how- Table 6. Significant risk factors for pelvic insufficiency fracture. Risk Factor Details Corresponding Study Corticosteroid Use Significant Risk Factor [1] No Significant Relationship Found in Pelvic Cancer Patients [6,8,9,14] Concurrent Chemotherapy Significant Relationship Found only in Rectal Cancer Patients [17] Osteoporosis Significant Risk Factor [14,15,17] ≥55 years [8] Mean age 69 years in the PIF groups and 59 years in the unaffected group [5] >70 years [3] Old Age >75 years [15] ≤49 kg [4] <50 kg [3] <55 kg [8] <23 kg/m2 [15] Median BMI 26 kg/m2 in the PIF group and 28 kg/m2 in the unaffected group [6] Low Body Weight Median BMI 25.9 kg/m2 in PIF group and 27.2 kg/m2 in the unaffected group [14] Menopausal Status Post-menopausal [2,5] History of HRT History of HRT who were not Currently Using [14] ever, all cases received fracture-site specific doses that ranged between 40 - 45 Gy, which is below the previous- ly suggested threshold. Nevertheless, all cases received significant doses to sites of PIF which was necessary due to the location of their cancers. Future studies are needed to determine whether dose-specific thresholds do in fact exist in order to factor into pre-treatment risk stratifica- tion. In patients with known risk factors for osteoporosis or for radiation-induced PIF, potential interventions to pre- vent PIF should be based on standard treatments to pro- mote bone health and may be initiated before, during or after RT. One approach, recommended by Oh et al., would be to prevent PIF after RT through combined treatment of os- teoporosis [8]. Osteoporosis often remains undetected in cancer patient until fracture occurs [19]. Studies have found that bone density testing is performed in only 3% - 32% of high risk patient [20,21]. Guidelines published by the US Preventive Services Task Force recommend screening of women aged 65 years or older or in younger women with 10-year cumulative fracture risk of 9.3% or greater, which would include all women with gyneco- logic malignancy receiving pelvic radiation therapy [22]. Screening should include a baseline bone marrow density (BMD) measurement with repeat testing as early as two years after a normal initial BMD. BMD is classified according to T-score, the number of standard deviations above or below the mean BMD for healthy adults. A T-score of −2.5 or less is classified as osteoporosis; between −2.5 and −1.0 is considered low bone density (osteopenia); and −1 or greater is consi- dered normal. Based on BMD scores, medications to decrease frac- ture risk may be indicated. Based on a recent AHRQ comparative effectiveness review, there is limited evi- dence supporting a potential benefit of vitamin D and calcium (alone or in combination) in lowering fracture risk. With regard to other interventions, bisphosphonates, denosumab, raloxifene, and teriparatide reduce vertebral fracture risk, but only alendronate, risedronate, zole- dronic acid, and denosumab have proven to reduce hip fracture risk, which is an important point to consider in patients after pelvic RT who are more likely to have PIF develop the sacrum and femoral neck than in the verte- bral bodies. Raloxifene does not reduce the risk of hip or other nonvertebral fractures. Most osteoporosis interve- netions have possible adverse effects, which should be taken into account in when deciding what treatment would be best suited for PIF prophylaxis [23]. 4.3. What Diagnostic Imaging Should Be Ordered for High Risk Patients Presenting with Acute Pelvic or Hip Pain? Diagnosing pelvic insufficiency fractures in gynecologic Open Access IJCM  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 41 oncology patients is often difficult due to symptoms and radiographic findings that can be mistaken for recurrent disease or bony metastasis. Consistent with findings in the current study, most cases present with pelvic, lower back, and/or hip pain, but some patients may be asymp- tomatic [15]. When a patient presents with these symp- toms, it is important understand the limitations of each imaging modality and more importantly, to know which modality is most likely to both detect PIFs and