 J. Biomedical Science and Engineering, 2013, 6, 47-60 JBiSE http://dx.doi.org/10.4236/jbise.2013.612A007 Published Online December 2013 (http://www.scirp.org/journal/jbise/) Effect of cardiac ventricular mechanical contraction on the characteristics of the ECG: A simulation study Ismail Adeniran1, Jules C. Hancox2, Henggui Zhang1,3* 1School of Physics and Astronomy, The University of Manchester, Manchester, United Kingdom 2Department of Physiology and Cardiovascular Laboratories, School of Medical Sciences, Bristol, United Kingdom 3School of Computer Sciences and Technology, Harbin Institute of Technology, Harbin, China Email: *henggui.zhang@Manchester.ac.uk Received 22 October 2013; revised 25 November 2013; accepted 8 December 2013 Copyright © 2013 Ismail Adeniran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Introduction: The 12-lead electrocardiogram (ECG) is the most widely-used tool for the detection and di- agnosis of cardiac conditions including myocardial infarction and ischemia. It has therefore been a focus of cardiac modeling. However, the most contempo- rary in silico ECG investigations of the intact heart have assumed a static heart and ignored the mecha- nical contraction that is an essential component of cardiac function. The aim of this study was to utilize electromechanically coupled human ventricle models to explore the consequences of ventricular mechanical contraction on the ECG profiles. Methods and Re- sults: Biophysically detailed human ventricular cell models incorporating contractile activity and a stretch- activated current (Isac) were incorporated into a 3D human ventricular model within a human torso, from which 12-lead ECGs were computed at a stimulation rate of 1 Hz. Compared to the static model, ventricu- lar contraction without Isac had little effect on the QRS complex, but shifted the T-wave peak leftwards and reduced its peak amplitude. With Isac, ventricular mechanical contraction increased the QRS duration by 23% and QT interval by 5%. Conclusion: Me- chanical contraction of the heart has a significant effect on the morphology and characteristics of the ECG particularly on the T-wave. The alteration of the cell membrane kinetics by stretch via Isac further exacerbates these effects. Our simulation data suggest that mechanical contraction should be considered in the interpretation of ECGs in pathological conditions, especially those in which mechanical contraction of the heart is impaired. Keywords: Ventricles; Mechanical Contraction; Electrophysiology; Simulation; ECG 1. INTRODUCTION The 12-lead electrocardiogram (ECG) is used to under- stand a person’s cardiac electrical activity by measuring the electrical potential on the body surface in order to obtain diagnostic information on the status of the heart. Common ECG monitoring’ uses include the detection of complex arrhythmias, shortened or prolonged QT inter- vals, ST-segment elevation and ischemia monitoring [1-3]. The 12-lead ECG system consists of six frontal plane leads (Lead I, Lead II, Lead III, aVR, aVL and aVF) and six chest leads (V1 - V6) [1-3]. Though being the most widely-used cardiac diagnostic tool, the ECG has some shortcomings, e.g., low sensitivity for detecting acute inferior myocardial infarction (its sensitivity is only approximately 60%) [4]. Complex ECG patterns associated with left bundle branch block (LBBB), ventri- cular-paced rhythm (VPR) and left ventricular hypertro- phy (LVH) reduce the ability of the ECG to detect acute coronary ischemic change and acute myocardial infarc- tion [5]. Biophysically detailed computer models of the heart that relate cardiac cellular mechanisms to a clinical mea- surement of ECG may establish a correlation atlas be- tween ECG characteristics and various pathological con- ditions. This may be beneficial for mitigating the short- comings of the use of clinical ECGs for diagnosing car- diac diseases. However, the majority of cardiac model- ling studies have focused mainly on the electrical activity of the heart, with an assumption that the heart is station- ary in the thorax [6-10]. This assumption ignores the fact that the heart is a mechanical pump, which undergoes rhythmic mechanical contractions in response to cardiac electrical excitation waves. The mechanical contraction alters the geometry of the heart as well as the cardiac *Corresponding author. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 48 electrical excitation via the mechanism of mechano- electric feedback (MEF) [11-14]. It can be anticipated that the motion of the heart dur- ing the cardiac cycle alters the relative position of the ECG leads on the body surface to the electrical signal sources in the heart, as well as the anisotropic conductiv- ity of the electrical propagation within the torso; all of which may have influences on the ECG, therefore, pro- ducing differences to the ECG characteristics is compar- ed to a stationary heart, particularly during the T-wave when the heart is subjected to maximum systolic pressure [2,15]. In addition, in response to changes in volume load or contractile state (changing geometry), the heart regu- lates its electrical activity via MEF [11,16,17], which activates stretch-activated channels (SACs) [18,19] that regulate cardiac cell action potentials (APs), such as prolongation or shortening [13,20,21] of AP duration (APD), changes of AP morphology and AP refractory properties (such as the diastolic depolarisation and pre- mature excitation) [11,22-24]. However, it is incomplete- ly understood how these changes of cardiac geometry and electrophysiology influence the body surface ECG. Therefore, the aim of this study was to investigate the consequences of ventricular wall motion on the 12-lead ECG in the absence and presence of a stretch-activated current (Isac) during the cardiac cycle. 2. MATERIAL AND METHODS 2.1. Single Cell Electromechanical Model For simulating electrophysiology (EP), we utilized the O’Hara-Rudy (ORd) human ventricular single cell model [25], which was developed from undiseased human ven- tricle data and recapitulates human ventricular cell elec- trical and membrane channel properties, as well as the transmural heterogeneity of ventricular action potential (AP) across the ventricular wall [25]. The ORd model also reproduces Ca2+ versus voltage-dependent inactiva- tion of L-type Ca2+ current and Ca2+/calmodulin-de- pendent protein kinase II (CaMK) modulated rate de- pendence of Ca2+ cycling [25]. For simulating cellular mechanical properties, we used the Rice et al. myofila- ment (MM) model [26]. This model was chosen as it is based on the cross-bridge cycling model of cardiac mus- cle contraction and is able to replicate a wide range of experimental data including steady-state force-sarcomere length (F-SL), force-calcium and sarcomere length-cal- cium relationships [26]. The intracellular calcium concentration i 2 Ca from the EP model was used as the coupling link to the MM model. i produced as dynamic output from the EP model during the time course of the AP served as input to the MM model from which the amount of Ca2+ bound to troponin was calculated. The formulation of the myoplasmic Ca2+ concentration in the EP model is: 2 Ca 2 , , dCa 2 d2 cap iCaipCaCabNaCa imyo nsr ss updiff Ca myo myo A II I tF vv JJ vv v (1) where Cai is the buffer factor for i , IpCa is the sarcolemmal Ca2+ pump current, ICab is the Ca2+ back- ground current, INaCa,i is the myoplasmic component of Na+/Ca2+ exchange current, Acap is capacitive area, F is the Faraday constant, vmyo is the volume of the myoplas- mic compartment, vnsr is the volume of the network sar- coplasmic reticulum compartment, vss is the volume of the subspace compartment, Jup is the total Ca2+ uptake flux, via SERCA pump from myoplasm to the network sarcoplasmic reticulum and Jdiff,Ca is the flux of the diffu- sion of Ca2+ from the subspace to the myoplasm. 