 International Journal of Clinical Medicine, 2013, 4, 561-570 Published Online December 2013 (http://www.scirp.org/journal/ijcm) http://dx.doi.org/10.4236/ijcm.2013.412097 Open Access IJCM 561 Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India* Mohammed Salman1,2#, Shruti Dasgupta1,3, Cletus J. M. D’Souza1,2, D. Xaviour1, B. V. Raviprasad1, Jayashankar Rao1, G. L. Lakshmi1 1Anthropological Survey of India, Southern Regional Centre, Mysore, India; 2Department of Studies in Biochemistry, University of Mysore, Mysore, India; 3Department of Studies in Biotechnology, University of Mysore, Mysore, India. Email: #Salman.biochem@gmail.com Received October 24th, 2013; revised November 20th, 2013; accepted December 5th, 2013 Copyright © 2013 Mohammed Salman et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Obje ctive: The study aims to explore the prevalence of hypertension and its impact on Type 2 diabetes in a Mysore population of the Indian subcontinent. Methods: 636 participants volunteered for the study. Anthropometric measure- ments and blood pressure were recorded while plasma was analyzed for biochemical markers. The IDF and JNC 7 di- agnostic criteria were followed to define diabetes and hypertension. Statistical Analyses: One-way an alysis o f v aria nce, χ2-test and Logistic regression analysis were performed to assess differences of the mean, proportion and the independ- ent effect of hypertension on the development of type 2 diabetes. Results: Hypertension was observed to be prevalent in 37.1% of the studied population with an insignificant gender difference. Rate of occurrence of hypertensives was found to be significantly higher in type 2 diabetes (51.9%), obese subjects (45.2%), long-term smokers (49%) and alcohol addicts (48%) than control groups. The risk of development of diabetes was significantly higher in hypertensives than normotensive. However, when creatinine and blood urea nitrogen were included in the model, the significance was nul- lified. Conclusions: The prevalence of type 2 diabetes and hypertension is increasing at an alarming rate. This study reveals that the significance of hypertension as a parameter in predicting the risk of type 2 diabetes was influenced by the renal function and lipid profile. Keywords: Hypertension; Type 2 Diabetes; Prevalence; Kidney Dysfunction; Mortality; Mysore Population; India 1. Introduction The incidence of Type 2 Diabetes (T2D) is increasing globally from 2.8% in 2000 to 4.4% in 2030 [1]. The prevalence of T2D in Asian Indians ranges from 2.7% in rural India to 14% in urban India. India has the highest number of diabetes in the world [2,3]. The National Ur- ban Diabetes Survey reported 12.1% of diabetes and 14% of impaired glucose tolerance [4]. The prevalence of hy- pertension (HTN) among adults is expected to rise by 60% resulting in a total of 1.56 billio n affected individu- als by 2025. Approximately 70% of diabetics are hyper- tensives, as diabetics are pr one to HTN twice more likely than normoglycemic individuals [5]. Similarly, the pres- ence of HTN precedes the onset of diabetes mellitus (DM) [6,7]; and among diabetics, HTN develops into diabetes nephropathy and retinopathy. The co-occurrence of HTN and T2D affects up to 60% of patients leading to higher risk of developing cardiovascular morbidity and mortal- ity [8]. Though cardiovascular risks are common in both, in conjunction they accelerate cardiac, cerebral and renal dysfunctions [9]. The United Kingdom Prospective Dia- betes Study (UKPDS) revealed that blood pressure con- trol helps to avoid cardiovascular complications in pa- tients with T2D [10]. The decrease in mean systolic blood pressure by 10 mm/Hg reduces the risks of devel- oping complications in diabetes by 12%, mortality by 15%, myocardial infraction by 11% and microvascular complications by 13% among diabetics respectively [11]. The prevalence varies across different ethnic and reli- gious groups in Asia; the co-occurrence of diabetes with HTN shows an increasing trend and has become an epi- demic of a great concern [12]. About 50% of diabetes cases in India show the co-occurrence of HTN [13,14]. *Competing Interests: the author(s) declare that they have no competing interests. #Corresponding author.  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 562 Diabetes in other Asian countries such as Saudi Arabia (53%) [15,16], Jordan (72.4%) [17], Oman (21.5%), Turkey (32%), Bahrain (38%) and Taiwan (39%) [18-21] also shows a similar trend. In addition, studies reported higher tendency of HTN among UK Afro-Caribbean (82%) [22], UK Caucasian (74%), Italian (74.4%) and Spanish diabetes (7 3%) [23-25]. Few epidemiological studies asserting incidence rates of T2D and HTN have been carried out in various sectors of Karnataka. In the rural population of Davanagere, 18.3% of HTN has been reported, where males recorded a higher prevalence rate (19.1%) than females (17.5%) [26]. Heritability of HTN in families of Tumkur popula- tion was reported, wherein the young normotensive with a positive family history of HTN had significantly higher blood pressure [27]. In Karnataka, the prevalence of T2D has been observed to be 3.77% of Suttur population [28], 10.0% in Kolar popu lation [29], 16% of the Udup i popu- lation [30] and 17.3% in Dharwad urban population [31]. The incidence of obesity among T2D of Mysore popula- tion was reported [32], while the awareness of diabetes and their attitude to patients of Bijapur have been re- ported [33]. But there is no known record of the preva- lence of HTN among T2D or vice versa, implying how frequent HTN exacerbate s T2D in southern India. Currently, there are limited epidemiological studies edifying the relationship between T2D and HTN in In- dian context. There is an ongoing debate regarding the consideration of high blood pressure over other meta- bolic components (co njointly involved in T2D and HTN), as a predictor of T2D in Indians. Further, social and cul- tural diversity in India necessitates the exploration of the mentioned relationship in various sections of this country. Therefore we hypothesize that the risk of incidence of T2D is higher in the subjects with HTN. The present study aims to assess the prevalence of HTN among T2D subjects and its contribution in the occurrence of T2D in Mysore population of Karnataka in South India. 