Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2011, 2, 35-42 doi:10.4236/ajps.2011.21004 Published Online March 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS 35 Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove Plants, Sonneratia caseolaris and S. alba Raiki Yamamoto1, Yoshifumi Kawana1, Reiko Minagawa2, Hamako Sasamoto2 1Graduate School of Environment and Information Sciences, Yokohama National University, Yokohama, Japan; 2Faculty of Envi- ronment and Information Sciences, Yokohama National University, Yokohama, Japan. Email: sasamoto@ynu.ac.jp Received December 1st, 2010; revised December 13th, 2010; accepted December 29th, 2010. ABSTRACT The effects of five salt ingredients of sea water, KCl, NaCl, CaCl2, MgCl2 and MgSO4, on induction of cell pro liferation in Sonneratia caseo laris were investigated. Proliferation was examined in tissue explants derived from such as leaves, cotyledons, and hypocotyls using a small-scale liquid culture method. Ad dition of 12.5-25 mM of MgCl2 was unique in stimulating cell proliferation in all tissues of S. caseolaris. Otherwise, differen t effects of salts were ob served among the three tissues. In hypocotyl culture, 25-50 mM of NaCl and CaCl2 stimulated cell divisions. Tolerance to 100 mM of MgSO4 was observed in leaves. Three osmotica lly active compounds commonly used in tissue cultu re, sorbitol, manni- tol and glycinebetain e, were also tested to assess the importance of osmotic effects on cell proliferatio n. No significant stimulation by these was observed over a wide range of concentrations. Data were compared with those of cotyledon cultures of another mangrove, S. alba, which exhibits no stimulation by MgCl2, stimulation by KCl and tolerance to NaCl. Mechanisms for adaptation of mangrove plants to various and high salts were discussed by comparing the dif- ferences in reaction to salts in cultures of two Sonneratia mangrove species of the same genera growing different salt environment. Keywords: Halophilic, Ions, Salt Tolerant, Sonneratiaceae, S. caseolaris 1. Introduction Mangrove plants are mainly tree species that grow in tropical and subtropical brackish coastal regions. This group includes more than 100 species from different families [1]. Micropropagation of these species is needed for conservation of mangrove forests threatened by po- tential destruction [2]. Biotechnological breeding tech- niques such as somatic hybridization of trees [3,4], may be promising tools for a year-round supply to replenish saplings and protect salty soil environment from deserti- fication [5]. Studies on salt injury to crops are an increasingly im- portant topic as soil damage from salt accumulation is proceeding [6]. Clarifying salt-tolerant mechanisms in cell and tissue culture systems of mangrove plants, and introduction of their characteristics through somatic hy- bridization or genetic transformation into crop species are also interesting themes to investigate. Mangrove plants offer both theoretical and biotechnological potential for improving plant growth in high salt environments, how- ever, information on their physiology, biochemistry, and molecular biology remains limited. The development of in vitro culture systems is important for complementation of whole plant studies. In 2001, the successful establish- ment of a suspension culture from leaf explants of Bru- guiera sexangula (Rhisophoraceae) was reported [7,8]. Protoplast fusion studies [9] and molecular biological work on salt-tolerance related genes has since been per- formed [10]. In order to expand our knowledge of man- groves, we recently established a suspension culture from cotyledon explants of the mangrove, Sonneratia alba (Sonneratiaceae), with subsequent study of