2 Ca Cai is formulated as: ,CMDN ,TRPN 22 22 ,CMDN ,TRPN 1 CMDN TRPN 1 Ca Ca Cai mm mm ii KK KK (2) where [CMDN] and [TRPN] are the calmodulin and tro- ponin Ca2+ buffers in the myoplasm respectively, and Km,CMDN and Km,TRPN are the half-saturation concentra- tions of calmodulin and troponin respectively. In the original ORd model, Eq.2 considers Ca2+ bind- ing to both calmodulin and troponin. However, as the MM model implements actual regulatory sites for the apparent Ca2+ binding to troponin, Eq.2 was modified to: ,CMDN 2 2 ,CMDN 1 CMDN 1 Ca Cai m mi K K (3) Now, the EP model only handles Ca2+ binding to calmodulin with the MM model handling Ca2+ binding to troponin. The flux of the binding of Ca2+ to troponin via the MM model was incorporated into the EP model via Eq.1 as follows: 2 , , dCa 2 d2 1000 cap iCaipCaCabNaCa imyo Trop nsr ss updiff Ca myo myo A II I tF J vv JJ vv v (4) where JTro p is the flux of Ca2+ binding to troponin. The combination of all state variables from the EP model Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 49 with the MM model and the substitution of Eq.3 and Eq.4 for Eqs.1 and 2 yielded a human ventricular myo- cyte electromechanical cell model. 2.2. Stretch-Activated Current In accord with previous studies [19,27-31], we incorpo- rated a stretch-activated current (Isac) into the electrome- chanics model using the following formulation: acsac mmsac IGPVE (5) where Gsac and Esac are the maximum channel conduc- tance and reversal potential of the SAC respectively. In the electromechanics model, Esac was typically set to −6.3 mV and describes the experimentally observed de- polarising effect of the channel [32,33]. Vm is the mem- brane potential and Pm is the channel’s open probability modelled as: 12 1 1e e m k P , (6) where and 1/2 are the strain (with an explicit depend- ence on the sarcomere length) and half-activation strain respectively, ke = 0.02 [19,29,34] is the activation slope. The SAC is assumed to be permeable to Na+, K+ and Ca2+ [19,30,35] in the ratio 1:1:1 with Isac therefore de- fined as: ,, acsacNasac Ksac Ca IIII (7) where Isac,Na, Isa c,K and Isac,Ca are the contributions of Na+, K+ and Ca2+ to Isac. 2.3. Tissue Mechanics Model We modelled cardiac tissue mechanics within the theo- retical framework of nonlinear elasticity [36,37] as an in- homogeneous, anisotropic, nearly incompressible nonlin- ear material similar to previous studies [27,38-42]. We used a two-field variational principle with the deforma- tion u and the hydrostatic pressure p as the two fields [37,43,44]. p is utilised as the Lagrange multiplier to enforce the near incompressibility constraint. Thus, the total potential energy functional for the mechanics problem is formulated as: ,, int ext upup u (8) where , int up is the internal potential energy or total strain energy of the body and ext u is the ex- ternal potential energy or potential energy of the external loading of the body. As in previous studies [38,41,42,45], in the absence of body forces, and assuming that the body is always in instantaneous equilibrium and no iner- tia effects, the coordinates of the deformed body satisfies the steady-state equilibrium equation with near incom- pressibility enforced. According to standard variational principles, equilib- rium is derived by searching for critical points of (Eq.8) in suitable admissible displacement and pressure spaces and ˆ Uˆ . The corresponding Euler-Lagrange equa- tions resulting from (Eq.8) lead to solving the problem [44,46-49]: Find (u,p) in ˆˆ UP such that: ˆ,:d d det ˆ :dd , d WxId uvx F pIduvxgvsvu F U (9) ˆ det1 d0,qIdu xqp P ˆˆ (10) where and U are the admissible variation spaces for the displacements and the pressures, respectively. Id u is the deformation gradient, v is a test function and is the material stored energy function and corresponds to the density of elastic energy locally stored in the body during the deformation. ˆ W With the axes of the geometry aligned to the underly- ing tissue microstructure [50,51], the second Piola- Kirchhoff stress tensor S, obtained from the directional derivative of (Eq.8) in the direction of an arbitrary vir- tual displacement and which relates a stress to a strain measure [37,43] and a manipulation of Eq.9 is defined as: 1 Active Tension 1 2MN MN NM WW SpC EE S (11) where W is a strain energy function that defines the con- stitutive behaviour of the material, E is the Green-La- grange strain tensor that quantifies the length changes in a material fibre and angles between fibre pairs in a de- formed solid, C is the Right-Cauchy green strain tensor, p is a Lagrange multiplier (referred to as the hydrostatic pressure in the literature) used to enforce incompressibil- ity of the cardiac tissue, SActive Tension is a stress tensor in- corporating active tension from the electromechanics cell model and enables the reproduction of the three physio- logical movements of the ventricular wall: longitudinal shortening, wall thickening and rotational twisting [52- 58]. For the strain energy function W, we used the Guc- cione constitutive law [59] given by: 1eQ WC (12) where 222 2 113223323 41221 1331 2 2 QCE CEEE CEE EE 2 (13) Following previous work [27,60], , 10.831 kPaC Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 50 2, 3, 4 14.31C4.49C10C . ij are the compo- nents of the Green-Lagrange strain tensor. E 2.4. Tissue Electrophysiology Model The monodomain representation [61-63] of cardiac tissue was used for the electrophysiology model with a modifi- cation (the incorporation of the Right Cauchy Green de- formation tensor C), which allows the monodomain equation to take into account the effect of the deforming tissue, similar to previous studies [38,42,64]: stim I TT DV TT nD V xT VENTRICLES 1 dDCV d m ion V CI t T VV (14) where Cm is the cell capacitance per unit surface area, V is the membrane potential, Iion is the sum of all trans- membrane ionic currents from the electromechanics sin- gle cell model, Istim is an externally applied stimulus and D is the diffusion tensor. In simulations, intracellular conductivities in the fibre, cross-fibre and sheet direc- tions were set to 3.0, 0.1 and 0.31525 ms·mm‒1 respec- tively. These gave a conduction velocity of 65 cm·s‒1 in the fibre direction along multiple cells, which is close to the value 70 cm·s‒1 observed in the fibre direction in human myocardium [65]. 2.5. Tor so Model The electrical potential in the torso (Figure 1(B)) is ob- tained via the Poisson equation: 0 0 (15) where DT is the torso conductivity and VT is the electrical potential in the torso. The torso is modelled as a passive conductor surrounded by air. Consequently, the normal component of its outer surface is zero leading to the first boundary condition: (16) where n is the outward unit normal on the torso surface, T is the outer surface of the torso. The torso is a con- ductor surrounding the ventricles. Therefore, from the conservation of charge and current, on the boundary be- tween the ventricles and the torso, the normal component of the current in the ventricles must equal the normal component of the current in the surrounding torso. Since there cannot be a discontinuity in the potentials on the boundary of two directly connected volume conductors, i.e., the ventricles and the torso, this condition implies the following boundary condition: VE NTRICLES (17) where VVENTRICLES is the electrical potential on the sur- face between the ventricles and the torso. The torso con- ductivity was set to 0.3 ms·mm‒1. 