2. Materials and Methods 2.1. Study Population This case-control study was conducted among partici- pants in the diabetes health check-up programs organized by Amrita Kripa Polyclinic and Lion’s Club of Mysore (R) in Mysore district of Karnataka State, India, during 2010 to 2011 including both non-diabetes and diabetes patients, without any mental impairment. 2.2. Sample Size A total of 654 subjects volunteered and gave consent to participate in the study, out of which 636 were included in the study. The subjects including 343 males and 293 females, aged between 30 - 80 years were enrolled for the study. Subjects with abnormal renal or chronic liver dysfunction were excluded from the study. 2.3. Sampling Procedure The study protocol was reviewed and approved by the Institutional Ethics Committee, Kolkata and also the Ethical Committee of University of Mysore. Informed consents were obtained from each participant in the study. The study was conducted according to the ethical guide- lines for biomedical research on human populations (http://icmr.nic.in/ethical_guidelines, ICMR 7). Each par- ticipant of the study was about 12 hours of fasting period before the collection of blood. 5 ml blood sample was collected in 10 ml BD vacutainer by a phlebotomist, stored at 4˚C and transported to the laboratory immedi- ately for further processing. Postprandial plasma glucose was measured after 2 hours of administering 75-grams of glucose to the subjects (OGTT, WHO, 1999). 2.4. Data Collection Questionnaire: Data was collected on standardized questionnaire that included personal information, life- style, habitual behaviors (smoking and alcohol intake), clinical history of associated complications and blood pressure was recorded under the supervision of a physi- cian. Anthropometry: Height, weight, waist and hip cir- cumference were measured by physical anthropologists using anthr opometer (Ho ltaine, UK) and digital weighing machine (Tanita Corporation, Tokyo, Japan) as per WHO international manual [34]. Waist circumference (WC) was measured at the midpoint at the bottom of the rib cage and the top of the lateral border of the iliac crest during minimal respiration. Laboratory Examination: Fasting plasma glucose (FPG), Glycated Haemoglobin (HbA1c), High-density lipoprotein (HDL), Low-density lipoprotein (LDL), Total Cholesterol (CHO), Triglycerides (TRIG), Creatinine (CRE), Blood urea nitrogen (BUN) and Postprandial glu- cose (PPG) were measured on Auto analyzer EM 360 (Transasia, ERBA Mannheim, Germany). Operational Definitions: Body mass index (BMI) was calculated as weight in kilograms divided by the squared value of height in meters (kg/m2). BMI was categorized as normal (<25 kg/m2), overweight (>25 and < 30 kg/m2), and obese (>30 kg/m2) [35]. Blood pressure (Systolic and Diastolic) of each subject was measured using a standardized sphygmomanometer (Elko, India), in supine position. An average of two readings of both systolic (SBP) and diastolic blood pressure (DBP) was taken. HTN was defined following the criteria of the Joint National Co mmittee on Prevention, Detection, Eva- luation and Treatment of High Blood Pressure (JNC 7 Open Access IJCM 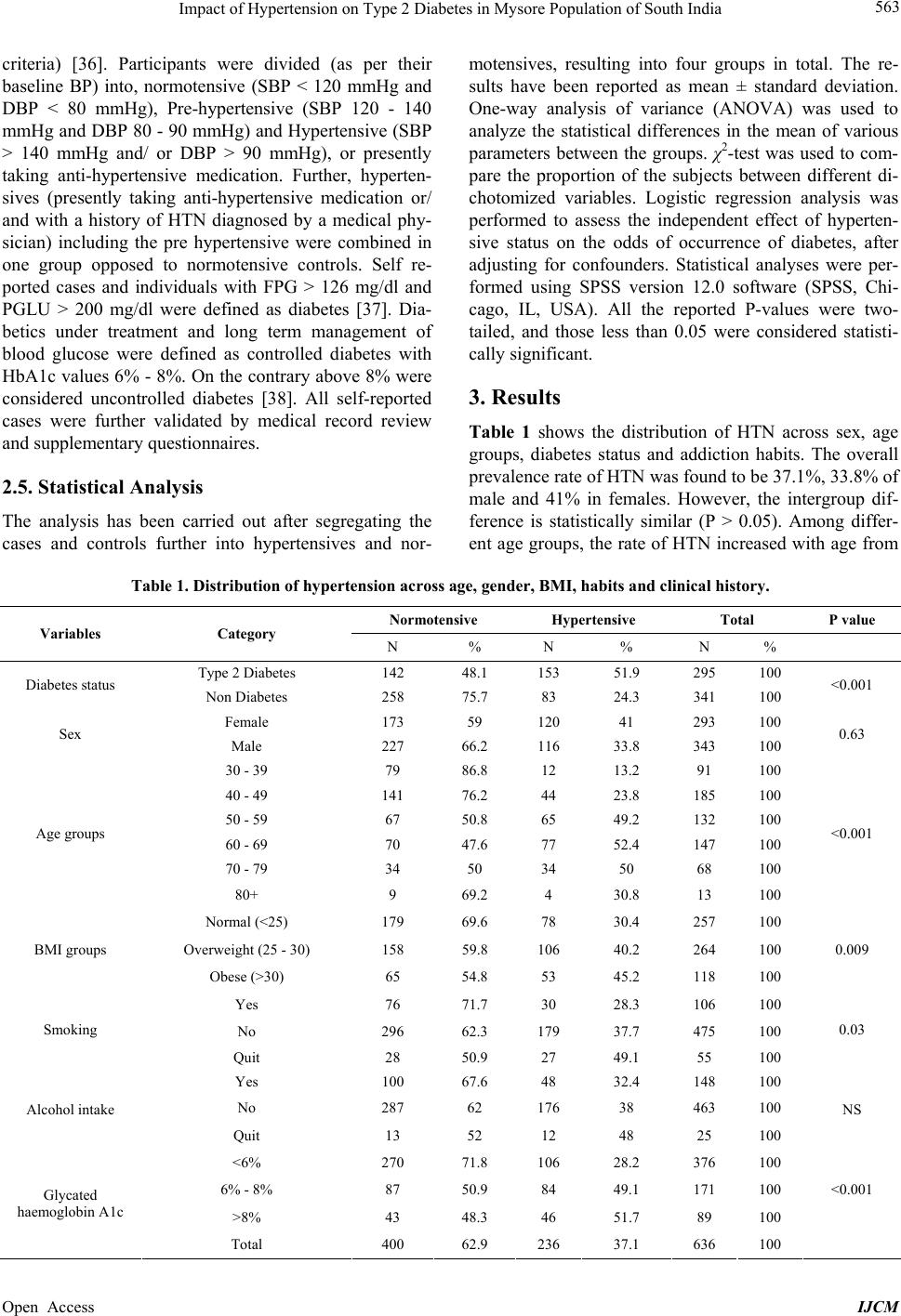 Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India Open Access IJCM 563 criteria) [36]. Participants were divided (as per their baseline BP) into, normotensive (SBP < 120 mmHg and DBP < 80 mmHg), Pre-hypertensive (SBP 120 - 140 mmHg and DBP 80 - 90 mmHg) and Hypertensive (SBP > 140 mmHg and/ or DBP > 90 mmHg), or presently taking anti-hypertensive medication. Further, hyperten- sives (presently taking anti-hypertensive medication or/ and with a history of HTN diagnosed by a medical phy- sician) including the pre hypertensive were combined in one group opposed to normotensive controls. Self re- ported cases and individuals with FPG > 126 mg/dl and PGLU > 200 mg/dl were defined as diabetes [37]. Dia- betics under treatment and long term management of blood glucose were defined as controlled diabetes with HbA1c values 6% - 8%. On the contrary above 8% were considered uncontrolled diabetes [38]. All self-reported cases were further validated by medical record review and supplementary questionnaires. 2.5. Statistical Analysis The analysis has been carried out after segregating the cases and controls further into hypertensives and nor- motensives, resulting into four groups in total. The re- sults have been reported as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to analyze the statistical differences in the mean of various parameters between the groups. χ2-test was used to com- pare the proportion of the subjects between different di- chotomized variables. Logistic regression analysis was performed to assess the independent effect of hyperten- sive status on the odds of occurrence of diabetes, after adjusting for confounders. Statistical analyses were per- formed using SPSS version 12.0 software (SPSS, Chi- cago, IL, USA). All the reported P-values were two- tailed, and those less than 0.05 were considered statisti- cally significant. 