its organo- genic potential [11]. The effects of four main salt ingre- dients of sea water on S. alba were investigated and its halophilic nature and tolerance to NaCl were determined. They were compared with suspension cultures of B. sex- angula, and tobacco BY-2 cells [12]. S. alba grows at the  Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 36 most seaside coast of the Iriomote Island, Okinawa, Ja- pan [13], while B. sexangula grows in more in-land areas of Thailand [14]. To investigate the mechanisms of salt tolerance, a comparison of salt-tolerant and salt-intolerant plants, or cells of similar genetic background but different salt tol- erance, is required. The latter investigation is problematic, because obtaining a specific tree mutant is very difficult. In the last few years, we have studied another mangrove plant, S. caseolaris, which is in the same genera as S. alba and shares its habitat in the in-land area of man- grove forests of Myanmar and Thailand [14]. Some cell proliferation was obtained from cotyledons and hypocot- yls of S. caseolaris using the same hormonal conditions as for S. alba [11]. The objective of this study was to investigate the cell proliferation of different types of explants from S. case- olaris in response to KCl, NaCl, CaCl2, MgCl2, MgSO4, which are components of sea water. The results were compared with cell proliferation from S. alba cotyledon explants. One potential effect of adding salts to a culture system is the osmotic effect on cells. In order to assess the importance of osmotic potential on the proliferation of mangrove cells, several osmotically active compounds, sorbitol, mannitol and glycinebetaine, which are com- monly used in tissue systems, were also tested. A small- scale liquid culture method [11] was used which mini- mized the volume of plant material required. 2. Materials and Methods 2.1. Materials Fruits of S. caseolaris were collected at the Khu Khut, Songkla or at Khanom river Basin, Si Thammarat Prov- ince, Thailand, and those of S. alba were collected from Iriomote island, Okinawa, Japan. Aseptic procedures for S. alba were replicated from Kawana et al. [11]. Seeds of S. caseolaris were disinfected by treatment with deter- gent, soaked in 70% ethanol solution for 1 min, washed with water, soaked in 3% sodium hypochlorite solution (NaClO) for 1 hr, and finally washed with sterile water three times. Disinfected seeds were germinated on 5 ml of 0.8% w/v agar medium (tissue culture grade, Wako Chemicals Co. Ltd) in 15-ml test tubes under a 16 hr photoperiod (80 μmol m-2s-1), at 24˚C. After two to five months of culture, sterile hypocotyls and cotyledons were cut into two to three, 1 mm-wide sections for culture. As leaves did not develop in sterile culture, additional seeds were planted in a mixture of sand, vermiculite and soil in 1/10000a Wagner’s pots (ICW-2, ICM Co. Ltd, Tsukuba, Japan) in a container and watered weekly. The plants of S. caseolaris were maintained in a green house in natural light conditions at 15-45˚C. After six months to three years of growth, the top four leaves of each plant were harvested. Leaves were washed with water and de- tergent, sterilized with 1% NaClO for 30 min to 1 hr and washed with sterile water three times. They were then cut into 1 mm-wide sections in a manner similar to that for the cotyledons. 