2.6. Computing the 12-Lead ECGs We computed the ECGs according to the standard defini- tions [3,66] (Figure 1(B)). The limb leads were calcu- lated as follows: Lead Lead Lead ARA LRA LL I II III A where LA, LL and RA refer to the left arm, left leg and right arm respectively and X is the potential of the ap- propriate lead. The augmented limb leads were calcu- lated as: 1 Lead Lead 2 1 Lead Lead 2 1Lead Lead 2 aVF III aVL III aVRI II The precordial leads, V1 - V6 are located over the left chest as shown in Figure 1(B). Their potentials are measured directly from their locations. 2.7. Computational Methods 2.7.1. Ge ometry and Meshes The 3D simulations were carried out on a DT-MRI re- constructed anatomical human ventricle geometry (Fig- ure 1(A)), incorporating anisotropic fibre orientation (Figure 1(A)), from a healthy 34-year old male. This had a spatial resolution of 0.2 mm and approximately 24.2 million nodes in total and was segmented into distinct ENDO (60%), MCELL (30%) and EPI (10%) regions (Figure 1(A)). The chosen cell proportion in each region reflects experimental data for cells spanning the left ven- tricular wall of the human heart [67]. The conditional activation sites were determined empirically across the ventricle wall and were validated by reproducing the activation sequence and QRS complex in the measured 64-channel ECG [68] of that person (Figure 1(A)). 2.7.2. Solving the Electrome chanics Problem The electromechanics problem consists of two sub- problems: the electrophysiology problem and the me- chanics problem. The electrophysiology problem Eq.10 was solved with a Strang splitting method [69] ensuring that the solution is second-order accurate. It was discre- tised in time using the Crank-Nicholson method [70], which is also second-order accurate and discretised in space with Finite Elements [48,49,70,71]. Iion in Eq.10 epresents the single cell electromechanics model from r Copyright © 2013 SciRes. OPEN ACCESS 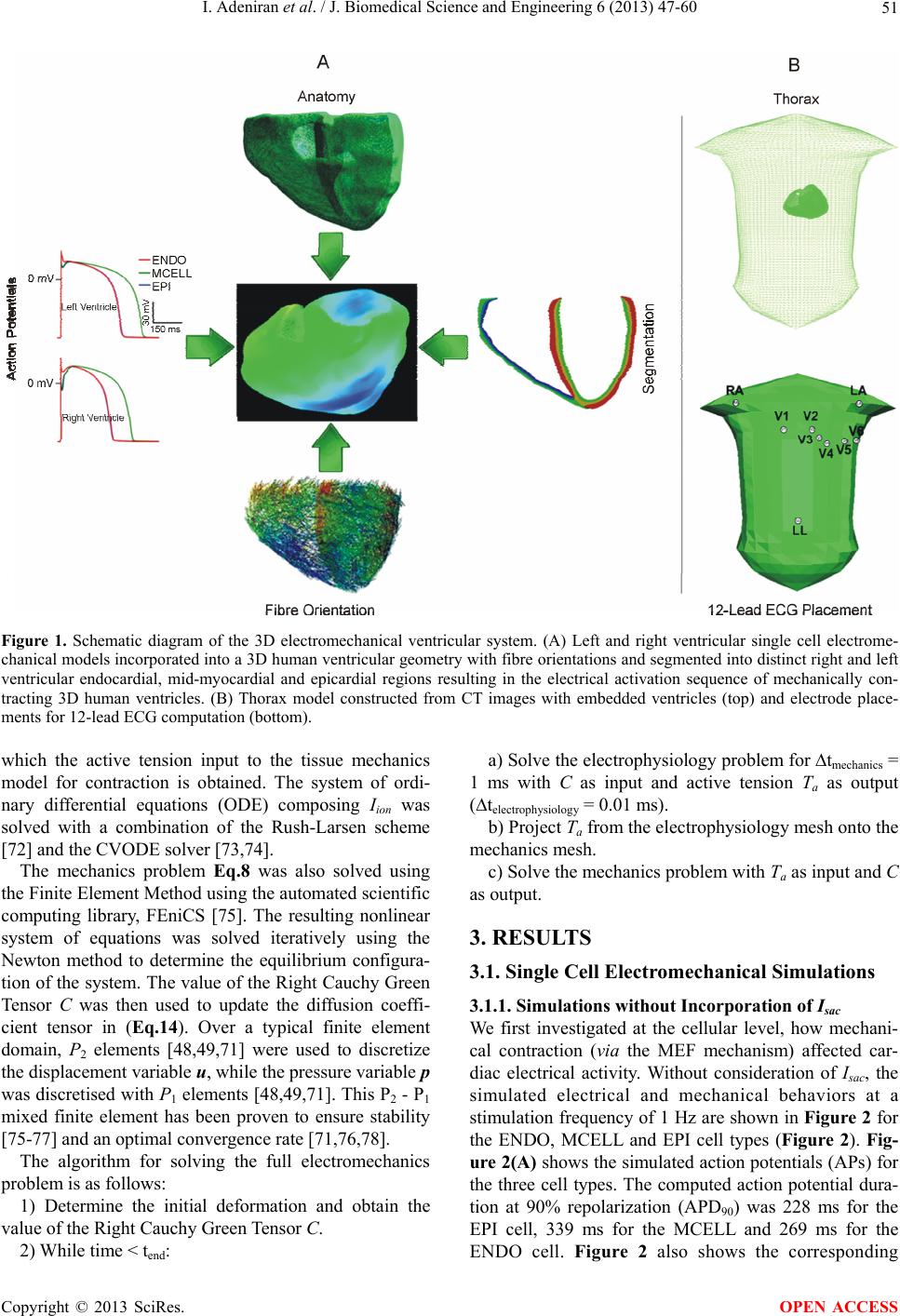 I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 Copyright © 2013 SciRes. 51 Figure 1. Schematic diagram of the 3D electromechanical ventricular system. (A) Left and right ventricular single cell electrome- chanical models incorporated into a 3D human ventricular geometry with fibre orientations and segmented into distinct right and left ventricular endocardial, mid-myocardial and epicardial regions resulting in the electrical activation sequence of mechanically con- tracting 3D human ventricles. (B) Thorax model constructed from CT images with embedded ventricles (top) and electrode place- ments for 12-lead ECG computation (bottom). which the active tension input to the tissue mechanics model for contraction is obtained. The system of ordi- nary differential equations (ODE) composing Iion was solved with a combination of the Rush-Larsen scheme [72] and the CVODE solver [73,74]. a) Solve the electrophysiology problem for tmechanics = 1 ms with C as input and active tension Ta as output (telectrophysiology = 0.01 ms). b) Project Ta from the electrophysiology mesh onto the mechanics mesh. The mechanics problem Eq.8 was also solved using the Finite Element Method using the automated scientific computing library, FEniCS [75]. The resulting nonlinear system of equations was solved iteratively using the Newton method to determine the equilibrium configura- tion of the system. The value of the Right Cauchy Green Tensor C was then used to update the diffusion coeffi- cient tensor in (Eq.14). Over a typical finite element domain, P2 elements [48,49,71] were used to discretize the displacement variable u, while the pressure variable p was discretised with P1 elements [48,49,71]. This P2 - P1 mixed finite element has been proven to ensure stability [75-77] and an optimal convergence rate [71,76,78]. c) Solve the mechanics problem with Ta as input and C as output. 3. RESULTS 3.1. Single Cell Electromechanical Simulations 3.1.1. Si m ulations without I n cor porati on o f Isac We first investigated at the cellular level, how mechani- cal contraction (via the MEF mechanism) affected car- diac electrical activity. Without consideration of Isac, the simulated electrical and mechanical behaviors at a stimulation frequency of 1 Hz are shown in Figure 2 for the ENDO, MCELL and EPI cell types (Figure 2). Fig- ure 2(A) shows the simulated action potentials (APs) for the three cell types. The computed action potential dura- tion at 90% repolarization (APD90) was 228 ms for the EPI cell, 339 ms for the MCELL and 269 ms for the ENDO cell. Figure 2 also shows the corresponding The algorithm for solving the full electromechanics problem is as follows: 1) Determine the initial deformation and obtain the value of the Right Cauchy Green Tensor C. 2) While time < tend: OPEN ACCESS 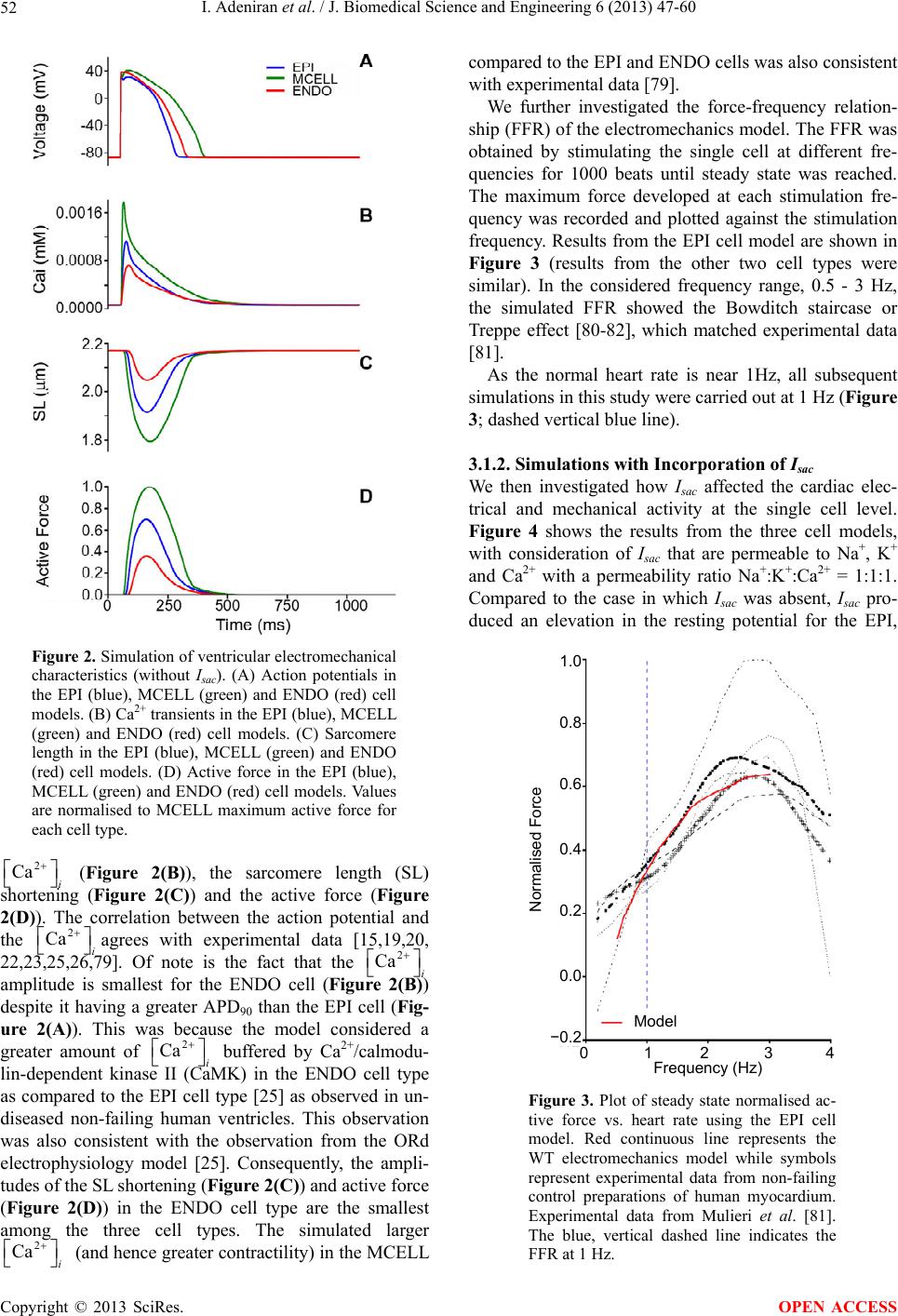 I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 52 Figure 2. Simulation of ventricular electromechanical characteristics (without Isac). (A) Action potentials in the EPI (blue), MCELL (green) and ENDO (red) cell models. (B) Ca2+ transients in the EPI (blue), MCELL (green) and ENDO (red) cell models. (C) Sarcomere length in the EPI (blue), MCELL (green) and ENDO (red) cell models. (D) Active force in the EPI (blue), MCELL (green) and ENDO (red) cell models. Values are normalised to MCELL maximum active force for each cell type. 2 Ca 2 Ca i (Figure 2(B)), the sarcomere length (SL) shortening (Figure 2(C)) and the active force (Figure 2(D)). The correlation between the action potential and the i agrees with experimental data [15,19,20, 22,23,25,26,79]. Of note is the fact that the i 2 Ca amplitude is smallest for the ENDO cell (Figure 2(B)) despite it having a greater APD90 than the EPI cell (Fig- ure 2(A)). This was because the model considered a greater amount of i buffered by Ca2+/calmodu- lin-dependent kinase II (CaMK) in the ENDO cell type as compared to the EPI cell type [25] as observed in un- diseased non-failing human ventricles. This observation was also consistent with the observation from the ORd electrophysiology model [25]. Consequently, the ampli- tudes of the SL shortening (Figure 2(C)) and active force (Figure 2(D)) in the ENDO cell type are the smallest among the three cell types. The simulated larger (and hence greater contractility) in the MCELL compared to the EPI and ENDO cells was also consistent with experimental data [79]. 2 Ca We further investigated the force-frequency relation- ship (FFR) of the electromechanics model. The FFR was obtained by stimulating the single cell at different fre- quencies for 1000 beats until steady state was reached. The maximum force developed at each stimulation fre- quency was recorded and plotted against the stimulation frequency. Results from the EPI cell model are shown in Figure 3 (results from the other two cell types were similar). In the considered frequency range, 0.5 - 3 Hz, the simulated FFR showed the Bowditch staircase or Treppe effect [80-82], which matched experimental data [81]. As the normal heart rate is near 1Hz, all subsequent simulations in this study were carried out at 1 Hz (Figure 3; dashed vertical blue line). 3.1.2. Simulations with Incorporation of Isac We then investigated how Isac affected the cardiac elec- trical and mechanical activity at the single cell level. Figure 4 shows the results from the three cell models, with consideration of Isac that are permeable to Na+, K+ and Ca2+ with a permeability ratio Na+:K+:Ca2+ = 1:1:1. Compared to the case in which Isac was absent, Isac pro- duced an elevation in the resting potential for the EPI, 0 1 2 3 4 Frequency (Hz) 1.0 0.8 0.6 0.4 0.2 0.0 −0.2 Model Normalised Force 2 Ca i Figure 3. Plot of steady state normalised ac- tive force vs. heart rate using the EPI cell model. Red continuous line represents the WT electromechanics model while symbols represent experimental data from non-failing control preparations of human myocardium. Experimental data from Mulieri et al. [81]. The blue, vertical dashed line indicates the FFR at 1 Hz. Copyright © 2013 SciRes. OPEN ACCESS 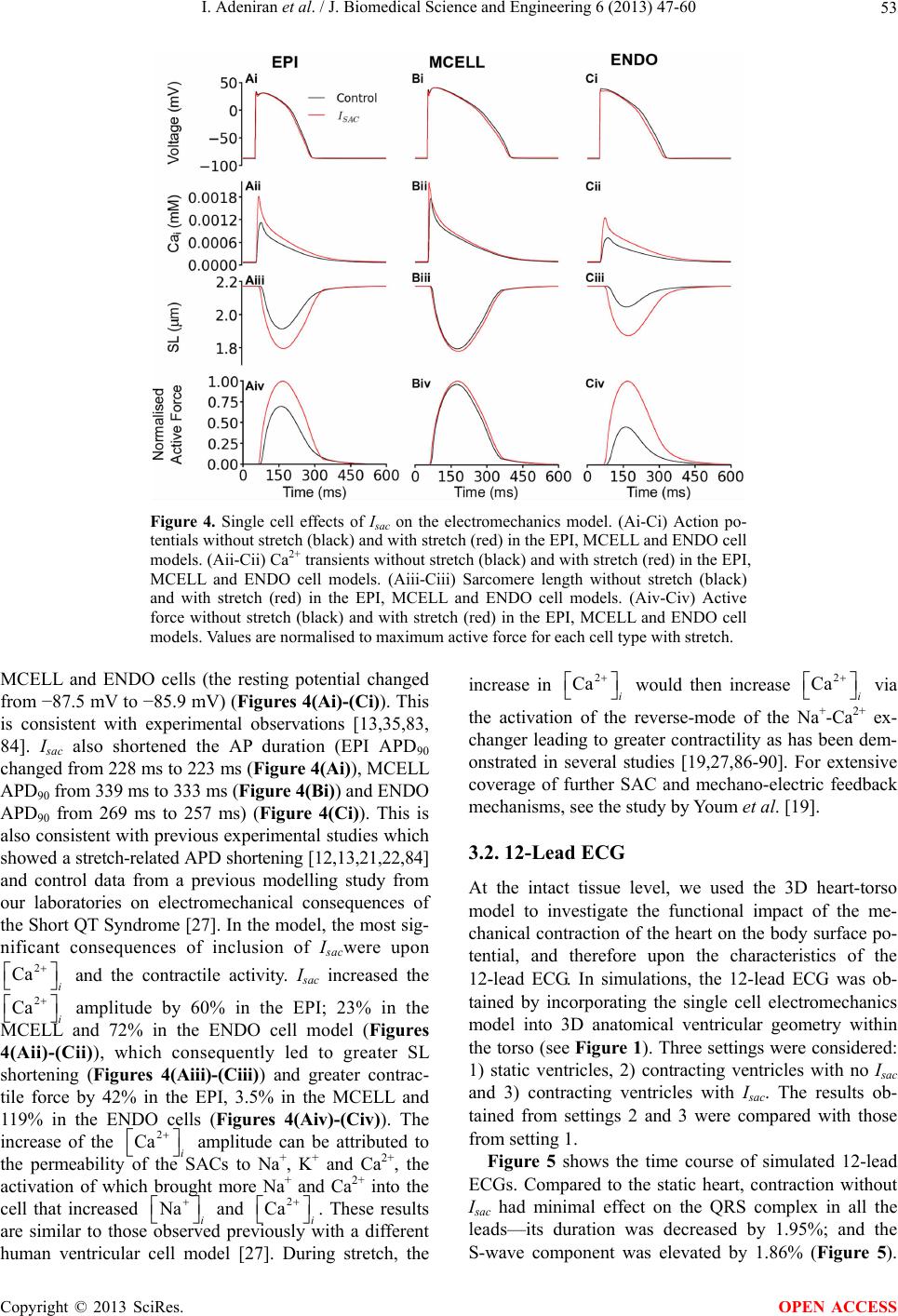 I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 Copyright © 2013 SciRes. 