 International Journal of Clinical Medicine, 2013, 4, 20-30 Published Online December 2013 (http://www.scirp.org/journal/ijcm) http://dx.doi.org/10.4236/ijcm.2013.412A2004 Open Access IJCM Reactive Arthritis: From Clinical Features to Pathogenesis Ethelina Cargnelutti1,2, María Silvia Di Genaro1,2* 1Division of Immunology, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, San Luis, Argentina; 2Laboratory of Immunopathology, Multidisciplinary Institute of Biological Investigations-San Luis (IMIBIO-SL), National Council of Scientific and Technical Investigations (CONICET), San Luis, Argentina. Email: *sdigena@unsl.edu.ar Received October 27th, 2013; revised November 20th, 2013; accepted December 10th, 2013 Copyright © 2013 Ethelina Cargnelutti, María Silvia Di Genaro. This is an open access article distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Ethelina Cargnelutti, María Silvia Di Genaro. All Copyright © 2013 are guarded by law and by SCIRP as a guardian. ABSTRACT Reactive arthritis (ReA) is a sterile synovitis which occurs after a gastrointestinal or urogenital infection. ReA belongs to Spondyloarthritis (SpA), a group of diseases that share several clinical and radiological features including familiar clustering, absence of rheumatoid factor and association with HLA-B27. Clinically, ReA is characterized by an asym- metric arthritis predominantly affecting the lower limbs, often associated with urethritis, conjunctiv itis and other extra- articular symptoms. The ReA prevalen ce depends on the incidence of causative pathogen s. The ReA diagnosis is based on clinical features and serological tests to evidence previous infection. Different treatment including antibiotics, dis- ease modifying antirheumatic drugs (DMARs) and biologic agents has been recommended. Even though knowing that infections trigger th e jo int inf lammation , th e ReA pathog enesis remains to be poo r ly understoo d. Sev eral ani mal models and in vitro studies have been used to elucidate the mechanisms involved in ReA development. In this sense, HLA-B27 transgenic rat or mice have been used to explain the role of this molecule in SpA aetiopathogenesis. Moreover, the in- fectious model of Yersinia-induced ReA in rodents has shed some lights on the relationship between host genetic sus- ceptibility to infection and abnormal immune response in ReA development. Understanding the immune mediators triggering ReA will contribute to find a specific treatment for this arthritis. In this review, we focus on clinical features, epidemiology, treatment, and the different attempts to understand the pathogenesis of ReA. Keywords: Reactive Arthritis; HLA-B27; Spondyloarthritis; Yersinia-Induced ReA; Therapy 1. Introduction Reactive arthritis (ReA) is arthritis that arises following a gastrointestinal or urogenital infection. It is a form of Spondyloarthritis (SpA), a group of diseases with com- mon features including inflammatory arthritis (generally an asymmetrical oligoarthritis), absence of rheumatoid factor and genetic association with the human leukocyte antigen (HLA)-B27. In addition to ReA, SpA also in- cludes ankylosing spondylitis (AS), psoriatic arthritis (PsA), arthritis related to inflammatory bowel disease (IBD-SpA) and undifferentiated SpA (U-SpA) [1]. Nevertheless, at present it exist a discussion whether this classification represent alternative presentatio ns of one entity with het- erogeneous phenotype [2]. Currently, according to the Assessment of Spondylo Arthritis International Society (ASAS) classification criteria (2009-2011), the SpA is classified as axial and peripheral arthritis [3]. The term “ReA” was introduced by Avohen et al. in 1969 to describe arthritis induced by Yersinia entero- colitica [4]. Moreover, the clinical features of this dis- ease were characterized and the diagnosis of the preced- ing infection through serological methods was establish ed [5]. The name ReA involves the immunological origin of this arthritis in which microorganisms do not en ter in the joint cavity and antibiotic treatment has no effect on its development or outcome [6]. Even though none cultiva- ble microorganism has been isolated from the joints of patients with ReA, bacterial antigens have been demon- strated in synovial fluid or tissue using different tech- niques, indicating deficient clearance of the inducer bac- teria [7-10]. In addition, Chlam ydi a trachomatis mRNA has been detected in the joints of patients with post-ve- *Corresponding author.  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 21 nereal ReA, raising the possibility that viable forms of this microorganism may be present [11,12]. A controversy exists in relation to the clinical find ings to diagnose ReA [13]. According to the 4th International Workshop on Reactive Arthritis (Berlin, 1999), the term ReA must apply to a patient with typical clinical features of this disease and in those whose preceding infection was caused by the classic microorganisms involved in their development. The minimum time interval between the gastrointestinal/genitourinary infection symptoms and arthritis should be of 1 - 7 days, maximum 4 weeks. It is advisable for the investigation of microorganisms induc- ers of ReA by culturing urine/feces or through serologi- cal methods [14]. The current diagnosis of ReA is per- formed considering clinical features, radiologic examina- tion and laboratory tests. However, for diagnosis of ReA, there is not a single laboratory test, and the radiological images do not help much in diagnosing an acute episode. The investigations performed to ReA diagnosis, and also to make a differential diagnosis are based on hematologic, microbiologic, serologic and radiologic findings, and on synovial fluid studie s ( Table 1). 