2.2. Liquid Culture Disinfected cotyledons of S. caseolaris and S. alba, as well as hypocotyls and leaves of the former were cut in a Petri dish under axenic conditions. Two or three sections were placed in a 10 mL flat-bottomed culture tube (Ma- ruemu No.3) with 1 mL liquid Murashige & Skoog’s (MS) [15] basal medium containing 0.1 μM 2,4-di-chlo- rophenoxyacetic acid (2,4-D) and 3% w/v of sucrose or glucose (when cited in text), and covered with autoclaved translucent film (BioHazard Bag 86.1199, Assist Co. Ltd.). The pH was adjusted to 5.8 before sterilization at 121˚C, 20 min. All cultures were incubated in the dark at 30˚C on a rotary shaker at 100 rpm. When cited in the text, 10, 12.5, 25, 50, 100, or 200 mM of KCl, NaCl, CaCl2, MgCl2, or MgSO4 was added to the above media. Sorbitol, mannitol or glycinebetaine at 25, 50, 100, 200 or 400 mM were also added. The cultures were observed under an inverted microscope (Olympus CK40). 2.3. Data Description In S. caseolaris, two methods of quantifying the degree of cell proliferation were used. First, the total area of proliferated mass per explant was calculated using the image analysis program, Image J [16] to analyze digital photographs taken through the inverted microscope (Fig. 1). As this method is limited to a single two-dimensional representation of each cell mass, we also described cell proliferation at the cut surface of the explants in terms of a system of grades based on direct observation under an inverted microscope. In S. caseolaris, Grado 0, flat sur- face with no reaction; Grade 1, one cell layer appeared from the cut surface; Grade 2, two or three cells layers were present; Grade 3, 4-5 layers of proliferated cells could be found on the cut surface but the area of prolif- eration was more localized; Grade 4, 4-5 cells layer of proliferated cells covering a larger area of the cut surface; and Grade 5, more proliferation than 4. In S. alba, prolif- eration from both cut surfaces was assessed in assigning the numbers of grade (0, 1-4). All data were averaged per explant from data of 4 to 8 explants in 2 to 4 tubes. A student t-test was performed on the difference of mean values between the control (i.e. without added salts and osmotic compounds) and each treatment at P < 0.05. 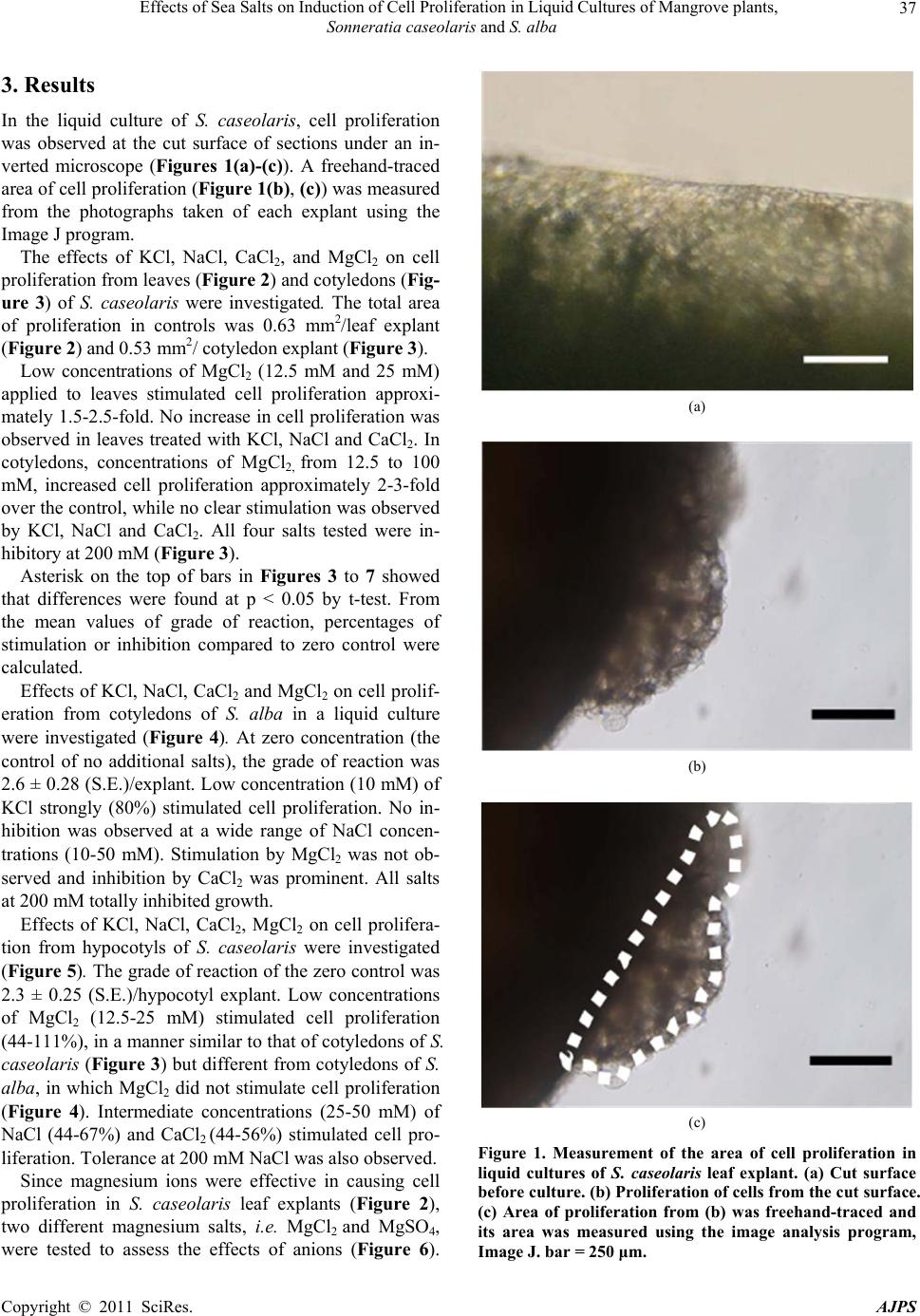 Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 37 3. Results In the liquid culture of S. caseolaris, cell proliferation was observed at the cut surface of sections under an in- verted microscope (Figures 1(a)-(c)). A freehand-traced area of cell proliferation (Figure 1(b), (c)) was measured from the photographs taken of each explant using the Image J program. The effects of KCl, NaCl, CaCl2, and MgCl2 on cell proliferation from leaves (Figure 2) and cotyledons (Fig- ure 3) of S. caseolaris were investigated. The total area of proliferation in controls was 0.63 mm2/leaf explant (Figure 2) and 0.53 mm2/ cotyledon explant (Figure 3). Low concentrations of MgCl2 (12.5 mM and 25 mM) applied to leaves stimulated cell proliferation approxi- mately 1.5-2.5-fold. No increase in cell proliferation was observed in leaves treated with KCl, NaCl and CaCl2. In cotyledons, concentrations of MgCl2, from 12.5 to 100 mM, increased cell proliferation approximately 2-3-fold over the control, while no clear stimulation was observed by KCl, NaCl and CaCl2. All four salts tested were in- hibitory at 200 mM (Figure 3). Asterisk on the top of bars in Figures 3 to 7 showed that differences were found at p < 0.05 by t-test. From the mean values of grade of reaction, percentages of stimulation or inhibition compared to zero control were calculated. Effects of KCl, NaCl, CaCl2 and MgCl2 on cell prolif- eration from cotyledons of S. alba in a liquid culture were investigated (Figure 4). At zero concentration (the control of no additional salts), the grade of reaction was 2.6 ± 0.28 (S.E.)/explant. Low concentration (10 mM) of KCl strongly (80%) stimulated cell proliferation. No in- hibition was observed at a wide range of NaCl concen- trations (10-50 mM). Stimulation by MgCl2 was not ob- served and inhibition by CaCl2 was prominent. All salts at 200 mM totally inhibited growth. Effects of KCl, NaCl, CaCl2, MgCl2 on cell prolifera- tion from hypocotyls of S. caseolaris were investigated (Figure 5). The grade of reaction of the zero control was 2.3 ± 0.25 (S.E.)