53 Figure 4. Single cell effects of Isac on the electromechanics model. (Ai-Ci) Action po- tentials without stretch (black) and with stretch (red) in the EPI, MCELL and ENDO cell models. (Aii-Cii) Ca2+ transients without stretch (black) and with stretch (red) in the EPI, MCELL and ENDO cell models. (Aiii-Ciii) Sarcomere length without stretch (black) and with stretch (red) in the EPI, MCELL and ENDO cell models. (Aiv-Civ) Active force without stretch (black) and with stretch (red) in the EPI, MCELL and ENDO cell models. Values are normalised to maximum active force for each cell type with stretch. MCELL and ENDO cells (the resting potential changed from −87.5 mV to −85.9 mV) (Figures 4(Ai)-(Ci)). This is consistent with experimental observations [13,35,83, 84]. Isac also shortened the AP duration (EPI APD90 changed from 228 ms to 223 ms (Figure 4(Ai)), MCELL APD90 from 339 ms to 333 ms (Figure 4(Bi)) and ENDO APD90 from 269 ms to 257 ms) (Figure 4(Ci)). This is also consistent with previous experimental studies which showed a stretch-related APD shortening [12,13,21,22,84] and control data from a previous modelling study from our laboratories on electromechanical consequences of the Short QT Syndrome [27]. In the model, the most sig- nificant consequences of inclusion of Isacwere upon increase in 2 Ca i would then increase via 2 Ca i the activation of the reverse-mode of the Na+-Ca2+ ex- changer leading to greater contractility as has been dem- onstrated in several studies [19,27,86-90]. For extensive coverage of further SAC and mechano-electric feedback mechanisms, see the study by Youm et al. [19]. 3.2. 12-Lead ECG At the intact tissue level, we used the 3D heart-torso model to investigate the functional impact of the me- chanical contraction of the heart on the body surface po- tential, and therefore upon the characteristics of the 12-lead ECG. In simulations, the 12-lead ECG was ob- tained by incorporating the single cell electromechanics model into 3D anatomical ventricular geometry within the torso (see Figure 1). Three settings were considered: 1) static ventricles, 2) contracting ventricles with no Isac and 3) contracting ventricles with Isac. The results ob- tained from settings 2 and 3 were compared with those from setting 1. 2 Ca i 2 and the contractile activity. Isac increased the Ca i amplitude by 60% in the EPI; 23% in the MCELL and 72% in the ENDO cell model (Figures 4(Aii)-(Cii)), which consequently led to greater SL shortening (Figures 4(Aiii)-(Ciii)) and greater contrac- tile force by 42% in the EPI, 3.5% in the MCELL and 119% in the ENDO cells (Figures 4(Aiv)-(Civ)). The increase of the i amplitude can be attributed to the permeability of the SACs to Na+, K+ and Ca2+, the activation of which brought more Na+ and Ca2+ into the cell that increased i and i . These results are similar to those observed previously with a different human ventricular cell model [27]. During stretch, the 2 Ca Na 2 Ca Figure 5 shows the time course of simulated 12-lead ECGs. Compared to the static heart, contraction without Isac had minimal effect on the QRS complex in all the leads—its duration was decreased by 1.95%; and the S-wave component was elevated by 1.86% (Figure 5). OPEN ACCESS 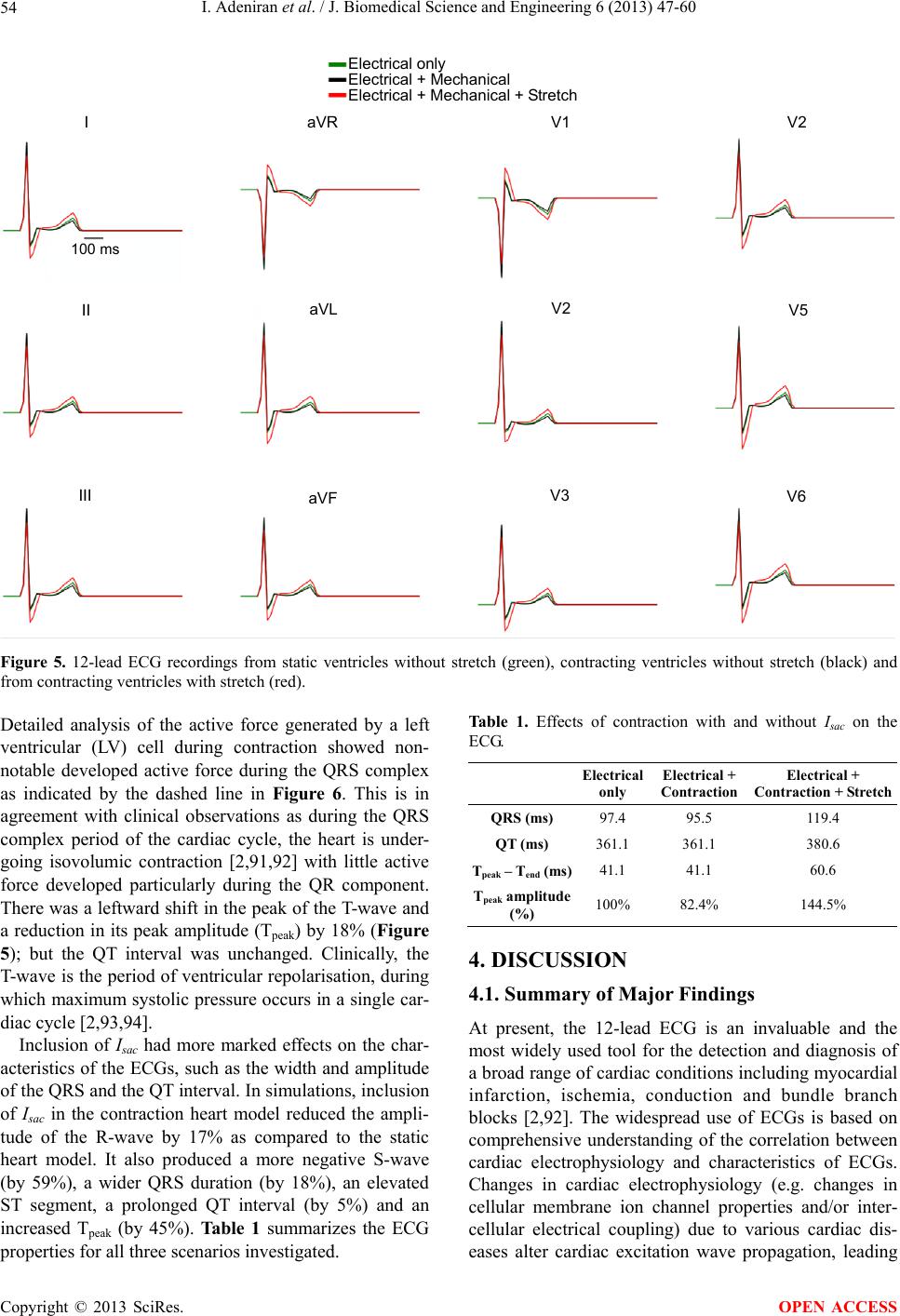 I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 54 Electrical only Electrical + Mechanical Electrical + Mechanical + Stretch I II III aVR aVL aVF V3 V2 V1 V2 V5 V6 100 ms Figure 5. 12-lead ECG recordings from static ventricles without stretch (green), contracting ventricles without stretch (black) and from contracting ventricles with stretch (red). Detailed analysis of the active force generated by a left ventricular (LV) cell during contraction showed non- notable developed active force during the QRS complex as indicated by the dashed line in Figure 6. This is in agreement with clinical observations as during the QRS complex period of the cardiac cycle, the heart is under- going isovolumic contraction [2,91,92] with little active force developed particularly during the QR component. There was a leftward shift in the peak of the T-wave and a reduction in its peak amplitude (Tpeak) by 18% (Figure 5); but the QT interval was unchanged. Clinically, the T-wave is the period of ventricular repolarisation, during which maximum systolic pressure occurs in a single car- diac cycle [2,93,94]. Inclusion of Isac had more marked effects on the char- acteristics of the ECGs, such as the width and amplitude of the QRS and the QT interval. In simulations, inclusion of Isac in the contraction heart model reduced the ampli- tude of the R-wave by 17% as compared to the static heart model. It also produced a more negative S-wave (by 59%), a wider QRS duration (by 18%), an elevated ST segment, a prolonged QT interval (by 5%) and an increased Tpeak (by 45%). Ta b l e 1 summarizes the ECG properties for all three scenarios investigated. Table 1. Effects of contraction with and without Isac on the ECG. Electrical only Electrical + Contraction Electrical + Contraction + Stretch QRS (ms) 97.4 95.5 119.4 QT (ms) 361.1 361.1 380.6 Tpeak – Tend (ms)41.1 41.1 60.6 Tpeak amplitude (%) 100% 82.4% 144.5% 4. DISCUSSION 4.1. Summary of Major Findings At present, the 12-lead ECG is an invaluable and the most widely used tool for the detection and diagnosis of a broad range of cardiac conditions including myocardial infarction, ischemia, conduction and bundle branch blocks [2,92]. The widespread use of ECGs is based on comprehensive understanding of the correlation between cardiac electrophysiology and characteristics of ECGs. Changes in cardiac electrophysiology (e.g. changes in cellular membrane ion channel properties and/or inter- cellular electrical coupling) due to various cardiac dis- eases alter cardiac excitation wave propagation, leading Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 55 Electrical only Electrical + Mechanical Electrical + Mechanical + Stretch A B 100 ms Figure 6. ECG and active force recordings from a left ventricular cell in static ventricles without stretch (green), contracting ventricles without stretch (black) and from contracting ventricles with stretch (red). to an altered electrical field in and around the heart that varies with time during the cardiac cycle [2,15,92]. Such a changed electrical field surrounding the heart is re- flected by changes in the ECG characteristics. Another important characteristic of the heart is the muscle con- traction and relaxation, which also varies with time dur- ing the cardiac cycle [2,15,92]. In addition, in response to mechanical events such as stretch and volume load, the heart regulates its own electrical activity via MEF [2,18,19]; which includes the activation of SACs [18,19, 95]. So far, how cardiac mechanical contraction influ- ences ECG characteristics is less well-understood. In the present study, we have incorporated biophysically de- tailed coupled electromechanical ventricular cell models into a 3D-anatomical human ventricle situated within a human thorax to investigate the effects of mechanical contraction with and without Isac on the 12-lead ECG. Our simulations suggest that: 1) contraction without con- sideration of Isac has no significant effect on the QRS complex, no effect on the QT interval, but shifts the T-wave leftwards and reduces Tpeak (Figure 5); 2) con- traction with consideration of Isac reduces the R-wave amplitude, widens the QRS complex duration, increases Tpeak significantly, shifts the T-wave rightwards and in- creases the QT interval (Figure 5). These findings are dependent on the degree of stretch and are influenced by the magnitude of Isac. Several aspects of our findings merit more detailed discussion. 