3. Results Table 1 shows the distribution of HTN across sex, age groups, diabetes status and addiction habits. The overall prevalence rate of HTN was found to be 37.1%, 33.8% of male and 41% in females. However, the intergroup dif- ference is statistically similar (P > 0.05). Among differ- ent age groups, the rate of HTN increased with age from Table 1. Distribution of hypertension across age, gender, BMI, habits and clinical history. Normotensive Hypertensive Total P value Variables Category N % N % N % Type 2 Diabetes 142 48.1 153 51.9 295 100 Diabetes status Non Diabetes 258 75.7 83 24.3 341 100 <0.001 Female 173 59 120 41 293 100 Sex Male 227 66.2 116 33.8 343 100 0.63 30 - 39 79 86.8 12 13.2 91 100 40 - 49 141 76.2 44 23.8 185 100 50 - 59 67 50.8 65 49.2 132 100 60 - 69 70 47.6 77 52.4 147 100 70 - 79 34 50 34 50 68 100 Age groups 80+ 9 69.2 4 30.8 13 100 <0.001 Normal (<25) 179 69.6 78 30.4 257 100 Overweight (25 - 30) 158 59.8 106 40.2 264 100 BMI groups Obese (>30) 65 54.8 53 45.2 118 100 0.009 Yes 76 71.7 30 28.3 106 100 No 296 62.3 179 37.7 475 100 Smoking Quit 28 50.9 27 49.1 55 100 0.03 Yes 100 67.6 48 32.4 148 100 No 287 62 176 38 463 100 Alcohol intake Quit 13 52 12 48 25 100 NS <6% 270 71.8 106 28.2 376 100 6% - 8% 87 50.9 84 49.1 171 100 >8% 43 48.3 46 51.7 89 100 <0.001 Glycated haemoglobin A1c Total 400 62.9 236 37.1 636 100 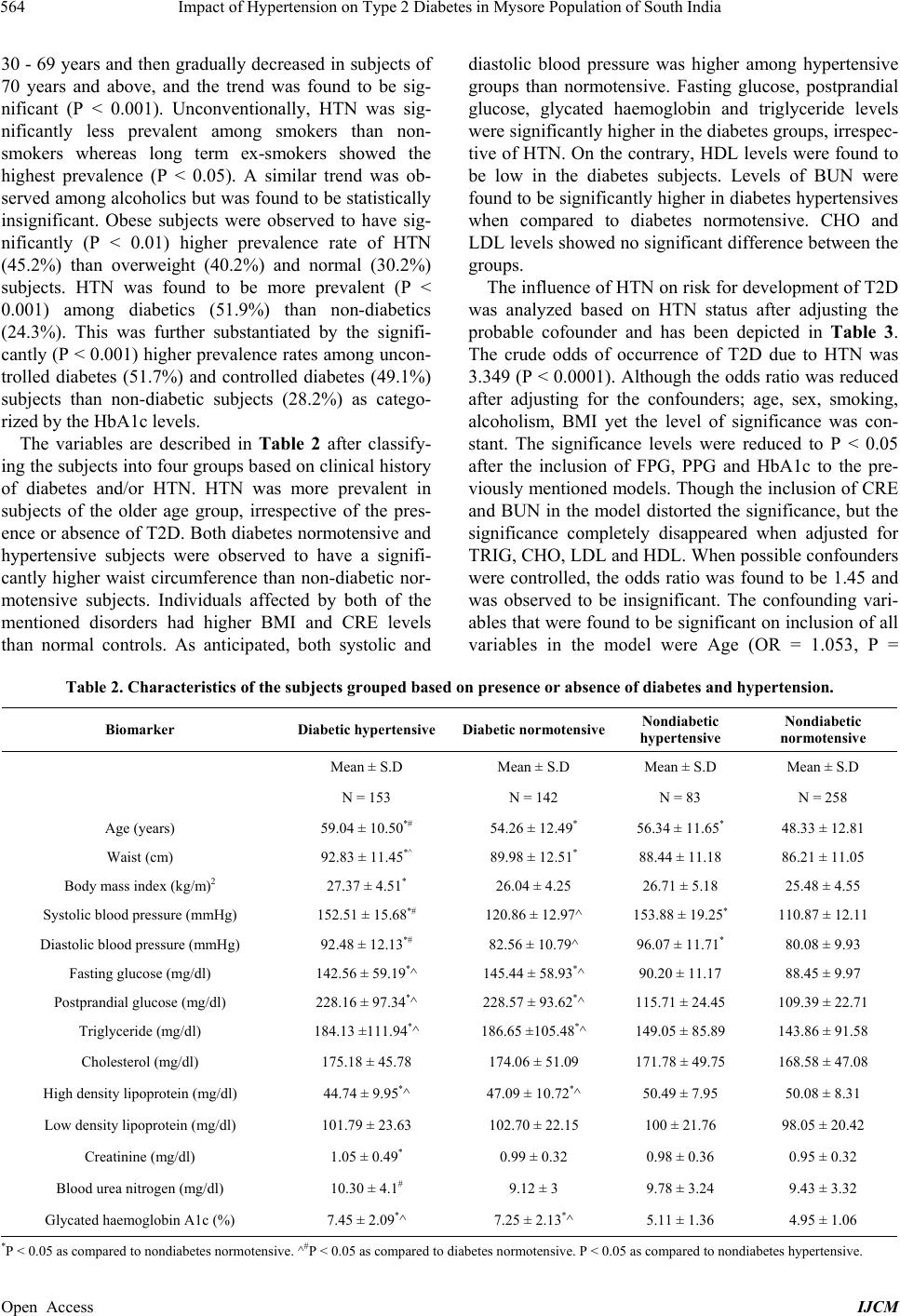 Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 564 30 - 69 years and then gradually decreased in subjects of 70 years and above, and the trend was found to be sig- nificant (P < 0.001). Unconventionally, HTN was sig- nificantly less prevalent among smokers than non- smokers whereas long term ex-smokers showed the highest prevalence (P < 0.05). A similar trend was ob- served among alcoholics but was found to be statistically insignificant. Obese subjects were observed to have sig- nificantly (P < 0.01) higher prevalence rate of HTN (45.2%) than overweight (40.2%) and normal (30.2%) subjects. HTN was found to be more prevalent (P < 0.001) among diabetics (51.9%) than non-diabetics (24.3%). This was further substantiated by the signifi- cantly (P < 0.001) higher prevalence rates among uncon- trolled diabetes (51.7%) and controlled diabetes (49.1%) subjects than non-diabetic subjects (28.2%) as catego- rized by the HbA1c levels. The variables are described in Table 2 after classify- ing the subjects into four groups based on clinical history of diabetes and/or HTN. HTN was more prevalent in subjects of the older age group, irrespective of the pres- ence or absence of T2D. Both diabetes normotensive and hypertensive subjects were observed to have a signifi- cantly higher waist circumference than non-diabetic nor- motensive subjects. Individuals affected by both of the mentioned disorders had higher BMI and CRE levels than normal controls. As anticipated, both systolic and diastolic blood pressure was higher among hypertensive groups than normotensive. Fasting glucose, postprandial glucose, glycated haemoglobin and triglyceride levels were significantly higher in the diabetes groups, irrespec- tive of HTN. On the contrary, HDL levels were found to be low in the diabetes subjects. Levels of BUN were found to be significantly higher in diabetes hypertensives when compared to diabetes normotensive. CHO and LDL levels showed no significant difference between the groups. The influence of HTN on risk for development of T2D was analyzed based on HTN status after adjusting the probable cofounder and has been depicted in Table 3. The crude odds of occurrence of T2D due to HTN was 3.349 (P < 0.0001). Although the odds ratio was reduced after adjusting for the confounders; age, sex, smoking, alcoholism, BMI yet the level of significance was con- stant. The significance levels were reduced to P < 0.05 after the inclusion of FPG, PPG and HbA1c to the pre- viously mentioned models. Though the inclusion of CRE and BUN in the model distorted the significance, but the significance completely disappeared when adjusted for TRIG, CHO, LDL and HDL. When possible confounders were controlled, the odds ratio was found to be 1.45 and was observed to be insignificant. The confounding vari- ables that were found to be significant on inclus ion of all variables in the model were Age (OR = 1.053, P = Table 2. Characteristics of the subjects gr ouped based on presence or absence of diabetes and hypertension. Biomarker Diabetic hypertensive Diabetic normotensiveNondiabetic hypertensive Nondiabetic normotensive Mean ± S.D Mean ± S.D Mean ± S.D Mean ± S.D N = 153 N = 142 N = 83 N = 258 Age (years) 59.04 ± 10.50*# 54.26 ± 12.49* 56.34 ± 11.65* 48.33 ± 12 .81 Waist (cm) 92.83 ± 11.45*^ 89.98 ± 12.51* 88.44 ± 11.18 86.21 ± 11.05 Body mass index (kg/m)2 27.37 ± 4.51* 26.04 ± 4.25 26.71 ± 5.18 25.48 ± 4.55 Systolic blood pressure (mmHg) 152.51 ± 15.68*# 120.86 ± 12.97^ 153.88 ± 19.25* 110.87 ± 12.11 Diastolic blood pressure (mmHg) 92.48 ± 12.13*# 82.56 ± 10.79^ 96.07 ± 11.71* 80.08 ± 9.93 Fasting glucose (mg/dl) 142.56 ± 59.19*^ 145.44 ± 58.93*^ 90.20 ± 11.17 88.45 ± 9.97 Postprandial glucose (mg/dl) 228.16 ± 97.34*^ 228.57 ± 93.62*^ 115.71 ± 24.45 109.39 ± 22.71 Triglyceride (mg/dl) 184.13 ±111.94*^ 186.65 ±105.48*^ 149.05 ± 85.89 143.86 ± 91.58 Cholesterol (mg/dl ) 175.18 ± 45.78 174.06 ± 51.09 171.78 ± 49.75 168.58 ± 47.08 High density lipoprotein (mg/dl) 44.74 ± 9.95*^ 47.09 ± 10.72*^ 50.49 ± 7.95 50.08 ± 8.31 Low density lipoprotein (mg/dl) 101.79 ± 23.63 102.70 ± 22.15 100 ± 21.76 98.05 ± 20.42 Creatinine (mg/dl) 1.05 ± 0.49* 0.99 ± 0.32 0.98 ± 0.36 0.95 ± 0.32 Blood urea nitrogen (mg/dl ) 10.30 ± 4.1# 9.12 ± 3 9.78 ± 3.24 9.43 ± 3.32 Glycated haem o g l o bi n A 1 c (%) 7.45 ± 2.09*^ 7.25 ± 2. 