/hypocotyl explant. Low concentrations of MgCl2 (12.5-25 mM) stimulated cell proliferation (44-111%), in a manner similar to that of cotyledons of S. caseolaris (Figure 3) but different from cotyledons of S. alba, in which MgCl2 did not stimulate cell proliferation (Figure 4). Intermediate concentrations (25-50 mM) of NaCl (44-67%) and CaCl2 (44-56%) stimulated cell pro- liferation. Tolerance at 200 mM NaCl was also observed. Since magnesium ions were effective in causing cell proliferation in S. caseolaris leaf explants (Figure 2), two different magnesium salts, i.e. MgCl2 and MgSO4, were tested to assess the effects of anions (Figure 6). (a) (b) (c) Figure 1. Measurement of the area of cell proliferation in liquid cultures of S. caseolaris leaf explant. (a) Cut surface before culture. (b) Proliferation of cells from the cut surface. (c) Area of proliferation from (b) was freehand-traced and its area was measured using the image analysis program, Image J. bar = 250 μm. 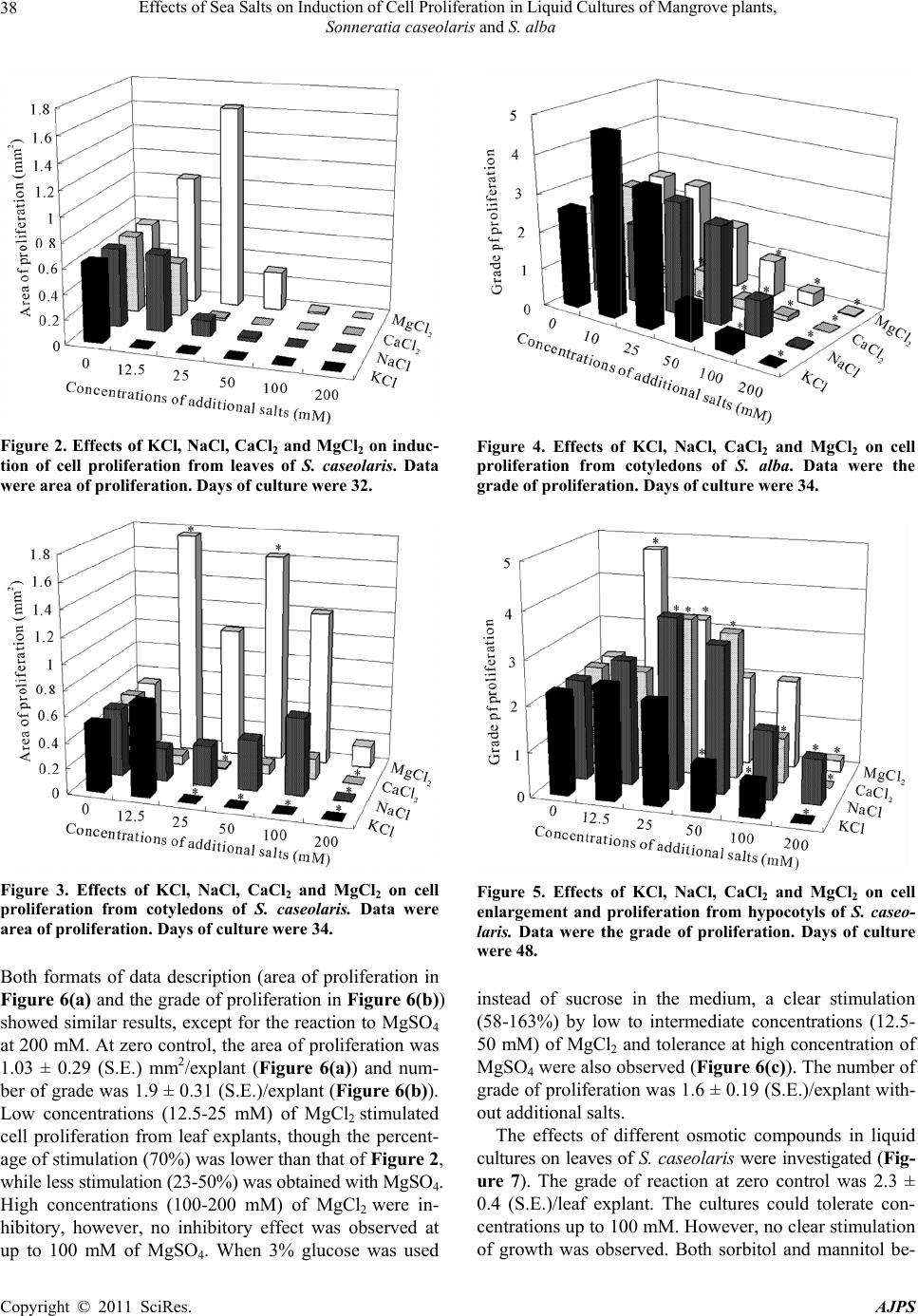 Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 38 Figure 2. Effects of KCl, NaCl, CaCl2 and MgCl2 on induc- tion of cell proliferation from leaves of S. caseolaris. Data were area of proliferation. Days of culture were 32. Figure 3. Effects of KCl, NaCl, CaCl2 and MgCl2 on cell proliferation from cotyledons of S. caseolaris. Data were area of proliferation. Days of culture were 34. Both formats of data description (area of proliferation in Figure 6(a) and the grade of proliferation in Figure 6(b)) showed similar results, except for the reaction to MgSO4 at 200 mM. At zero control, the area of proliferation was 1.03 ± 0.29 (S.E.) mm2/explant (Figure 6(a)) and num- ber of grade was 1.9 ± 0.31 (S.E.)/explant (Figure 6(b)). Low concentrations (12.5-25 mM) of MgCl2 stimulated cell proliferation from leaf explants, though the percent- age of stimulation (70%) was lower than that of Figure 2, while less stimulation (23-50%) was obtained with MgSO4. High concentrations (100-200 mM) of MgCl2 were in- hibitory, however, no inhibitory effect was observed at up to 100 mM of MgSO4. When 3% glucose was used Figure 4. Effects of KCl, NaCl, CaCl2 and MgCl2 on cell proliferation from cotyledons of S. alba. Data were the grade of proliferation. Days of culture were 34. Figure 5. Effects of KCl, NaCl, CaCl2 and MgCl2 on cell enlargement and proliferation from hypocotyls of S. caseo- laris. Data were the grade of proliferation. Days of culture were 48. instead of sucrose in the medium, a clear stimulation (58-163%) by low to intermediate concentrations (12.5- 50 mM) of MgCl2 and tolerance at high concentration of MgSO4 were also observed (Figure 6(c)). The number of grade of proliferation was 1.6 ± 0.19 (S.E.)/explant with- out additional salts. The effects of different osmotic compounds in liquid cultures on leaves of S. caseolaris were investigated (Fig- ure 7). The grade of reaction at zero control was 2.3 ± 0.4 (S.E.)/leaf explant. The cultures could tolerate con- centrations up to 100 mM. However, no clear stimulation of growth was observed. Both sorbitol and mannitol be-  Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 39 Figure 6. Comparison of the effects of MgCl2 and MgSO4 on cell proliferation from leaves of S. caseolaris. The control medium is MS containing 0.1 μM 2,4-D and 3% sucrose (a, b) and 3% glucose (c). Days of culture were 68. Data were the area of proliferation (a) and grade of proliferation (b, c). gan to produce significant inhibitory effects at 200 mM and they became totally inhibitory to growth at 400 mM. When the effect of sorbitol in combination with 3% su- crose was investigated in liquid culture of cotyledons (Figure 8), the grade of reaction at zero control was 2.4 ± 0.26 (S.E.)