4.2. Mechanistic Insights With mechanically contracting ventricles without con- sideration of Isac, the QRS complex was not affected sig- nificantly, either in duration (1.95% decrease) or mor- phology (Figure 5). Contraction during the QRS com- plex is isovolumic [2,91,92], thus explaining the insig- nificant effect of contraction on the ECG during this pe- riod. Contraction of each myocyte in the intact tissue is not necessarily isometric, however, as each undergoes different length changes with time. Some myocytes con- tract isotonically, some isometrically whilst others con- tract eccentrically [2,91,92,94]. Therefore, even though the ventricular volume does not change substantially, the ventricle chamber geometry changes considerably [2,94, 96-98]. Consequently, compared to a static heart, the position of the cardiac electrical sources, the distance of the ventricles from the body surface and the varying anisotropic conductivity due to MEF are altered in a mechanically contracting heart; hence, there was a 1.95% decrease in QRS duration without Isac. With considera- tion of Isac, these differential changes in myocyte lengths and hence ventricular geometry are exacerbated; hence, there was a greater and more significant effect of con- traction with Isac on the QRS complex (Figure 5). By the Frank-Starling law, with the increased stretch of the myocardial fibers during diastole by Isac, contractility would increase [2,94,99]. During the T-wave, ventricular contraction attains a maximum, after which ventricular pressure declines with ventricular repolarisation causing a decline in the active force of the myocytes [2,94,96-98]. The ventricular pressure during this period is ~30% greater than during the QRS complex [2,94,96-98]. Therefore, during con- traction, the aforementioned changes in ventricular ge- ometry, the distance of the ventricles from the body sur- face and the varying anisotropic conductivity due to MEF are altered markedly compared to a static heart. This results in a marked effect on the T-wave; without Isac, it is shifted leftwards and the Tpeak is reduced in am- plitude by 18%. With Isac, the myocardial fibers are stretched during diastole leading to increased contractil- ity, which in turn increases systolic pressure [2,94,99] leading to a greater effect on the T-wave (Figure 5); Tpeak is increased in amplitude significantly by ~45% and QT Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 56 interval is increased by ~5%. 4.3. Relevance to Previous Studies Our simulations suggest that ventricular contraction al- ters 12-lead ECG morphology. This is consistent with previous studies [100-103]. In their study, Wei et al. [100] used MRI time sequences of the motion of a human ven- tricular geometry to compute 12-lead ECGs by mounting the geometry in a model of the human body. They found that ventricular motion reduced Tpeak with minimal change to the QRS complex. However, they did not con- sider the incorporation of Isac. Xia et al. [101] also ob- tained similar results with a human whole heart geometry mounted in a human torso but their simulations lacked the use of biophysically detailed electromechanical sin- gle cell models and the incorporation of Isac. Therefore, the present work is the first study to investigate the ef- fects of Isac on the characteristics of simulated body sur- face 12-lead ECGs using anatomically detailed and me- chanically contracting ventricles. The findings from the present study improve our understanding of the effects of electrical-mechanical coupling on the characteristics of ECG. This was achieved by 1) using biophysically de- tailed human ventricular myocyte models [25] coupled with the Rice et al. myofilament model [26]; employed with and without Isac and 2) by demonstrating the impor- tance of ventricular motion on the morphology, proper- ties and subsequently interpretation of the 12 lead ECG. 4.4. Limitations In addition to acknowledged limitations of both the ORd electrophysiology model [25] and the Rice et al. [26] myofilament model, Isac density was based on prior stud- ies [19,28-31], due to lack of experimental data on the Isac from human ventricular myocytes. Additionally, the simulations here were performed at a single, physiologi- cally relevant frequency (1 Hz), but rate-dependent dif- ferences in MEF have not been pursued in this initial study Experimentally observed effects of cycle length (restitution curve) [104] have also not been studied. These rate-dependent phenomena would constitute a val- uable future line of investigation. It should also be ac- knowledged that Isac. may not be the only mechanism responsible for MEF (e.g. [105]) and that due to an ab- sence of functional data in human myocytes, the elec- tromechanical model lacks stretch sensitive K+ channels (e.g. TREK). The model also lacks interactions between myocytes and fibroblasts, which have been proposed to contribute to ventricular MEF [106,107]. Whilst it would be useful for such components to be incorporated into future models, the advantage of the approach adopted here is that it has been possible to isolate and attribute with confidence electrical changes to the incorporation of Isac. Finally, the use of a ventricular computational fluid dynamics model to determine pressure boundary condi- tions would allow a more realistic pressure profile. Al- though it is important that these potential limitations are stated, they do not fundamentally influence the principal conclusions of this study. 5. CONCLUSION With the use of a biophysically detailed electromechani- cal human ventricular single cell model incorporated into a thorax-mounted human ventricular geometry, we have shown that the morphology and properties of the ECG are dependent on ventricular contraction. We have also shown that cellular stretch incorporated at the single cell level as an SAC has a significant influence on the ECG in a mechanically contracting ventricle. 6. ACKNOWLEDGEMENTS This work was supported by project grants from Engineering and Physical Science Research Council UK (EP/J00958X/1; EP/I029826/1), the British Heart Foundation (FS/08/021), the Natural Science Founda- tion of China (61179009) and the University of Manchester. REFERENCES [1] Drew, B.J., Califf, R.M., Funk, M., Kaufman, E.S., Kru- coff, M.W., Laks, M.M., Macfarlane, P.W., Sommargren, C., Swiryn, S., Van Hare, G.F. and American Heart As- sociation (2005) AHA scientific statement: Practice stan- dards for electrocardiographic monitoring in hospital set- tings: An American Heart Association Scientific State- ment from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Com- puterized electrocardiology and the American Associa- tion of Critical-Care Nurses. Journal of Cardiovascular Nursing, 20, 76-106. http://dx.doi.org/10.1097/00005082-200503000-00003 [2] Bonow, R.O., Mann, D.L., Zipes, D.P. and Libby, P. (2011) Braunwald’s heart disease: A textbook of cardio- vascular medicine, 2-volume set: Expert consult premium edition-enhanced online features and print. 