1 3*^ 5.11 ± 1.36 4.95 ± 1.06 *P < 0.05 as com pared to nondiabe tes normotensi ve. ^#P < 0.05 as compared to diabetes normotensive. P < 0.05 as compared to nondiabetes hypertensive. Open Access IJCM 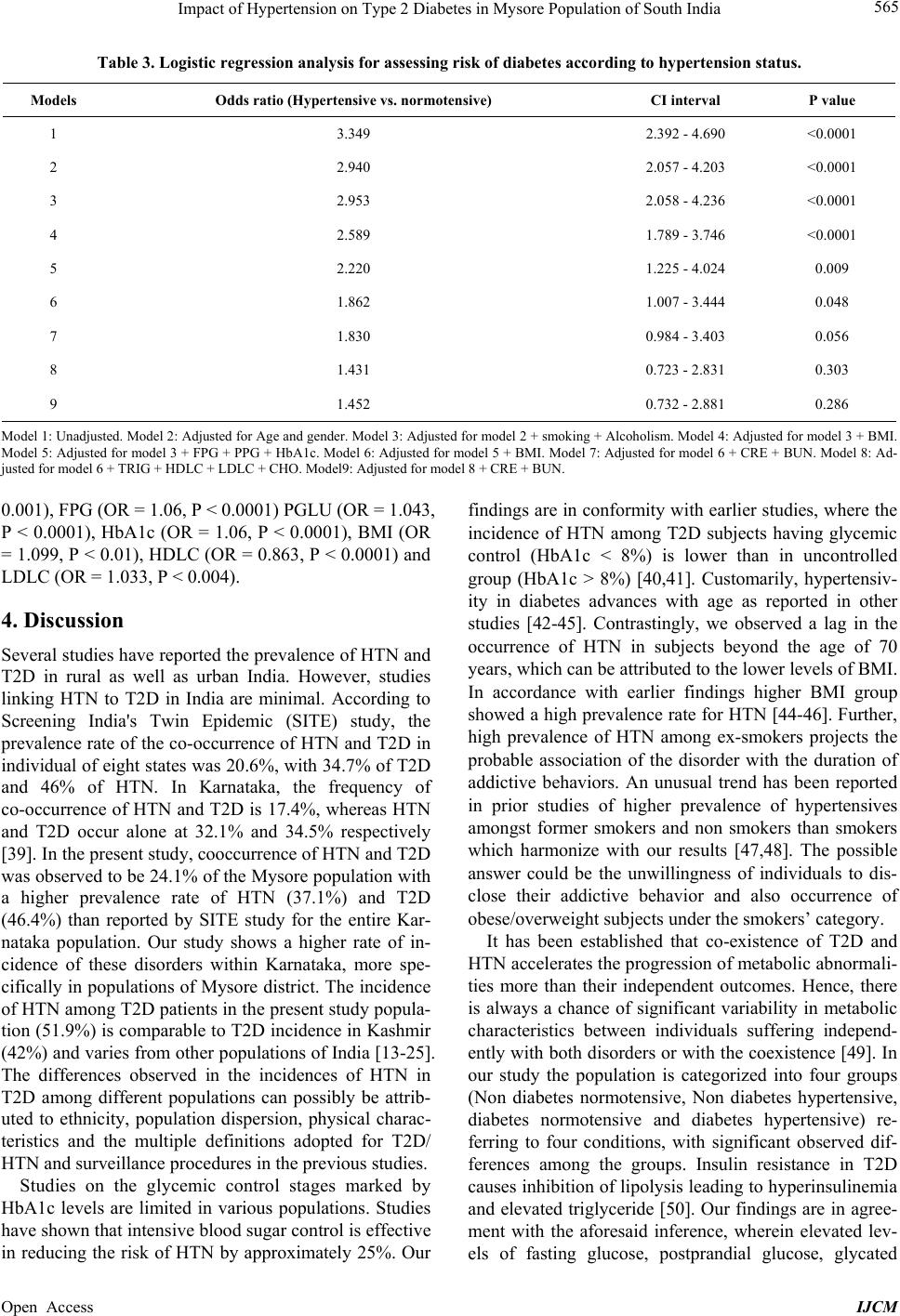 Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 565 Table 3. Logistic regression analysis for assessing risk of diabetes according to hypertension status. Models Odds ratio (Hypertensive vs. normotensive) CI interval P value 1 3.349 2.392 - 4.690 <0.0001 2 2.940 2.057 - 4.203 <0.0001 3 2.953 2.058 - 4.236 <0.0001 4 2.589 1.789 - 3.746 <0.0001 5 2.220 1.225 - 4.024 0.009 6 1.862 1.007 - 3.444 0.048 7 1.830 0.984 - 3.403 0.056 8 1.431 0.723 - 2.831 0.303 9 1.452 0.732 - 2.881 0.286 Model 1: Unadjusted. Model 2: Adjusted for Age and gender. Model 3: Adjusted for model 2 + smoking + Alcoholism. Model 4: Adjusted for model 3 + BMI. Model 5: Adjusted for model 3 + FPG + PPG + HbA1c. Model 6: Adjusted for model 5 + BMI. Model 7: Adjusted for model 6 + CRE + BUN. Model 8: Ad- justed for m odel 6 + TRIG + HDLC + LDLC + CHO. Model9: Adjusted for model 8 + CRE + BUN. 0.001), FPG (OR = 1.0 6, P < 0.0001) PGLU (OR = 1.043, P < 0.0001), HbA1c (OR = 1.06, P < 0.0001), BMI (OR = 1.099, P < 0.01), HDLC (OR = 0.863, P < 0.00 01) and LDLC (OR = 1.033, P < 0.004). 4. Discussion Several studies have reported the prevalence of HTN and T2D in rural as well as urban India. However, studies linking HTN to T2D in India are minimal. According to Screening India's Twin Epidemic (SITE) study, the prevalence rate of the co-occurrence of HTN and T2D in individual of eight states was 20.6%, with 34.7% of T2D and 46% of HTN. In Karnataka, the frequency of co-occurrence of HTN and T2D is 17.4%, whereas HTN and T2D occur alone at 32.1% and 34.5% respectively [39]. In the present study, cooccurrence of HTN and T2D was observed to be 24.1% of the Mysore population with a higher prevalence rate of HTN (37.1%) and T2D (46.4%) than reported by SITE study for the entire Kar- nataka population. Our study shows a higher rate of in- cidence of these disorders within Karnataka, more spe- cifically in populations of Mysore district. The incidence of HTN among T2D patients in the present study popula- tion (51.9%) is comparable to T2D incidence in Kash mir (42%) and varies from other populations of India [13-25]. The differences observed in the incidences of HTN in T2D among different populations can possibly be attrib- uted to ethnicity, population dispersion, physical charac- teristics and the multiple definitions adopted for T2D/ HTN and surveillance procedu res in the previous stud ies. Studies on the glycemic control stages marked by HbA1c levels are limited in various populations. Studies have shown that intensive blood sugar control is effective in reducing the risk of HTN by approximately 25%. Our findings are in conformity with earlier studies, where the incidence of HTN among T2D subjects having glycemic control (HbA1c < 8%) is lower than in uncontrolled group (HbA1c > 8%) [40,41]. Customarily, hypertensiv- ity in diabetes advances with age as reported in other studies [42-45]. Contrastingly, we observed a lag in the occurrence of HTN in subjects beyond the age of 70 years, which can be attributed to the lower levels of BMI. In accordance with earlier findings higher BMI group showed a high prev alence rate for HTN [44-46]. Further, high prevalence of HTN among ex-smokers projects the probable association of the disorder with the duration of addictive behaviors. An unusual trend has been reported in prior studies of higher prevalence of hypertensives amongst former smokers and non smokers than smokers which harmonize with our results [47,48]. The possible answer could be the unwillingness of individuals to dis- close their addictive behavior and also occurrence of obese/overweight subjects under the smokers’ category. It has been established that co-existence of T2D and HTN accelerates the progression of metabolic abnormali- ties more than their independent outcomes. Hence, there is always a chance of significant variability in metabolic characteristics between individuals suffering independ- ently with both disorders or with th e coexistence [49]. In our study the population is categorized into four groups (Non diabetes normotensive, Non diabetes hypertensive, diabetes normotensive and diabetes hypertensive) re- ferring to four conditions, with significant observed dif- ferences among the groups. Insulin resistance in T2D causes inhibition of lipo lysis leading to hyperinsulinemia and elevated triglyceride [50]. Our findings are in agree- ment with the aforesaid inference, wherein elevated lev- els of fasting glucose, postprandial glucose, glycated Open Access IJCM  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 566 haemoglobin, triglyceride levels of FPG, PPG, HbA1c, TRIG and low levels of HDL levels were observed in diabetes hypertensives and normotensive groups. Our finding is in concordance with earlier studies, suggesting the association of T2D with dyslipidemia, particularly with high triglycerides accompanied with a simultaneous decrease in HDL cholesterol [51]. Positive association between HTN and abnormal lipid profiles in rural popu- lation of Bagalkot in Karnataka [52] and among hospital patients have been reported in previous studies on Indian populat i on [ 53,54]. Elevated BUN has been reported to be a marker for activating the sympathetic nervous system and an un- regulated rennin angiotensin system. Thus increased lev- els of BUN in T2D with HTN distinctly reveals the risk of renal and atherosclerotic complications in diabetes hypertensives than diabetes normotensive [55-59]. In the present study, the co-incidence of T2D and HTN as de- fined by higher BMI and CRE