/cotyledon explant. Tolerance up to 200 mM of sorbitol was observed and 400 mM sorbitol was in- hibitory. The pattern is very different from those of the four salts, i.e. KCl, NaCl, MgCl2 and CaCl2. Glycinebe- taine totally inhibited growth in cultures of cotyledons and leaves of S. caseolaris at any concentrations (data not shown). Figure 7. Effects of sorbitol and mannitol in combination with 3% sucrose on cell proliferation from leaves of S. case- olaris. Days of culture were 35. Data were the grade of pro- liferation. Figure 8. Effect of sorbitol in combination with 3% sucrose on cell proliferation from cotyledons of S. caseolaris. Data were the grade of proliferation. Days of culture were 37. 4. Discussion 4.1. Differences in Response to Added Salts between S. caseolaris and S. alba Tolerance to a wide range (10-50 mM) of concentrations of NaCl was observed in cotyledon cultures of S. alba but not in cotyledon cultures of S. caseolaris. The fact that S. alba grows along the most seaside coast while S. caseolaris grows more in-land, may have contributed to the different responses observed. The salinity of the latter growing area was lower than that of the former [17]. In the suspension culture derived from cotyledons of S. alba, stimulation of growth was observed by NaCl at the same concentrations [12]. S. alba would have developed mechanisms to deal with the key component of sea water, i.e. sodium ions in order to achieve proper growth and development. Sea water and the mangrove growing soil contain substantial amount of other salts [18]. Induction  Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 40 of cell proliferation was greatly stimulated by KCl in cotyledon culture of S. alba but not in any of the S. case- olaris tissues. In the established suspension culture of S. alba, increase of growth was obtained at low concentra- tion (10 mM) of KCl [12]. On the contrary, low concen- trations (12.5-25 mM) of MgCl2 greatly stimulated cell induction from all three tissues in S. caseolaris, but not in the cotyledons of S. alba. No data of hypocotyls or leaves of S. alba were available in this report, because the growth of big seedlings was difficult in S. alba. To explore the mechanisms of salts tolerance, the develop- ment of in vitro culture systems is important for com- plementation of whole plant studies. S. alba and S. case- olaris are two excellent mangrove species of the same genera showing different responses to different salts. These can be cultured in the same MS basal medium containing 3% of sucrose and 0.1 μM of 2,4-D, while modified amino acid basal medium was employed for the culture of B. sexangula of different family [8]. 4.2. Differences in Tissue Responses to Salts in S. caseolaris Differences were found in the reaction to different salts depending on the source of tissue tested, i.e. leaves, cotyledons, or hypocotyls. Stimulation by NaCl was ob- served in cultures of hypocotyls, but not in cotyledons or leaves. Similarly, CaCl2 was stimulatory to hypocotyl culture but had little effect on other tissues of S. caseo- laris. Cotyledons and hypocotyls were more tolerant to MgCl2 than leaves. In nature, young seedlings of Son- neratia species sometimes grow under water but leaves are rarely submerged. Thus, we would expect cotyledons and hypocotyls to be more tolerant tissues to salty water than leaves. The observed difference in tolerance to NaCl depending on the origin of tissues, i.e. viviparous seed- lings and leaves, was also reported