9th Edition, Saunders. [3] Malmivuo, J. and Plonsey, R. (1995) Bioelectromagnet- ism: principles and applications of bioelectric and bio- magnetic fields. Oxford University Press, New York. http://dx.doi.org/10.1093/acprof:oso/9780195058239.001 .0001 [4] Menown, I.B., Mackenzie, G. and Adgey, A.A. (2000) Optimizing the initial 12-lead electrocardiographic diag- nosis of acute myocardial infarction. European Heart Journal, 21, 275-283. ttp://dx.doi.org/10.1053/euhj.1999.1748 [5] Brady, W.J., Chan, T.C. and Pollack, M. (2000) Electro- cardiographic manifestations: Patterns that confound the EKG diagnosis of acute myocardial infarction-left bundle Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 57 branch block, ventricular paced rhythm, and left ventri- cular hypertrophy. Journal of Emergency Medicine, 18, 71-78. http://dx.doi.org/10.1016/S0736-4679(99)00178-X [6] Sovilj, S., Magjarevic, R., Lovell, N.H. and Dokos, S. (2013) A simplified 3D model of whole heart electrical activity and 12-lead ECG generation. Computational and Mathematical Methods in Medicine, 2013, Article ID: 134208. [7] Keller, D.U.J, Seemann, G., Weiss, D.L., Farina, D., Ze- helein, J. and Dossel, O. (2007) Computer based model- ing of the congenital long-QT 2 syndrome in the Visible Man torso: From genes to ECG. Proceedings of the 29th Annual International Conference of the IEEE EMBS, Lyon, 23-26 August 2007, 1410-1413. [8] Boulakia, M., Cazeau, S., Fernández, M.A., Gerbeau, J.-F. and Zemzemi, N. (2010) Mathematical modeling of elec- trocardiograms: A numerical study. Annals of Biomedical Engineering, 38, 1071-1097. http://dx.doi.org/10.1007/s10439-009-9873-0 [9] Potse, M., Dubé, B. and Vinet, A. (2009) Cardiac anisot- ropy in boundary-element models for the electrocardio- gram. Medical & Biological Engineering & Computing, 47, 719-729. http://dx.doi.org/10.1007/s11517-009-0472-x [10] Potse, M., Dube, B. and Gulrajani, R.M. (2003) ECG simulations with realistic human membrane, heart, and torso models. Proceedings of the 25th Annual Interna- tional Conference of the IEEE EMBS, Cancun, 17-21 September 2003, 70-73. [11] Lab, M.J. (1996) Mechanoelectric feedback (transduction) in heart: Concepts and implications. Cardiovascular Re- search, 32, 3-14. [12] Taggart, P. (1996) Mechano-electric feedback in the hu- man heart. Cardiov a scular Research, 32, 38-43. [13] Taggart, P. and Sutton, P.M. (1999) Cardiac mechano- electric feedback in man: Clinical relevance. Progress in Biophysics and Molecular Biology, 71, 139-154. http://dx.doi.org/10.1016/S0079-6107(98)00039-X [14] Kelly, D., Mackenzie, L., Hunter, P., Smaill, B. and Saint, D.A. (2006) Gene expression of stretch-activated chan- nels and mechanoelectric feedback in the heart. Clinical and Experimental Pharmacology and Physiology, 33, 642-648. http://dx.doi.org/10.1111/j.1440-1681.2006.04392.x [15] Katz, A. (2010) Physiology of the heart. 5th Edition, Lippincott Williams & Wilkins, Philadelphia. [16] Franz, M.R. (1996) Mechano-electrical feedback in ven- tricular myocardium. Cardiovascular Research, 32, 15- 24. [17] Lab, M.J. (1982) Contraction-excitation feedback in myo- cardium. Physiological basis and clinical relevance. Cir- culation Research, 50, 757-766. http://dx.doi.org/10.1161/01.RES.50.6.757 [18] Ward, M.-L. and Allen, D.G. (2010) Stretch-activated channels in the heart: Contribution to cardiac perform- ance. In: Kamkin, A. and Kiseleva, I., Ed., Mechanosen- sitivity of the Heart Mechanosensitivity in Cells and Tis- sues, Springer, Dordrecht, 141-167. [19] Youm, J.B., Han, J., Kim, N., Zhang, Y.-H., Kim, E., Leem, C.H., Kim, S.H. and Earm, Y.E. (2005) Role of stretch-activated channels in the heart: Action potential and Ca2+ transients. In: Kamkin, A. and Kiseleva, I., Ed., Mechanosensitivity in Cells and Tissues, Academia, Mos- cow. [20] Belus, A. and White, E. (2003) Streptomycin and intra- cellular calcium modulate the response of single guinea- pig ventricular myocytes to axial stretch. The Journal of Physiology, 546, 501-509. http://dx.doi.org/10.1113/jphysiol.2002.027573 [21] Zeng, T., Bett, G.C. and Sachs, F. (2000) Stretch-activat- ed whole cell currents in adult rat cardiac myocytes. American Journal of Physiology. Heart and Circulatory Physiology, 278, H548-H557. [22] Dean, J.W. and Lab, M.J. (1989) Effect of changes in load on monophasic action potential and segment length of pig heart in situ. Cardiovascular Research, 23, 887- 896. http://dx.doi.org/10.1093/cvr/23.10.887 [23] Franz, M.R., Burkhoff, D., Yue, D.T. and Sagawa, K. (1989) Mechanically induced action potential changes and arrhythmia in isolated and in situ canine hearts. Car- diovascular Research, 23, 213-223. http://dx.doi.org/10.1093/cvr/23.3.213 [24] Hansen, D.E. (1993) Mechanoelectrical feedback effects of altering preload, afterload, and ventricular shortening. American Journal of Physiology, 264, H423-H432. [25] O’Hara, T., Virág, L., Varró, A. and Rudy, Y. (2011) Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLOS Computational Biology, 7, Article ID: e1002061. http://dx.doi.org/10.1371/journal.pcbi.1002061 [26] Rice, J.J., Wang, F., Bers, D.M. and de Tombe, P.P. (2008) Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary dif- ferential equations. Biophysical Journal, 95, 2368-2390. http://dx.doi.org/10.1529/biophysj.107.119487 [27] Adeniran, I., Hancox, J. and Zhang, H. (2013) In silico investigation of the short QT syndrome, using human ventricle models incorporating electromechanical coupl- ing. Front Physiology, 4, 166. [28] Panfilov, A.V., Keldermann, R.H. and Nash, M.P. (2005) Self-organized pacemakers in a coupled reaction-diffu- sion-mechanics system. Physical Review Letters, 95, Ar- ticle ID: 258104. http://dx.doi.org/10.1103/PhysRevLett.95.258104 [29] Lunze, K., Stålhand, J. and Leonhardt, S. (2010) Model- ing of stretch-activated sarcolemmal channels in smooth muscle cells. Proceedings of the World Congress on Me- dical Physics and Biomedical Engineering IFMBE, Mu- nich, 7-12 September 2009, 740-743. [30] Kuijpers, N.H.L. (2008) Cardiac electrophysiology and mechanoelectric feedback. Ph.D. Thesis, Eindhoven Uni- versity of Technology, Eindhoven. [31] Kohl, P. and Sachs, F. (2001) Mechanoelectric feedback in cardiac cells. Philosophical Transactions of the Royal Society A, 359, 1173-1185. http://dx.doi.org/10.1098/rsta.2001.0824 Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 58 [32] Trayanova, N., Li, W., Eason, J. and Kohl, P. (2004) Effect of stretch-activated channels on defibrillation effi- cacy. Heart Rhythm, 1, 67-77. http://dx.doi.org/10.1016/j.hrthm.2004.01.002 [33] Kohl, P., Hunter, P. and Noble, D. (1999) Stretch-induced changes in heart rate and rhythm: Clinical observations, experiments and mathematical models. Progress in Bio- physics and Molecular Biology, 71, 91-138. http://dx.doi.org/10.1016/S0079-6107(98)00038-8 [34] Zabel, M., Koller, B.S., Sachs, F. and Franz, M.R. (1996) Stretch-induced voltage changes in the isolated beating heart: importance of the timing of stretch and implica- tions for stretch-activated ion channels. Cardiovascular Research, 32, 120-130. [35] Kamkin, A., Kiseleva, I. and Isenberg, G. (2000) Stretch- activated currents in ventricular myocytes: Amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovascular Research, 48, 409-420. http://dx.doi.org/10.1016/S0008-6363(00)00208-X [36] Marsden, J.E. and Hughes, T.J.R. (1994) Mathematical foundations of elasticity. Dover Publications, New York. [37] Holzapfel, G.A. (2000) Nonlinear solid mechanics: A continuum approach for engineering. John Wiley & Sons Ltd., Chichester. [38] Pathmanathan, P. and Whiteley, J.P. (2009) A numerical method for cardiac mechanoelectric simulations. Annals of Biomedical Engineering, 37, 860-873. http://dx.doi.org/10.1007/s10439-009-9663-8 [39] Costa, K.D., Holmes, J.W. and Mcculloch, A.D. (2001) Modelling cardiac mechanical properties in three dimen- sions. Philosophical Transactions of the Royal Society A, 359, 1233-1250. http://dx.doi.org/10.1098/rsta.2001.0828 [40] Hunter, P.J., Nash, M.P. and Sands, G.B. (1997) Compu- tational mechanics of the heart. In: Panfilov, A.V. and Holden, A.V., Eds., Computational Biology of the Heart. Wiley, West Sussex, 345-407. [41] Niederer, S.A. and Smith, N.P. (2008) An improved nu- merical method for strong coupling of excitation and contraction models in the heart. Progress in Biophysics and Molecular Biology, 96, 90-111. http://dx.doi.org/10.1016/j.pbiomolbio.2007.08.001 [42] Whiteley, J.P., Bishop, M.J. and Gavaghan, D.J. (2007) Soft tissue modelling of cardiac fibres for use in coupled mechano-electric simulations. Bulletin of Mathematical Biology, 69, 2199-2225. http://dx.doi.org/10.1007/s11538-007-9213-1 [43] Bonet, J. and Wood, R.D. (2008) Nonlinear continuum mechanics for finite element analysis. 