levels strengthens the no- tion that obesity and renal dysfunction are predictors of T2D associated with HTN. Our study indicates that HTN plays a major role in the development of T2D, after the confounding ef fects of age, sex, BMI, glycemic index (FPG, PPG and HbA1c), lipid profile, CRE and other relevant factors had been adjusted. Our result concurs with the recent findings that aging, obesity, dysglyceamia and dyslipidemia co-exist with HTN and T2D [17,60,61]. Studies reported a precise and prominent role of HTN in the prevalence of T2D with crude relative risks of 2.34 (CI of 2.16 - 2.73) [62] and 2.65 (CI of 1.88 - 3.73) [63], relatively lower than the ratio obtained in our study. This evidently suggests that Mysore populatio n is at a higher risk of T2D due to HTN. Previous studies on Asian populations proposed that baseline hyperglycemia and BMI are potential covariates determining the association of HTN with T2D. Thus, baseline HTN may be a potential predictor for incident diabetes, if the onset of diabetes is defined using pa- rameters like FPG and PPG levels [61]. However, it has been concluded that obesity and metabolic syndrome does not explain the entire association between BP and incidence of T2D [63]. Hence, besides BMI, lipid profile and Glycemic index, we included CRE and BUN into these models. Accumulation of BUN and CRE is a direct indicator of renal dysfunction. Thus higher levels of these are observed in hypertensives, more precisely in untreated hypertensives and diabetes nephropathy cases [64]. Furthermore, it has been reported that renal failure rate is two to three times higher in patients of diabetes hypertensives than in non diabetes hypertensives [65]. Therefore, inclusion of these predictors can ascertain the association between T2D and HTN. Lack of correction for the predictor variables related to renal dysfunction can be one of the reasons for discrepancies in the earlier studies [61,62]. 5. Limitations and Strengths This study is the first of its kind in South Indian popula- tion. Glycemic index is defined more explicitly in this study by performing OGTT and HbA1c estimations compared to earlier studies, which failed to include glu- cose tolerance [62,66]. Some of the major limitations of this study are complete exclusion of the effects of resid- ual confounding due to measurement errors, i.e. in the assessment of confounding factors or unmeasured dietary, social and economic factors. Data could not be stratified gender wise because of small sample size. The sample population was heterogeneous consisting of both urban and rural inhabitants, thus findings cannot be generalized to other populations of India. Although clinical data are available, duration and history of diabetes and HTN was not taken into consideration for the study. Some of the salient markers like micro albumin, urea and insulin are beyond the scope of this paper. Non availability of in- formation regarding the treatment for HTN in subjects of the hypertensive group, to see the effect of treatment of diabetes, was one of the lacunae. 6. Conclusion The high prevalence rate of T2D and HTN is major con- cerns in Mysore population. HTN plays a key role in the progression of T2D and is associated with vascular com- plications. Among hypertensives, BMI, Glycemic index, lipid profile and kidney dysfunction, markers are poten- tial predictors of T2D. The assessment of nephropathic markers besides analyzing metabolic components and blood pressure management is better approaches to pre- vent the risk of development of T2D in hypertensives. 7. Authors’ Contributions MDS conceived and executed the study. SDG helped in sample collection, laboratory work and statistical analy- sis. CDS supervised the research, proof read the draft. DDX , BVR, JSR and LGL contributed in formulation of data collection. All authors read and approved the final manuscript. 8. Acknowledgements The authors express gratitude to the Director, Anthropo- logical Survey of India, Ministry of Culture, Government of India, Kolkata for sponsoring the project. We express thanks to Deputy Director and staff of the Anthropologi- cal Survey of India for extending technical and adminis- trative support. We are grateful to all the participants of the study. We are indebted to the Lions Club of Mysore, Drs. Vikas Modi, Pushpa Jogihalli and Ramakrishna of Open Access IJCM  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 567 Amritakripa Hospital, Rupanagar, Mysore for holding medical camps in Mysore. We appreciate the assistance provided by the paramedical staff in the collection of blood samples. Thanks are also due to Abrar Alam, Ran- jith, Pratiksha Bhat, Amith, Suprabha Samanta, Deepti Lokanath, and Sanjukta Mukherjee for their coop eration. REFERENCES [1] G. S. Wild, G. Roglic, A. Green, R. Sicree and H. King, “Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030,” Diabetes Care, Vol. 27, No. 5, 2004, pp. 1047-1053. http://dx.doi.org/10.2337/diacare.27.5.1047 [2] B. Basnayar and L. Rajapasha, “Cardiovascular and In- fectious Diseases in South Asia: The Double Whammy,” British Medical Journals, Vol. 328, No. 7443, 2004, p. 781. http://dx.doi.org/10.1136/bmj.328.7443.781 [3] D. Yach, C. Hawkes, C. L. Gould and K. J. Hofman, “The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control,” Journal of the American. Medical Association, Vol. 291, No. 21, 2004, pp. 2616-2622. http://dx.doi.org/10.1001/jama.291.21.2616 [4] A. Ramachandran, C. Snehalatha, A. Kapur, V. Vijay, V. Mohan, A. K Das, P. V. Rao, C. S. Yajnik, K. M. Pra- sanna Kumar and J. D. Nair, “Diabetes Epidemiology Study Group in India (DESI): High Prevalence of Diabe- tes and Impaired Glucose Tolerance in India: National Urban Diabetes