for callus induction of B. sexangula [7]. Effects of salts can be explained partly as the osmotic effects of component ions. However, MgCl2 and CaCl2 were not always inhibitory compared to NaCl and KCl [12], implying that cations themselves are important in mangrove cells. This is the first report where effects of high concentra- tions of MgSO4 were investigated, and tolerance of leaves of S. caseolaris was found in liquid culture, while low concentrations of MgSO4 were not stimulatory com- pared with MgCl2. Tolerance to MgSO4 was also found in media containing glucose (Figure 6(c)), which sugar stimulated cell proliferation from leaves of S. caseolaris in solid culture [19]. Although differences in ion disso- ciation can result in different osmotic potentials, the re- sults in Figure 6 suggest anions may have specific roles to play. Both anions are ingredients of sea water and fur- ther studies are needed to ascertain their function. 4.3. Effects of Osmotic Compounds in S. caseolaris The presence of salts naturally found in sea water can change the osmotic environment surrounding plants. Changes in the osmotic environment have a substantial effect on plant growth and development. It has been shown that osmotic stress enhances somatic embryo for- mation in carrot [20] and mannitol can induce somatic embryogenesis in vegetative tissues of Arabidopsis [21]. In this study, several common osmotica were used to assess the importance of the osmotic environment on cell proliferation of mangrove species. As shown in Figure 7, neither mannitol nor sorbitol had a significant stimula- tory effect on cell proliferation of S. caseolaris, and be- came inhibitory to growth at 200 mM. In leaf culture of a Rhizophoraceae mangrove, Rhizophora stylosa, sorbitol at 0.2-0.4 M was stimulatory for callus induction, while NaCl was inhibitory at the same osmotic potential [22]. Mannitol and glycinebetaine are known as naturally oc- curring osmotica in S. alba [23] and an Avicenniaceae mangrove, Avicennia marina [24], respectively. The gly- cinebtaine had a negative effect on growth even at low concentrations (data not shown). These suggest that changes in osmotic conditions alone are not sufficient to promote growth. The effects of salts cannot be described only by their effects as osmoticum. Both cations and anions of various salts may have specific functions to play in promoting growth and development of mangrove plants. 4.4. Data Description Method In this report, in addition to recording the response as the numbers of grade of proliferation, which was used in the previous report in culture of S. alba [11], we introduced another data description method, the area of cell prolif- eration measured using an image analysis program. No critical difference in the effects of salts was found be- tween two methods of data description. Although both methods yielded similar results, the grade method is pre- ferred as one can identify small changes in the explant, especially at high salt concentrations, and the standard errors were smaller than the area method. The area method can only account for two dimensional changes in an explant and the process is time consuming. The use of small volume culture method as detailed in this study enables the assessment of changes of explants to differ- ent tested variables. Since the sources of seed materials are limited, this greatly increases the efficiency of the study.  Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 41 4.5. Conclusions Mangrove plants have adapted to grow in an environ- ment with high salts. This unique property is also re- flected in the cell cultures generated from the mangrove species, Sonneratia caseolaris and S. alba. The ability of mangrove cell cultures to grow in the presence of high salts indicates the unique adaptation and metabolism of mangrove cells. It is important to note that different sea water components, KCl, NaCl, CaCl2, MgCl2 and MgSO4, elicit different responses from different mangrove spe- cies. This result indicates that different mangrove species have different metabolic adaptations to various salts in the environment. The ability of both mangrove species to tolerate high levels of magnesium ions indicates that magnesium ions may have a vital metabolic role to play within mangrove cells. Cell cultures generated from dif- ferent tissues of the same plant react differently to vari- ous salts and salt concentrations. This observation sug- gests that different tissue types within the plant body responds to salts differently. Future biochemical and cell biological studies will provide further insight into the role of various ions on the growth of mangrove cultures. 5. Acknowlegements Authors thank Prof. K. Suzuki of the Yokohama National University for his kind supply of the fruits of S. caseo- laris. Authors also thank Prof. E. C. Yeung of the Uni- versity of Calgary for his critical reading of this manu- script. REFERENCES [1] P. B. Tomlinson, “The Botany of Mangroves,” Cam- bridge University Press, Cambridge, 1986. [2] S. Ogita, E. C. Yeung, H. Sasamoto, “Histological analy- sis in shoot organogenesis from hypocotyls explants of Kandelia candel (Rhizophoraceae),” Journal of Plant Research, Vol. 117, No. 6, December 2004, pp. 457-464. doi:10.1007/s10265-004-0180-4 [3] H. Sasamoto, Y. Wakita, S. Yokota, N. Yoshizawa, T. Katsuki, Y. Nishiyama, T. Yokoyama, M. Fukui, “Effects of electric cell fusion treatment among leaf protoplasts of Populus alba and Alnus firma on growth, leaf morphol- ogy, and RAPD pattern of eleven acclimatized plants,” In Vitro Cellular and Developmental Biology Plant, Vol. 42, No. 2, March 2006, pp. 174-178. doi:10.1079/IVP2005732 [4] Y. Kawana, H. Sasamoto, Y. Mochida, K. Suzuki, “Leaf protoplast isolation from eight mangrove species of three different families; Avicenniaceae, Rhizophoraceae and Sonneratiaceae,” Mangrove Science, Vol. 3, October 2004, pp. 25-31. [5] F. Kaai, Y. Kawana, H. Sasamoto, “The relation between recalcitrancy of a mangrove plant, Kandelia obovata, and high endogenous level of abscisic acid,” Plant Cell Tissue and Organ Culture, Vol. 94, No. 2, August 2008, pp. 125-130. doi:10.1007/s11240-008-9394-9 [6] FAO, “Global network on integrated soil management for sustainable use of salt-affected soils,” FAO Land and Plant Nutrition Management Service, 2005. http://www.fao.org/ag/AGL/ agII/spush/ [7] T. Mimura, M. Mimura, S. Washitani-Nemoto, K. Sakano, T. Shimmen, S. Siripatanadilok, “Efficient callus initia- tion from leaf of mangrove plant, Bruguiera sexangula in amino acid medium: Effect of NaCl on callus initiation,” Journal of Plant Research, Vol. 110, No. 1, March 1997, pp. 25-29. doi:10.1007/BF02506839 [8] M. Kura-Hotta, M. Mimura, T. Tsujimura, S. Washi- tani-Nemoto, T. Mimura, “High salt-treatment-induced Na+ extrusion and low salt-treatment-induced Na+ ac- cumulation in suspension-cultured cells of the mangrove plant Bruguiera sexangula,” Plant Cell and Environment, Vol. 24, No. 10, October 2001, pp. 1105-1112. doi:10.1046/j.0016-8025.2001.00761.x [9] H. Sasamoto, S. Ogita, T. Mimura, “Cell fusion and pro- toplast cultures in a mangrove plant, Bruguiera sexan- gula,” in Japanese. Proceedings of 111th Annual Meeting of the Japanese Forrest Society, April 2000, p. 603. [10] A. Yamada, T. Saitoh, T. Mimura, Y. Ozeki, “Expression of mangrove allene oxide cyclase enhances salt tolerance in Escherichia coli, yeast, and tobacco Cells,” Plant Cell Physiology, Vol. 43, No. 8, August 2002, pp. 