ade- quately differentiate them from pathologic fractures. Conventional radiographs are often inconclusive [11, 12] and bone scan is limited by low specificity, which requires an additional test to confirm diagnosis [11]. In a study comparing the rate of PIF detection using CT versus MRI, Cabarrus et al. found CT to be inferior to MRI. In this study of 64 subjects, CT diagnosed only 69% of fractures (89 of 129), while MRI diagnosed 99% of fractures (128 of 129) [24]. Findings in the current study are consistent with that of Cabarrus et al. When CT was used as the initial imaging study, it failed to diag- nose PIF in both instances where MRI subsequently de- tected PIF. In a more recent study which was previously described, Park et al. found that in 117 patients who underwent PET/CT within one year after completion of RT, 16.1% of PIF were identified. However, in 118 similar patients who received CT alone (unless they had a specific symp- toms like hip pain which would warrant an additional study such as MRI or bone scan), only 3.8% of PIF were identified [15]. PET/CT and MRI have superior detection rates com- pared to other modalities. Similarly, both modalities are able to distinguish between pathologic fractures and PIF. In the absence of comparative effectiveness studies, the decision to use one over the other should be based on institutional preference and availability. 4.4. In Cases Where PIF Is Diagnosed, What Are the Different Treatment Options? Treatment of pelvic insufficiency fractures typically be- gins with conservative management, emphasizing limited weight bearing, symptomatic pain relief, and physical therapy [25-28]. When expectant management is unsuc- cessful in resolving symptoms, surgical interventions may be considered. In reports of patients who have re- fractory pain after expectant management, clinical im- provements have been demonstrated after surgically in- vasive measures, such as prophylactic or medically indi- cated in situ fixation, percutaneous cement osteoplasty, and metallic stent scaffolding [26,29-33]. Moreover, conservative management has been shown to be thor- oughly effective, sometimes with NSAIDs and physical therapy alone, and improvement can typically be seen within weeks after PIF is diagnosed, with resolution of symptoms by 1 - 35 months [1,2,4,5,27,28]. In the cur- rent study, both expectant and surgical management were employed based on the given clinical scenario. At MGH, in cases where initial expectant management is ineffec- tive or where radiographic findings reveal progression of PIF, a multidisciplinary approach to management is ta- ken involving the patient, the primary gynecologic on- cologist, the radiation oncologist, and an experienced orthopedic surgeon. All patients in the current study, whether managed expectantly or surgically, had complete resolution of symptoms and had improved functional capacity by 12 month follow-up. 5. Conclusion Radiation-induced PIFs are an important complication associated with pelvic RT. Future research is needed to inform evidence based on clinical management of PIF in patients with gynecologic malignancies undergoing pel- vic radiation therapy. Key areas of opportunity identified in this review include: 1) developing optimal screening guidelines, 2) identifying high risk patients who would benefit from prophylaxis, 3) evaluating the comparative effectiveness of pharmacotherapies for prophylaxis in PIF, 4) evaluating the comparative effectiveness of PET/ CT versus MRI for screening and diagnosis, and 5) com- paring the morbidity associated with expectant mana- gement versus surgical interventions for treatment of patients with symptomatic fractures. REFERENCES [1] S. J. Huh, “Post Pelvic Radiotherapy Bony Changes,” The Journal of the Korean Society for Therapeutic Radiology and Oncology, Vol. 27, No. 1, 2009, pp. 1-9. http://dx.doi.org/10.3857/jkstro.2009.27.1.1 [2] V. Blomlie, E. K. Rofstad, K. Talle, et al., “Incidence of Radiation-Induced Insufficiency Fractures of the Female Pelvis: Evaluation with MR imaging,” American Journal of Roentgenology, Vol. 167, No. 5, 1996, pp. 1205-1210. http://dx.doi.org/10.2214/ajr.167.5.8911181 [3] S. Tokumara, T. Toita, M. Oguchi, et al., “Insufficiency Fractures After Pelvic Radiation Therapy for Uterine Cervical Cancer: An Analysis of Subjects in a Prospective Multi Institutional Trial,” International Journal of Radia- tion Oncology * Biology * Physics, Vol. 84, No. 2, 2012, pp. 195-200. http://dx.doi.org/10.1016/j.ijrobp.2012.03.042 [4] I. Ogino, N. Okmoto, Y. Ono, et al., “Pelvic Insufficiency Fractures in Postmenopausal Women with Advanced Cervical Cancer Treated by Radiotherapy,” Radiotherapy and Oncology, Vol. 68, No. 1, 2003, pp. 61-67. http://dx.doi.org/10.1016/S0167-8140(03)00128-2 [5] H. Ikushima, K. Osaki, S. Furutani, et al., “Pelvic Bone Complications Following Radiation Therapy of Gyneco- logic Malignancies: Clinical Evaluation of Radiation In- duced Pelvic Insufficiency Fractures,” Gynecologic On- Open Access IJCM  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases 42 cology, Vol. 103, No. 3, 2006, pp. 1100-1114. http://dx.doi.org/10.1016/j.ygyno.2006.06.038 [6] K. M. Schmeler, A. Jhingran, R. B. Iyer, et al., “Pelvic Fractures after Radiotherapy for Cervical Cancer: Impli- cations for Survivors,” Cancer, Vol. 116, No. 3, 2010, pp. 625-630. http://dx.doi.org/10.1002/cncr.24811 [7] N. N. Baxter, E. B. Habermann, J. E. Tepper, et al., “Risk of Pelvic Fractures in Older Women Following Pelvic Ir- radiation,” JAMA, Vol. 294, No. 20, 2005, pp. 2587-2593. http://dx.doi.org/10.1001/jama.294.20.2587 [8] D. Oh, S. J. Huh, H. Nam, et al., “Pelvic Insufficiency Fracture after Pelvic Radiotherapy for Cervical Cancer: Analysis of Risk Factors,” International Journal of Ra- diation Oncology * Biology * Physics, Vol. 70, No. 4, 2008, pp. 1183-1188. http://dx.doi.org/10.1016/j.ijrobp.2007.08.005 [9] J. W. Kwon, S. J. Huh, Y. C. Yoon, S. H. Choi, J. Y. Jung, D. Oh and B. K. Choe, “Pelvic Bone Complications after Radiation Therapy of Uterine Cervical Cancer: Evalua- tion with MRI,” American Journal of Roentgenology, Vol. 191, No. 4, 2008, pp. 987-994. http://dx.doi.org/10.2214/AJR.07.3634 [10] P. Bliss, C. Parsons and P. Blake, “Incidence and Possible Etiological Factors in the Development of Pelvic Insuffi- ciency Fractures Following Radical Radiotherapy,” Brit- ish Journal of Radiology, Vol. 69, No. 822, 1996, pp. 548-554. http://dx.doi.org/10.1259/0007-1285-69-822-548 [11] W. C. Peh, P. Khong, Y. Yin, W. Y. Ho, N. S. Evans, L. A. Gilula, H. W. Yeung and A. M. Davies, “Imaging of Pelvic Insufficiency Fractures,” Radiographics, Vol. 16, No. 2, 1996, pp. 335-348. [12] E. M. Lyders, C. T. Whitlow, M. D. Baker and P. P. Mor- ris, “Imaging and Treatment of Sacral Insufficiency Frac- tures,” American Journal of Neuroradiology, Vol. 31, No. 1, 2010, pp. 201-210. http://dx.doi.org/10.3174/ajnr.A1666 [13] S. J. Huh, B. Kim, M. K. Kang, et al., “Pelvic Insuffi- ciency Fractures after Pelvic Irradiation in Uterine Cervix Cancer,” Gynecologic Oncology, Vol. 86, No. 3, 2002, pp. 264-268. http://dx.doi.org/10.1006/gyno.2002.6756 [14] K. K. Shih, M. R. Folkert, M A. Kollmeier, N. R. Abu- Rustum, Y. Sonoda, M. M. Leitao, R. R. Barakat and K. M. Alektiar, “Pelvic Insufficiency Fractures in Patients with Cervical and Endometrial Cancer Treated with Postoperative Pelvic Radiation,” Gynecologic Oncology, Vol. 128, No. 3, 2013, pp. 540-543. http://dx.doi.org/10.1016/j.ygyno.2012.12.021 [15] S. Park, J. Kim, J. Lee, et al., “Pelvic Insufficiency Frac- ture after Radiotherapy in Patients with Cervical Cancer in the Era of PET/CT,” Journal of Radiation Oncology, Vol. 29, No. 4, 2011, pp. 269-276. http://dx.doi.org/10.3857/roj.2011.29.4.269 [16] P. J. Jenkins, D. J. Montefiore and S. J. Arnott, “Hip Complications Following Chemoradiationtherapy,” Vol. 7, 1995, pp. 123-126. [17] H. J. Kim, P. J. Boland, D. S. Meredith, E. Lis, Z. Zhang, W. Sih, et al., “Fractures of the Sacrum after Chemora- diation for Rectal Carcinoma: Incidence, Risk Factors, and Radiographic Evaluation,” International Journal of Radiation Oncology Biology & Physics, Vol. 84, No. 3, 2012, pp. 649-699. [18] N. Okonogi, J. I. Saitoh, Y. Suzuki, et al., “Changes in Bone Mineral Density in Uterine Cervical Cancer Patients after Radiation Therapy,” International