Survey,” Diabetologia, Vol. 44, No. 9, 2001, pp. 1094-1101. http://dx.doi.org/10.1007/s001250100627 [5] R. Klein, B. E. Klein, K. E. Lee, K. J. Cruickshanks and S. E. Moss, “The Incidence of Hypertension in Insulin De- pendent Diabetes,” Archives of Internal Medicine, Vol. 156, No. 6, 1996, pp. 622-627. http://dx.doi.org/10.1001/archinte.1996.00440060042005 [6] D. Conen, P. M. Ridker, S. Mora, J. E. Buring and R. J. Glynn, “Blood Pressure and Risk of Developing Type 2 Diabetes Mellitus: The Womens Health Study,” Euro- pean Heart Journal, Vol. 28, No. 23, 2007, pp. 2937- 2943. http://dx.doi.org/10.1093/eurheartj/ehm400 [7] P. W. Wilson, J. B. Meigs, L. Sullivan, C. S. Fox and D. M. Nathan and R. B. D’Agostino Sr, “Prediction of Inci- dent Diabetes Mellitus in Middle-Aged Adults: The Framingham Offspring Study,” Archives of Internal Me- dicine, Vol. 167, No. 10, 2007, pp. 1068-1074. http://dx.doi.org/10.1001/archinte.167.10.1068 [8] R. E. Aubert, L.S. Geiss, D. J. Ballard, B. Cocanougher and W. H. Herman, “Diabetes-Related Hospitalization and Hospital Utilization,” In: Diabetes in America, 2nd Edition, National Diabetes Information Clearinghouse, 1 Information Way, Bethesda, 1995, pp. 553-569. [9] N. M. Kaplan and E. Lieberman, “Hypertension and Dia- betes, Obesity, and Dyslipidaemia in Clinical Hyperten- sion,” 7th Edition, New Delhi, B.I. Waverly Pvt. Ltd, 1998, pp. 244-247. [10] UK Prospective Diabetes Study (UKPDS) Group, “Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: U KPDS 39,” British Medical Journals, Vol. 317, No. 7175, 1998, pp. 703-713. [11] UK Prospective Diabetes Study (UKPDS) Group, “Effi- cacy of Atenolol and Captopril in Reducing Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 39,” British Medical Journals, Vol. 317, No. 7160, 1998, pp. 713-720. http://dx.doi.org/10.1136/bmj.317.7160.713 [12] A. Ramachandran, R. C. Ma and C. Snehalatha, “Diabetes in Asia,” Lancet, Vol. 375, No. 9712, 2010, pp. 408-418. http://dx.doi.org/10.1016/S0140-6736(09)60937-5 [13] R. B. Singh, R. Beegom and V. Rastogi, “Clinical Char- acteristics and Hypertension among Known Patients of Non-Insulin Dependent Diabetes Mellitus in North and South Indians,” Journal of the Diabetic Association of India, Vol. 36, 1996, pp. 45-50. [14] S. Jain and J. C. Patel, “Diabetes and Hypertension,” Journal of the Diabetic Association of India, Vol. 23, 1983, pp. 83-86. [15] G. N. Dhobi, A. Majid, S. R. Masoodi, M. I. Bashir, A. I. Wani and A. H. Zargar, “Prevalence of Hypertension in Patients with New Onset Type 2 Diabetes Mellitus,” Journal of the Indian Medical Association, Vol. 106, No. 2, 2008, pp. 94-98. [16] D. Akbar, M. M. Ahmed and A. A. Algamdi, “Cardiovas- cular Risk Factors in Saudi and Non-Saudi Diabetics,” Saudi Medical Journal, Vol. 24, No. 6, 2003, pp. 686-687. [17] F. M. Mubarak, S. F. Erika, Y. J. Hashem and M. A. Kamel, “Hypertension among 1000 Patients with Type 2 Diabetes Attending a National Diabetes Center in Jor- dan,” Annals of Saudi Medicine, Vol. 28, No. 5, 2008, pp. 346-351. http://dx.doi.org/10.4103/0256-4947.51684 [18] S. Al-Moosa, S. Allin, N. Jemiai, J. Al-Lawati and E. Mossialos, “Diabetes and Urbanization in the Omani Po- pulation: An Analysis of National Survey Data,” Popula- tion Health Metrics, Vol. 24, No. 1, 2006, pp. 1-8. [19] I. Satman, T. Yilmaz, A. Sengül, S. Salman, F. Salman, S. Uygur, I. Bastar, Y. Tütüncü, M. Sargin, N. Dinççag, K. Karsidag, S. Kalaça, C. Ozcan and H. King, “Popula- tion-Based Study of Diabetes and Risk Characteristics in Turkey,” Diabetes Care, Vol. 25, No. 9, 2002, pp. 1551- 1556. http://dx.doi.org/10.2337/diacare.25.9.1551 [20] F. Al-Mahroos, K. Al-Roomi and P. M. McKeigue, “Re- lation of High Blood Pressure to Glucose Intolerance, Plasma Lipids and Educational Status in an Arabian Gulf Population,” International Journal of Epidemiology, Vol. 29, No. 1, 2000, pp. 71-76. http://dx.doi.org/10.1093/ije/29.1.71 [21] C. H. Tseng, “Higher Risk of Hypertension in Indigenous Type 2 Diabetic Patients in Taiwan,” Journal of Hyper- tension, Vol. 24, No. 9, 2006, pp. 1817-1821. http://dx.doi.org/10.1097/01.hjh.0000242406.76085.c4 [22] V. Baskar, D. Kamalakannan, M. R. Holland and B. M. Singh, “Does Ethnic Origin Have an Independent Impact on Hypertension and Diabetic Complications?” Diabetes, Obesity and Metabolism, Vol. 8, No. 2, 2006, pp. 214- 219. http://dx.doi.org/10.1111/j.1463-1326.2005.00485.x Open Access IJCM  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 568 [23] V. Baskar, D. Kamalakannan, M. R. Holland and B. M. Singh, “The Prevalence of Hypertension and Utilization of Antihypertensive Therapy in a District Diabetes Popu- lation,” Diabetes Care, Vol. 25, No. 11, 2002, pp. 2107- 2108. http://dx.doi.org/10.2337/diacare.25.11.2107 [24] M. Comaschi, C. Coscelli, D. Cucinotta, P. Malini, E. Manzato and A. Nicolucci, “Cardiovascular Risk Factors and Metabolic Control in Type 2 Diabetic Subjects At- tending Outpatient Clinics in Italy: The SFIDA (Survey of Risk Factors in Italian Diabetic Subjects by AMD) Study,” Nutrition, Metabolism and Cardiovascular Dis- eases, Vol. 15, No. 3, 2005, pp. 204-211. http://dx.doi.org/10.1016/j.numecd.2004.07.003 [25] F. J. Del Cañizo-Gómez and M. N. Moreira-Andrés, “Cardiovascular Risk Factors in Patients with Type 2 Diabetes. Do We Follow the Guidelines?” Diabetes Re- search and Clinical Practice, Vol. 65, No 2, 2004, pp. 125-133. http://dx.doi.org/10.1016/j.diabres.2003.12.002 [26] B. Y. Yuvraj, M. R. Nagendragowda and A. G. Umakan- tha, “Pevalence, Awaeness, Treatment and Control of Hy- pertension in Rural Areas of Davanagere,” Indian Journal of Community Medicine, Vol. 35, No. 1, 2010, pp. 138- 241. http://dx.doi.org/10.4103/0970-0218.62578 [27] G. Dayananda and