2nd Edition, Cam- bridge University Press, Cambridge. http://dx.doi.org/10.1017/CBO9780511755446 [44] Le Tallec, P. (1994) Numerical methods for nonlinear three-dimensional elasticity. In: Lions, J.L. and Ciarlet, P.G., Eds., Handbook of Numerical Analysis; Vol.3, Tech- niques of Scientific Computing (part 1); Numerical Meth- ods for Solids (part 1); Solution of Equations in R(n) (part 2), North-Holland, London. [45] Keldermann, R.H., Nash, M.P. and Panfilov, A.V (2009) Modeling cardiac mechano-electrical feedback using re- action-diffusion-mechanics systems. Physica D: Nonlin- ear Phenomena, 238, 1000-1007. http://dx.doi.org/10.1016/j.physd.2008.08.017 [46] Auricchio, F., Beirão da Veiga, L., Lovadina, C. and Rea- li, A. (2010) The importance of the exact satisfaction of the incompressibility constraint in nonlinear elasticity: Mixed FEMs versus NURBS-based approximations. Com- puter Methods in Applied Mechanics and Engineering, 199, 314-323. http://dx.doi.org/10.1016/j.cma.2008.06.004 [47] Braess, D. and Ming, P. (2005) A finite element method for nearly incompressible elasticity problems. Math Comp, 74, 25-52. http://dx.doi.org/10.1090/S0025-5718-04-01662-X [48] Braess, D. (2007) Finite elements: Theory, fast solvers, and applications in solid mechanics. 3rd Edition, Cam- bridge University Press, Cambridge. http://dx.doi.org/10.1017/CBO9780511618635 [49] Brenner, S.C. and Scott, R. (2010) The mathematical theory of finite element methods. 3rd Edition, Springer, New York. [50] Seemann, G., Keller, D.U.J., Weiss, D.L. and Dössel, O. (2006) Modeling human ventricular geometry and fiber orientation based on diffusion tensor MRI. Computers in Cardiology, 33, 801-804. [51] Legrice, I.J., Hunter, P.J. and Smaill, B.H. (1997) Lami- nar structure of the heart: A mathematical model. The American Journal of Physiology, 272, H2466-H2476. [52] Lilli, A., Baratto, M.T., Meglio, J.D., Chioccioli, M., Mag- nacca, M., Talini, E., Canale, M.L., Poddighe, R., Co- mella, A. and Casolo, G. (2013) Left ventricular rotation and twist assessed by four-dimensional speckle tracking echocardiography in healthy subjects and pathological remodeling: A single center experience. Echocardiogra- phy, 30, 171-179. http://dx.doi.org/10.1111/echo.12026 [53] Lorenz, C.H., Pastorek, J.S. and Bundy, J.M. (2000) De- lineation of normal human left ventricular twist through- out systole by tagged cine magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance, 2, 97- 108. http://dx.doi.org/10.3109/10976640009148678 [54] Tseng, W-Y.I., Reese, T.G., Weisskoff, R.M., Brady, T.J. and Wedeen, V.J. (2000) Myocardial fiber shortening in humans: Initial results of MR imaging. Radiology , 216, 128-139. http://dx.doi.org/10.1148/radiology.216.1.r00jn39128 [55] MacGowan, G.A., Shapiro, E.P., Azhari, H., Siu, C.O., Hees, P.S., Hutchins, G.M., Weiss, J.L. and Rademakers, F.E. (1997) Noninvasive measurement of shortening in the fiber and cross-fiber directions in the normal human left ventricle and in idiopathic dilated cardiomyopathy. Circulation, 96, 535-541. http://dx.doi.org/10.1161/01.CIR.96.2.535 [56] Coppola, B.A. and Omens, J.H. (2008) Role of tissue structure on ventricular wall mechanics. MCB Molecular and Cellular Biomechanics, 5, 183-196. [57] Cheng, A., Nguyen, T.C., Malinowski, M., Daughters, G.T., Miller, D.C. and Ingels Jr., N.B. (2008) Heteroge- neity of left ventricular wall thickening mechanisms. Cir- Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 59 culation, 118, 713-721. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.7446 23 [58] Bogaert, J. and Rademakers, F.E. (2001) Regional non- uniformity of normal adult human left ventricle. Ameri- can Journal of Physiology. Heart and Circulatory Physi- ology, 280, H610-H620. [59] Guccione, J.M., McCulloch, A.D. and Waldman, L.K. (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. Jour- nal of Biomechanical Engineering, 113, 42-55. http://dx.doi.org/10.1115/1.2894084 [60] Land, S., Niederer, S.A. and Smith, N.P. (2012) Efficient computational methods for strongly coupled cardiac elec- tromechanics. IEEE Transactions on Biomedical Engi- neering, 59, 1219-1228. http://dx.doi.org/10.1109/TBME.2011.2112359 [61] Colli Franzone, P., Pavarino, L.F. and Taccardi, B. (2005) Simulating patterns of excitation, repolarization and ac- tion potential duration with cardiac bidomain and mo- nodomain models. Mathematical Biosciences, 197, 35-66. http://dx.doi.org/10.1016/j.mbs.2005.04.003 [62] Potse, M., Dubé, B., Richer, J., Vinet, A. and Gulrajani, R.M. (2006) A comparison of monodomain and bidomain reaction-diffusion models for action potential propagation in the human heart. IEEE Transactions on Biomedical Engineering, 53, 2425-2435. http://dx.doi.org/10.1109/TBME.2006.880875 [63] Keener, J. and Sneyd, J. (2008) Mathematical physiology: II: Systems physiology. 2nd Edition, Springer, New York. [64] Nash, M.P. and Panfilov, A.V. (2004) Electromechanical model of excitable tissue to study reentrant cardiac arrhy- thmias. Progress in Biophysics and Molecular Biology, 85, 501-522. http://dx.doi.org/10.1016/j.pbiomolbio.2004.01.016 [65] Taggart, P., Sutton, P.M., Opthof, T., Coronel, R., Trim- lett, R., Pugsley, W. and Kallis, P. (2000) Inhomogeneous transmural conduction during early ischaemia in patients with coronary artery disease. Journal of Molecular and Cellular Cardiology, 32, 621-630. http://dx.doi.org/10.1006/jmcc.2000.1105 [66] Plonsey, R. and Barr, R.C. (2007) Bioelectricity: A quan- titative approach. 3rd Edition, Springer, New York. [67] Drouin, E., Charpentier, F., Gauthier, C., Laurent, K. and Le Marec, H. (1995) Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: Evidence for presence of M cells. Journal of the Ameri- can College of Cardiology, 26, 185-192. http://dx.doi.org/10.1016/0735-1097(95)00167-X [68] Keller, D.U.J., Kalayciyan, R., Dössel, O. and Seemann, G. (2009) Fast creation of endocardial stimulation pro- files for the realistic simulation of body surface ECGs. Proceedings of the World Congress on Medical Physics and Biomedical Engineering IFMBE, Munich, 7-12 Sep- tember 2009, 145-148. [69] Sundnes, J., Lines, G.T. and Tveito, A. (2005) An opera- tor splitting method for solving the bidomain equations coupled to a volume conductor model for the torso. Ma- thematical Biosciences, 194, 233-248. http://dx.doi.org/10.1016/j.mbs.2005.01.001 [70] Burnett, D.S. (1987) Finite element analysis: From con- cepts to applications. Addison Wesley, Massachusetts. [71] Ern, A. and Guermond, J.L. (2010) Theory and practice of finite elements. Springer, New York. [72] Rush, S. and Larsen, H. (1978) A practical algorithm for solving dynamic membrane equations. IEEE Transac- tions on Biomedical Engineering, 25, 389-392. http://dx.doi.org/10.1109/TBME.1978.326270 [73] Cohen, S. and Hindmarsh, A.C. (1996) CVODE, A stiff/ nonstiff ode solver in C. In: Holmes, L.M. Ed., Com- puters in Physics, American Institute of Physics Inc, New York, 138-143 [74] Hindmarsh, A.C., Brown, P.N., Grant, K.E., Lee, S.L., Serban, R., Shumaker, D.E. and Woodward, C.S. (2005) SUNDIALS: Suite of nonlinear and differential/algebraic equation solvers. ACM Transacti o ns on Mathematical Soft- ware, 31, 363-396. http://dx.doi.org/10.1145/1089014.1089020 [75] Logg, A., Mardal, K.A. and Wells, G. (2012) Automated solution of differential equations by the finite element method: The FEniCS book. Springer, New York. http://dx.doi.org/10.1007/978-3-642-23099-8 [76] Chamberland, E., Fortin, A. and Fortin, M. (2010) Com- parison of the performance of some finite element discre- tizations for large deformation elasticity problems. Com- puters & Structures, 88, 664-673. http://dx.doi.org/10.1016/j.compstruc.2010.02.007 [77] Haga, J.B., Osnes, H. and Langtangen, H.P. (2012) On the causes of pressure oscillations in low-permeable and low-compressible porous media. International Journal for Numerical and Analytical Methods in Geomechanics, 36, 1507-1522. http://dx.doi.org/10.1002/nag.1062 [78] Hughes, T.J.R. (2000) The finite element method: Linear static and dynamic finite element analysis. Dover Publi- cations, Dover. [79] McIntosh, M.A., Cobbe, S.M. and Smith, G.L. (2000) Heterogeneous changes in action potential and intracellu- lar Ca2+ in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovascular Research, 45, 397-409. http://dx.doi.org/10.1016/S0008-6363(99)00360-0 [80] Woodworth, R.S. (1902) Maximal contraction, ‘staircase’ contraction, refractory period, and compensatory pause, of the heart. American Journal of Physiology, 8, 213-249. [81] Mulieri, L.A., Hasenfuss, G., Leavitt, B., Allen, P.D. and Alpert, N.R. (1992) Altered myocardial force-frequency relation in human heart failure. Circulation, 85, 1743-1750. http://dx.doi.org/10.1161/01.CIR.85.5.1743 [82] Lakatta, E.G. (2004) Beyond Bowditch: The convergence of cardiac chronotropy and inotropy. Cell Calcium, 35, 629-642. http://dx.doi.org/10.1016/j.ceca.2004.01.017 [83] Boland, J. and Troquet, J. (1980) Intracellular action po- tential changes induced in both ventricles of the rat by an acute right ventricular pressure overload. Cardiovascular Research, 14, 735-740. http://dx.doi.org/10.1093/cvr/14.12.735 [84] Franz, M.R., Cima, R., Wang, D., Profitt, D. and Kurz, R. (19920 Electrophysiological effects of myocardial stretch Copyright © 2013 SciRes. OPEN ACCESS  I. Adeniran et al. / J. Biomedical Science and Engineering 6 (2013) 47-60 Copyright © 2013 SciRes. 60 OPEN ACCESS and mechanical determinants of stretch-activated arrhy- thmias. Circulation, 86, 968-978. http://dx.doi.org/10.1161/01.CIR.86.3.968 [85] Calkins, H., El-Atassi, R., Kalbfleisch, S., Langberg, J. and Morady, F. (1992) Effects of an acute increase in atrial pressure on atrial refractoriness in humans. Pacing and Clinical Electrophysiology, 15, 1674-1680. http://dx.doi.org/10.1111/j.1540-8159.1992.tb02954.x [86] Alvarez, B.V., Pérez, N.G., Ennis, I.L., Camilión de Hur- tado, M.C. and Cingolani, H.E. (1999) Mechanisms un- derlying the increase in force and Ca2+ transient that fol- low stretch of cardiac muscle: A possible explanation of the Anrep effect. Circulation Research, 85, 716-722. http://dx.doi.org/10.1161/01.RES.85.8.716 [87] Baartscheer, A., Schumacher, C.A., van Borren M.M.G.J, Belterman, C.N.W, Coronel, R. and Fiolet, J.W.T. (2003) Increased Na+/H+-exchange activity is the cause of in- creased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovascular Research, 57, 1015-1024. http://dx.doi.org/10.1016/S0008-6363(02)00809-X [88] Calaghan, S.C., Belus, A. and White, E. (2003) Do stretch- induced changes in intracellular calcium modify the electri- cal activity of cardiac muscle? Progress in Biophysics and Molecular Biology, 82, 81-95. http://dx.doi.org/10.1016/S0079-6107(03)00007-5 [89] Calaghan, S.C. and White, E. (1999) The role of calcium in the response of cardiac muscle to stretch. Progress in Biophysics and Molecular Biology, 71, 59-90. http://dx.doi.org/10.1016/S0079-6107(98)00037-6 [90] Barcenas-Ruiz, L., Beuckelmann, D.J. and Wier, W.G. (1987) Sodium-calcium exchange in heart: Membrane cur- rents and changes in [Ca2+]i. Science, 238, 1720-1722. http://dx.doi.org/10.1126/science.3686010 [91] Hall, J.E. and Guyton, A.C. (2010) Textbook of medical physiology. 12th Edition, Saunders, Philadelphia. [92] Rhoades, R. and Bell, D.R. (2013) Medical physiology: principles for clinical medicine. 4th Edition, Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadel- phia. [93] DiMino, T.L., Ivanov, A., Burke, J.F. and Kowey, P.R. (2006) Electrocardiography. In: Rosendorff, C., Ed., Es- sential Cardiology: Principles and Practice, Humana Press, Clifton, 117-138. http://dx.doi.org/10.1007/978-1-59259-918-9_8 [94] Klabunde, R.E. (2012) Cardiovascular physiology con- cepts. 2nd Edition, Lippincott Williams & Wilkins/Wol- ters Kluwer, Philadelphia. [95] Bett, G.C. and Sachs, F. (1997) Cardiac mechanosensiti- vity and stretch-activated ion channels. Trends in Cardio- vascular Medicine, 7, 4-8. http://dx.doi.org/10.1016/S1050-1738(96)00119-3 [96] Sengupta, P.P., Tajik, A.J., Chandrasekaran, K. and Khand- heria, B.K. (2008) Twist mechanics of the left ventricle: Principles and application. JACC: Cardiovascular Imaging, 1, 366-376. http://dx.doi.org/10.1016/j.jcmg.2008.02.006 [97] Sengupta, P.P., Khandheria, B.K. and Narula, J. (2008) Twist and untwist mechanics of the left ventricle. Heart Failure Clinics, 4, 315-324. http://dx.doi.org/10.1016/j.hfc.2008.03.001 [98] Hodt, A., Stugaard, M., Hisdal, J., Stranden, E., Atar, D. and Steine, K. (2012) Regional LV deformation in healthy in- dividuals during isovolumetric contraction and ejection phases assessed by 2D speckle tracking echocardiography. Clinical Physiology and Functional Imaging, 32, 372-379. http://dx.doi.org/10.1111/j.1475-097X.2012.01139.x [99] Lionel, H.O. (2006) Ventricular Function. In: Rosendorff, C., Ed., Essential Cardiology: Principles and Practice, Human Press, New Jersey, 37-54. [100] Wei, Q., Liu, F., Appleton, B., Xia, L., Liu, N., Wilson, S., Riley, R., Strugnel, W., Slaughter, R., Denman, R. and Crozier, S. (2006) Effect of cardiac motion on body sur- face electrocardiographic potentials: An MRI-based simu- lation study. Physics in Medicine and Biology, 51, 3405- 3418. http://dx.doi.org/10.1088/0031-9155/51/14/009 [101] Xia, L., Huo, M., Wei, Q., Liu, F. and Crozier, S. (2005) Analysis of cardiac ventricular wall motion based on a three-dimensional electromechanical biventricular model. Physics in Medicine and Biology, 50, 1901-1917. http://dx.doi.org/10.1088/0031-9155/50/8/018 [102] Smith, N.P., Buist, M.L. and Pullan, A.J. (2003) Altered T wave dynamics in a contracting cardiac model. Journal of Cardiovascular Electrophysiology, 14, S203-S209. http://dx.doi.org/10.1046/j.1540.8167.90312.x [103] Mao, H.D., Wang, L.W., Wong, C.L., Liu, H.F. and Shi, P.C. (2011) A coupled heart-torso framework for cardiac electrocardiographic simulation. Computing in Cardiology, 38, 225-228. [104] Horner, S.M., Dick, D.J., Murphy, C.F. and Lab, M.J. (1996) Cycle length dependence of the electrophysiolo- gical effects of increased load on the myocardium. Cir- culation, 94, 1131-1136. http://dx.doi.org/10.1161/01.CIR.94.5.1131 [105] Sung, D., Mills, R.W., Schettler, J., Narayan, S.M., Omens, J.H. and McCulloch, A.D. (2003) Ventricular filling slows epicardial conduction and increases action potential dura- tion in an optical mapping study of the isolated rabbit heart. Journal of Cardiovascular Electrophysiology, 14, 739-749. http://dx.doi.org/10.1046/j.1540-8167.2003.03072.x [106] Kohl, P., Camelliti, P., Burton, F.L. and Smith, G.L. (2005) Electrical coupling of fibroblasts and myocytes: Relevance for cardiac propagation. Journal of Electrocardiology, 38, 45-50. http://dx.doi.org/10.1016/j.jelectrocard.2005.06.096 [107] Thompson, S.A., Copeland, C.R., Reich, D.H. and Tung, L. (2011) Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation, 123, 2083-2093. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.0150 57
|