Journal of Radia- tion Oncology * Biology * Physics, Vol. 87, No. 5, 2013, pp. 968-974. http://dx.doi.org/10.1016/j.ijrobp.2013.08.036 [19] T. A. Guise, “Bone Loss and Fracture Risk Associated with Cancer Therapy,” Oncologist, Vol. 11, No. 10, 2006, pp. 1121-1131. http://dx.doi.org/10.1634/theoncologist.11-10-1121 [20] M. D. Smith, W. Ross and M. J. Ahern, “Tissing a Thera- peutic Window of Opportunity: An Audit of Patients At- tending a Tertiary Teaching Hospital with Potentially Os- teoporotic Hip and Wrist Fractures,” The Journal of Rheumatology, Vol. 28, No. 11, 2001, pp. 2504-2508. [21] C. A. Morris, H. Cheng, D. Cabral, et al., “Predictors of Screening and Treatment of Osteoporosis: A Structured Review of the Literature,” The Endocrinologist, Vol. 14, No. 2, 2004, pp. 70-75. http://dx.doi.org/10.1097/01.ten.0000123564.40707.84 [22] H. D. Nelson, E. M. Haney, T. Dana, C. Bougatsos and R. Chou, “Screening for Osteoporosis: An Update for the U. S. Preventive Services Task Force,” Annals of Internal Medicine, Vol. 153, No. 2, 2010, pp. 99-111. http://dx.doi.org/10.7326/0003-4819-153-2-201007200-0 0262 [23] C. J. Crandall, S. J. Newberry, A. Diamant, et al., “Treat- ment To Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis: Update of a 2007 Report, [Internet,” Comparative Effectiveness Reviews, No. 53, Agency for Healthcare Research and Quality (US), Rock- ville, 2012. http://www.ncbi.nlm.nih.gov/books/NBK92566/ [24] M. C. Cabarrus, A. Ambekar, Y. Lu, et al., “MRI and CT of Insufficiency Fractures of the Pelvis and the Proximal Femur,” American Journal of Roentgenology, Vol. 19, No. 4, 2008, pp. 995-1001. http://dx.doi.org/10.2214/AJR.07.3714 [25] P. Tai, A. Hammond, J. Van Dyk, et al., “Pelvic Fractures Following Irradiation of Endometrial and Vaginal Can- cers—A Case Series and Review of Literature,” Radio- therapy & Oncology, Vol. 56, No. 1, 2000, pp. 23-28. http://dx.doi.org/10.1016/S0167-8140(00)00178-X [26] E. Tsiridis, N. Upadhyay and P. V. Giannoudis, “Sacral Insufficiency Fractures: Current Concepts of Manage- ment,” Osteoporosis International, Vol. 17, No. 12, 2006, pp. 1716-1725. http://dx.doi.org/10.1007/s00198-006-0175-1 [27] A. Moreno, J. Clemente, C. Crespo, et al., “Pelvic Insuf- ficiency Fractures in Patients with Pelvic Irradiation,” In- ternational Journal of Radiation Oncology * Biology * Physics, Vol. 44, No. 1, 1999, pp. 61-66. http://dx.doi.org/10.1016/S0360-3016(98)00534-3 [28] J. Lin, E. Lachmann and W. Nagler, “Sacral Insuffi- ciency Fractures: A Report of Two Cases and a Review of the Literature,” Journal of Women’s Health and Gender Open Access IJCM  Pelvic Insufficiency Fractures after Chemoradiation for Gynecologic Malignancies: A Review of Seven Cases Open Access IJCM 43 Based Medicine, Vol. 10, No. 7, 2001, pp. 699-705. http://dx.doi.org/10.1089/15246090152563588 [29] J. Kamysz and M. Rechitsky, “Pubic Bone Cement Os- teoplasty for Pubic Insufficiency Fractures,” Journal of Vascular and Interventional Radiology, Vol. 19, No. 9, 2008, pp. 1386-1389. http://dx.doi.org/10.1016/j.jvir.2008.05.026 [30] J. Kamsyz, “Percutaneous Repair of a Nonunion Pubic Ramus Fracture Using a Metallic Stent Scaffold and Ce- ment Osteoplasty,” Journal of Vascular and Interven- tional Radiology, Vol. 21, No. 8, 2010, pp. 1313-1316. http://dx.doi.org/10.1016/j.jvir.2010.04.017 [31] D. Mears and J. Velyvis, “In Situ Fixation of Pelvic Nonunions Following Pathologic and Insufficiency Frac- tures,” Journal of Bone and Joint Surgery, Vol. 84, No. 5, 2002, pp. 721-728. [32] I. Papanastassiou, M. Setzer, M. Eleraky, et al., “Mini- mally Invasive Sacroiliac Fixation in Oncologic Patients with Sacral Insufficiency Fractures Using a Fluoroscopy- based Navigation System,” Journal of Spinal Disorders & Techniques, Vol. 24, No. 2, 2011, pp. 76-82. http://dx.doi.org/10.1097/BSD.0b013e3181df8e6b [33] D. Wahnert, M. Raschke and T. Fuchs, “Cement Aug- mentation of the Navigated Iliosacral Screw in the Treat- ment of Insufficiency Fractures of the Sacrum. A New Method Using Modified Implants,” International Ortho- pedics, Vol. 37, No. 6, 2013, pp. 1147-1150. http://dx.doi.org/10.1007/s00264-013-1875-8
|