M. Niranjan, “Blood Pressure Changes in Normotensive Subjects with and without Family His- tory of Hypertension,” Journal of Physiology and Bio- medical Sciences, Vol. 22, No. 1, 2009, pp. 35-37. [28] H. Basavanagowdappa, A. K. Prabhakar, P. Prasannaraj, K. C. Gurudev, Virupaksha and Suma, “Study of Preva- lence of Diabetes Mellitus and Impaired Fasting Glucose in a Rural Population,” International Journal of Diabetes Developing Countries, Vol. 25, No. 4, 2005, pp. 98-101. http://dx.doi.org/10.4103/0973-3930.27012 [29] C. Muninarayana, G. Balachandra, S. G. Hiremath, I. Krishna and N. S. Anil, “Prevalence and Awareness Re- garding Diabetes Mellitus in Rural Tamaka, Kolar,” In- ternational Journal of Diabetes Developing Countries, Vol. 30, No. 1, 2010, pp. 18-31. [30] C. R. Rao, V. G. Kamath, A. Shetty and A. Kamath, “A Study on the Prevalence of Type 2 Diabetes in Coastal Karnataka,” International Journal of Diabetes Develop- ing Countries, Vol. 30, No. 2, 2010, pp. 80-85. [31] S. Pushpa Patil, R. Umesh Dixit and B. H. Dhruvkumar, “Study of Diabetes in Dharwad—An Urban Area in In- dia,” Indian Journal of Science and Technology, Vol. 4, No. 11, 2011, pp. 1481-1483. [32] M. A. Shekar, H. M. Somashekar and B. S. Vishwanath, “Study of Incidence of Obesity in Newly Diagnosed Type 2 Diabetics Using Anthropometric Measurements,” In- ternational Journal of Diabetes Developing Countries, Vol. 25, No. 4, 2005, pp. 102-104. [33] C. K. Priyankaraj and M. M. Angadi, “Hospital-Based KAP Study on Diabetes in Bijapur, Karnataka,” Indian Journal of Medical Specialities, Vol. 1, No. 2, 2010, pp. 80-83. [34] World Health Organization, “Physical Status: The Use and Interpretation of Anthropometry. Report of the WHO Expert Committee,” World Health Organization Techni- cal Report Series, Vol. 854, Geneva, 1995, pp. 1-416. [35] World Health Organization, “Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Con- sultation,” World Health Organization Technical Report Series, Vol. 894, 2000, pp. 1-253. [36] A. V. Chobanian, G. L. Bakris, H. R. Black, W. C. Cush- man, L. A. Green, J. L. Izzo Jr., D. W. Jones, B. J. Ma- terson, S. Oparil, J. T. Wright Jr. and E. J. Roccella, “The Seventh Report of the Joint National Committee on Pre- vention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report,” Journal of the Ame- rican Medical Association, Vol. 289, No. 19, 2003, pp. 2560-2572. http://dx.doi.org/10.1001/jama.289.19.2560 [37] K. G. Alberti and P. Z. Zimmet, “Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complica- tions. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation,” Diabetic Medicine, Vol. 15, No. 7, 1998, pp. 539-553. http://dx.doi.org/10.1002/(SICI)1096-9136(199807)15:7< 539::AID-DIA668>3.0.CO;2-S [38] D. M. Nathan, D. E. Singer, K. Hurxthal and J. D. Good- son, “The Clinical Information Value of the Glycosylated Hemoglobin Assay,” New England Journal of Medicine, Vol. 310, No. 6, 1984, pp. 341-346. http://dx.doi.org/10.1056/NEJM198402093100602 [39] S. R. Joshi, B. Saboo, M. Vadivale, S. I. Dani, A. Mithal, U. Kaul, M. Badgandi, S. S. Iyengar, V. Viswanathan, N. Sivakadaksham, P. S. Chattopadhyaya, A. D. Biswas, S. Jindal, I.. A. Khan, B. K. Sethi, V. D. Rao and J. J. Dalal, “SITE Investigators Prevalence of Diagnosed and Undi- agnosed Diabetes and Hypertension in India Results from the Screening India’s Twin Epidemic (SITE) Study,” Dia- betes Technology and Therapeutics, Vol. 14, No. 1, 2012, pp. 8-15. http://dx.doi.org/10.1089/dia.2011.0243 [40] I. H. de Boer, B. Kestenbaum, T. C. Rue, M. W. Steffes, P. A. Cleary, M. E. Molitch, J. M. Lachin, N. S. Weiss and J. D. Brunzell, “Insulin Therapy, Hyperglycemia, and Hypertension in Type 1 Diabetes Mellitus,” Archives of Internal Medicine, Vol. 168, No. 17, 2008, pp. 1867- 1873. http://dx.doi.org/10.1001/archinternmed.2008.2 [41] Writing Team for the Diabetes Control and Compli- cations Trial/Epidemiology of Diabetes Interventions and Complications Research Group, “Sustained Effect of In- tensive Treatment of Type 1 Diabetes Mellitus on Devel- opment and Progression of Diabetic Nephropathy: The Epidemiology of Diabetes Interventions and Complica- tions (EDIC) Study,” Journal of the American Medical Association, Vol. 290, No. 16, 2003, pp. 2159-2167. http://dx.doi.org/10.1001/jama.290.16.2159 [42] T. M. Davis, I. M. Stratton, C. J. Fox, R. R. Holman and R. C. Turner, “UK Prospective Diabetes Study 22. Effect of Age at Diagnosis on Diabetic Tissue Damage during the First 6 Years of NIDDM,” Diabetes Care, Vol. 40, No. 9, 1997, pp. 1435-1441. http://dx.doi.org/10.2337/diacare.20.9.1435 [43] C. C. Cowie and M. I. Harris, “Physical and metabolic characteristics of persons with diabetes,” In: M. I. Harris, Ed., Diabetes in America, 2nd edition, National Diabetes Information Clearinghouse, 1 Information Way, Bethesda, 1995, pp. 117-164. [44] J. M. Sprafka, A. P. Bender and H. G. Jagger, “Preva- Open Access IJCM  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India 569 lence of Hypertension and Associated Risk Factors among Diabetic Individuals: The Three-City Study,” Dia- betes Care, Vol. 11, No. 1, 1988, pp. 17-22. http://dx.doi.org/10.2337/diacare.11.1.17 [45] “Hypertension in Diabetic Study (HDS): 1. Prevalence of Hypertension in Newly Presenting Type 2 Diabetic Pa- tients and the Association with Risk Factors for Cardio- vascular and Diabetic Complications,” Journal of Hy- pertension, Vol. 11, No. 3, 1993 pp. 309-317. http://dx.doi.org/10.1097/00004872-199303000-00012 [46] T. A. Hillier and K. L. Pedula, “Characteristics of an Adult Population with Newly Diagnosed Type 2 DM: The Relation of Obesity and Age of Onset,” Diabetes Care, Vol. 24, No. 9, 2001, pp. 1522-1527. http://dx.doi.org/10.2337/diacare.24.9.1522 [47] S. G. Manfred, J. Eliezer and L. Yair, “Blood Pressure in Smokers and Nonsmokers: Epide miologic Findings,” Ame- rican Heart Journal, Vol. 111, No. 5, 1986, pp. 932-940. http://dx.doi.org/10.1016/0002-8703(86)90645-9 [48] L. Duk-Hee, H. Myung-Hwa, K. Jang-Rak and R. J. David, “Effects of Smoking Cessation on Changes in Blood Pressure and Incidence of Hypertension A 4-Year Follow-Up Study,” Hypertension, Vol. 37, No. 2, 2001, pp. 194-198. http://dx.doi.org/10.1161/01.HYP.37.2.194 [49] W. O. Balogun and B. L. Salako, “Co-Occurrence of Dia- betes and Hypertension: Pattern and Factors Associated with Order of Diagnosis among Nigerians,” Annals of Ibadan Postgraduate Medicine, Vol. 9, No. 2, 2011, pp. 89-93. [50] J. M. Brun, “What Is the Purpose to Asses Trigliceride- mia during Treatment of NIDDM?” Diabetes and Me- tabolism, Vol. 23, No. 3, 1997, pp. 258-263. [51] G. M. Reaven, H. Lithell and L. Landsberg, “Hyperten- sion and Associated Metabolic Abnormalities—The Role of Insulin Resistance and the Sympathoadrenal System,” New England Journal of Medicine, Vol. 334, No. 6, 1996, pp. 374-381. http://dx.doi.org/10.1056/NEJM199602083340607 [52] R. Pramiladevi, S. M. Gooranavar, S. B. Biradar, M. C. Baragundi, S. A. Kora and M. Narayan, “Study of Lipid Profile in Hypertensive Patients in Rural Karnataka,” Journal of Pharmaceutical And Biomedical Sciences, Vol. 7, No. 18, 2011, pp. 1-6. [53] S. J. Jogalekar, “Co-Existence of Hypertension and Ab- normal Lipid Profile, a Hospital Based Retrospective Sur- vey,” Indian Heart Journal, Vol. 49, No. 3, 1997, pp. 275-278. [54] A. S. Hakim, S. A. Kamath and Mamata, “A Retrospec- tive Study of Lipid Profile in 500 Hypertensive Patients,” Journal of the Association of Physicians of India, Vol. 45, No. 12, 1997, pp. 943-944. [55] A. J. Kirtane, D. M. Leder, S. S. Waikar, G. M. Chertow, K. K. Ray, D. S. Pinto, D. Karmpaliotis, A. J. Burger, S. A. Murphy, C. P. Cannon, E. Braunwald and C. M. Gib- son, “Serum Blood Urea Nitrogen as an Independent Marker of Subsequent Mortality among Patients with Acute Coronary Syndromes and Normal to Mildly Re- duced Glomerular Filtration Rates,” Journal of the Ameri- can College of Cardiology, Vol. 45, No. 11, 2005, pp. 1781-1786. http://dx.doi.org/10.1016/j.jacc.2005.02.068 [56] R. Ostfeld, D. Mookherjee, M. Spinelli, D. Holtzman, A. Shoyeb, M. Schaefer, S. Doddamani, D. Spevack and Y. Du, “A Triglyceride/High-Density Lipoprotein Ratio >or = 3.5 Is Associated with an Increased Burden of Coronary Artery Disease on Cardiac Catheterization,” Journal of the Cardiometabolic Syndrome, Vol. 1, No. 1, 2006, pp. 13-15. http://dx.doi.org/10.1111/j.0197-3118.2006.05323.x [57] P. E. Tummala, X. L. Chen, C. L. Sundell, J. B. Laursen, P. Hammes, R. W. Alexander, D. G. Harrison and R. M. Medford, “Angiotensin II Induces Vascular Cell Adhe- sion Molecule-1 Expression in Rat Vasculature,” Circu- lation, Vol. 100, No. 11, 1999, pp. 1223-1229. http://dx.doi.org/10.1161/01.CIR.100.11.1223 [58] S. B. Manuck, J. R. Kaplan, M. R. Adams and T. B. Clarkson, “Effects of Stress and the Sympathetic Nervous System on Coronary Atherosclerosis in the Cynomolgus Macaque,” American Heart Journal, Vol. 116, No. 1, 1988, pp. 328-333. http://dx.doi.org/10.1016/0002-8703(88)90110-X [59] A. Rozanski, J. A. Blumenthal and J. Kaplan, “Impact of Psychological Factors on the Pathogenesis of Cardiovas- cular Disease and Implications for Therapy,” Circulation, Vol. 99, 1999, pp. 2192-2217. http://dx.doi.org/10.1161/01.CIR.99.16.2192 [60] V. S. Joe, P. Tiffany, J. K. Andrew, A. Mark, S. Stephen, L. Jean, A. Arnold and D. Pettitt, “High Rates of Co-Oc- currence of Hypertension, Elevated Low-Density Lipo- protein Cholesterol, and Diabetes Mellitus in a Large Managed Care Population,” American Journal of Man- aged Care, Vol. 10, No. 2, 2004, pp. 163-170. [61] Y. L. Won, H. K. Chang, J. R. Eun, B. P. Jeong, K. K. Young and Y. W. Sook, S. Kim and K. C. Sung, “The Effect of Body Mass Index and Fasting Glucose on the Relationship between Blood Pressure and Incident Dia- betes Mellitus: A 5-Year Follow-Up Study,” Hyperten- sion Research, Vol. 34, No. 10, 2011, pp. 1093-1097. http://dx.doi.org/10.1038/hr.2011.89 [62] T. W. Gress, F. J. Nieto, E. Shahar, M. R. Wofford and F. L. Brancati, “Hypertension and Antihypertensive Therapy as Risk Factors for Type 2 Diabetes Mellitus. Athero- sclerosis Risk in Communities Study,” New England Journal of Medicine, Vol. 342, No. 13, 2000, pp. 905- 912. http://dx.doi.org/10.1056/NEJM200003303421301 [63] C. David, M. R. Paul, M. Samia, E. B. Julie and J. G. Robert, “Blood Pressure and Risk of Developing Type 2 Diabetes Mellitus: The Women’s Health Study,” Euro- pean Heart Journal, Vol.28, No. 23, 2007, pp. 2937- 2943. http://dx.doi.org/10.1093/eurheartj/ehm400 [64] C. Josef, W. G. Laura, M. Geraldine, L. B. Fredrick, S. L. Andrew, J. Camille and J. K. Michael, “Prevalence of High Blood Pressure and Elevated Serum Creatinine Level in the United States,” Archives of Internal Medicine, Vol. 161, No. 9, 2001, pp. 1207-1216. http://dx.doi.org/10.1001/archinte.161.9.1207 [65] “Hypertension in Diabetes Study (HDS), II. Increased Risk of Cardiovascular Complications in Hypertensive Type 2 Diabetic Patients,” Journal of Hypertension, Vol. Open Access IJCM  Impact of Hypertension on Type 2 Diabetes in Mysore Population of South India Open Access IJCM 570 11, No. 3, 1993, pp. 319-325. http://dx.doi.org/10.1097/00004872-199303000-00013 [66] G. Hu, J. Pekka and T. Jaakko, “Joint Effects of History of Hypertension at Baseline and Type 2 Diabetes at Base- line and during Follow-Up on the Risk of Coronary Heart disease,” European Heart Journal, Vol. 28, No. 24, 2007, pp. 3059-3066. http://dx.doi.org/10.1093/eurheartj/ehm501
|