903-910. doi:10.1093/pcp/pcf108 [11] Y. Kawana, R. Yamamoto, Y. Mochida, K. Suzuki, S. Baba, H. Sasamoto, “Generation and maintenance of suspension cultures from cotyledons and their organo- genic potential of two mangrove species, Sonneratia alba and S. caseolaris,” Plant Biotechnology Reports, Vol. 1, No. 4, November 2007, pp. 219-226. doi:10.1007/s11816-007-0035-2 [12] Y. Kawana, H. Sasamoto, “Stimulation Effects of Salts on Growth in Suspension Culture of a Mangrove Plant, Sonneratia alba, Compared with Another Mangrove, Bruguiera sexangula and Non-Mangrove Tobacco BY-2 cells,” Plant Biotechnology, Vol. 25, No. 2, May 2008, pp. 151-155. doi:10.5511/plantbiotechnology.25.151 [13] A. Miyawaki, K. Suzuki, S. Suzuki, Y. Nakamura, Y. Murakami, Y. Tukagoshi, E. Nakata, “Phytosociological studies of mangrove vegetation in Japan. 1. Mangrove vegetation of Iriomote island,” Bull. Inst. Envir. Sci. Tech. Yokohama Nat. Univ. Vol. 9, 1982, pp. 77-89. [14] A. Miyawaki, S. Okuda, K. Suzuki, K. Fujiwara, Y. Na- kamura, Y. Murakami, K. Ohno, S. Suzuki, S. Sabhasri, “Phytosociological studies of mangrove vegetation in Thailand. In: A. Miyawaki, ed., Ecological studies on the vegetation of mangrove forests in Thailand, Published by Dept. of Vegetaion Science, Inst. Envir. Sci. Tech. Yo- kohama Nat. Univ., Yokohama, Japan, 1985. [15] T. Murashige, F. Skoog, “A revised medium for rapid growth and bioassay with tobacco tissue cultures,”  Effects of Sea Salts on Induction of Cell Proliferation in Liquid Cultures of Mangrove plants, Sonneratia caseolaris and S. alba Copyright © 2011 SciRes. AJPS 42 Physiol Plant, Vol. 15, No. 3, July 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x [16] Image J, NIH, USA, Internet Available: http://rsb.info.nih.gov/ij/ [17] N. C. Duke, “Mangrove floristics and biogeography,”In: A.I. Robertson, D.M. Alongi, Eds., Tropical mangrove ecosystems, American Geophysical Union, Washington, 1992, pp. 63-100. [18] T. Fukumoto, T. Nakamura, M. Suzuki, S. Ogita, T. Mi- mura, H. Sasamoto, “Different effects of four salts and pHs on protoplast cultures of a mangrove, Bruguiera sexangula suspension cells, Populus alba leaves and to- bacco BY-2 cells,” Plant Biotechnology, Vol. 21, No. 3, September 2004, pp. 177-182. doi:10.5511/plantbiotechnology.21.177 [19] R. Yamamoto, Y. Kawana, R. Minagawa, H. Sasamoto, “Effects of carbon and nitrogen sources on induction of cell proliferation in tissue cultures of a mangrove plant, Sonneratia caseolaris,” Mangrove Science, Vol. 6, Janu- ary 2009, pp. 1-8. [20] H. Kamada, K. Ishikawa, H. Saga, H. Harada, “Induction of somatic embryogenesis by osmotic stress in carrot,” Plant Tissue Culture Letters, Vol. 10, No. 1, April 1993, pp. 38-44. [21] M. Ikeda-Iwai, M. Umehara, S. Satoh, H. Kamada, “Stress-induced somatic embryogenesis in vegetative tis- sues of Arabidopsis thaliana,” Plant Journal, Vol. 34, No.1, April 2003, pp. 107-114. doi:10.1046/j.1365-313X.2003.01702.x [22] Y. Kawamitsu, K. Tokumaru, “Examination of the opti- mum callus induction medium in Rhizophora stylosa,” in Japanese. In: Reports of Research Institute for Subtropics, 2003, pp. 193-202. [23] E. Yasumoto, K. Adachi, M. Kato, H. Sano, H. Sasamoto, S. Baba, H. Ashihara, “Uptake of inorganic ions and compatible solutes in cultured mangrove cells during salt stress,” In Vitro Cellular Developmental Biology Plant, Vol. 35, No. 1, January1999, pp. 82-85. doi:10.1007/s11627-999-0015-z [24] H. Ashihara, K. Adachi, M. Otawa, E. Yasumoto, Y. Fukushima, M. Kato, H. Sano, H. Sasamoto, S. Baba, “Compatible solutes and inorganic ions in the mangrove plant Avicennia marina and their effects on the activities of enzymes,” Zeitschrift fur Naturforschung, Vol. 52c, No. 5/6, May 1997, pp. 433-440. |