2. Epidemiology The incidence and prevalence of ReA depends of geo- graphic region and the prevalence of causative pathogens. The ReA incidence is estimated to be 5 - 14/100,000 pa- tients aged 18 - 60 years [15,16]. Most patients are aged 20 - 40 and it is more common in Caucasians affecting equally men and women [16]. A population-based study in Oregon and Minnesota (US) reported a ReA incidence following documented enteric bacterial infections ranged from 0.6 to 3.1 cases per 100,000, depending upon the organism [17]. Two registry-based studies from Spain reported that 1.2% to 1.4% of all patients with SpA was diagnosed with ReA [18]. A recent epidemiological study in Argentina informed that from 402 patients with SpA aged 38.3 - 58 years, 25 (6.2%) had ReA [19]. In outbreaks triggered by a single source of infection, 0% - 22% of infected subjects developed subsequent Re A [20]. The HLA-B27 antigen is found in 30% - 70% of pa- tients with ReA, which is a lower frequency compare to others SpA, such as AS with 90% of patients positive for this antigen [21]. Patients with this molecule manifest a more severe arthritis with a tendency to progress to a chronic stage and also they have greater chance of de- veloping extra-articular symptoms [22]. At present, there are descript more than 100 isoforms of the HLA-B27 molecule (http://www.ebi.ac.uk/ipd/imgt/hla) that differ in the aminoacidic sequence. Most HLA-B27 molecules seem to be associated with SpA; however, there would be a hierarchy of association between the different sub- types of these molecules. Thus, HLA-B*2704 shows high- er association with SpA, followed by HLA-B*2705, HLA-B*2702 and HLA-B*2707, while HLA-B*2706 and HLA-B*2709 are less associated to these diseases [23]. Furthermore, there is a geographical distribution of these isoforms, with a prevalence of HLA-B*2704 and HLA-B*2707 for Asians, and HLA-B*2705 and HLA- B*2702 for Caucasians [24]. In regional studies in South America, the most frequent HLA-B27 isoforms are HLA- B*2705 and HLA-B*2702 [25-27]. In Argentina, it is estimated a prevalence of 4% of HLA-B27 in the general population [28]. A study in 11 Rheumatology Centers in Argentina reported in 405 patients with SpA that 50% of patients with ReA were positive for HLA-B27 [29]. Table 1. Methods and expected results for ReA diagnosis. Hematology Erythrocyt e se di mentation rate (ESR): usually elevated. C-reactive protein level (CRP): usually elevated. Complete blood c ell count: in the acute phase may show l eukocytosis. Rheumatoid factor: negative. Antinuclear antibody: negative. HLA-B27 testing: not diagnosis, but has prognostic value as positive results may indicate more serious disease. Microbiology Urine culture: may be positive for Chlamydia at the beginning of the infection. Stool culture: positive for Salmonella, Shigella or Yersinia whether obtained early. Synovial fluid studies Cell count: at early time of ReA is high and dominated by polymorphonuclears. Microscopy under polarized light: negative for urate crystals. Synovial fluid culture: negative. Serology Antibodies against Yersinia, Salmonella, Campylobacter, Chlamydia, Neisseria gonorrhoeae, Borrelia burgdorferi, and also against β-hemolytic streptococci should be determined and followed: positive for Salmonella, Shigella, Yersinia or Chalmydia. Radiological images Early disease: soft tissue swelling around affected joints that can represent large effusions; tendon swelling as in the calcaneal region. Chronic disease: bone and cartilage erosions with adjacent bone proliferation specially in the lower extremities; paravertebral ossification.  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 22 3. Clinical Features The classic clinical characteristics of ReA involve an axial joint arthritis, enthesitis and peripheral oligoarthritis (less than 5 joint affected) usually asymmetrically ac- companied by extra-articular symptoms [30]. The mus- culoskeletal symptoms are commonly acute and at be- ginning associated with systemic features such as malaise, fever, fatigue and weight loss [31]. Urogenital infection precedes 1 - 6 weeks the muscu- loskeletal symptoms. Their presentation varies from mild to severe with prostatitis or cervicitis. Howev er, it can be asymptomatic in both sex. It is often accompanied by other signs such as penis discharge in males, pain with urination, or hematuria [32]. On the other hand, acute diarrhea appears approximately one month before articu- lar manifestations in post-dysenteric ReA. Gastrointesti- nal symptoms are absent or mild in ReA triggered by Yersinia, unlike in patients with Sa lmonella and Campy- lobacter infections where symptoms are more severe and of longer du ration [33] . Joint inflammation could be axial, involving the lum- bar spine or sacroiliac joints, alternatively it is peripheral, commonly affecting the large joints of lower extremities, being knees, foot joints and ankles the most frequently involved. However, affectation of upper extremities (el- bow, shoulder) and polyarticular forms have been re- ported in which the subtalar, metatarsophalangeal and toe interphalangeal joints tend to be affected [34,35]. Some patients suffer from dactylitis which is a diffus e swelling of entire finger or toe, sometimes referred as “sauce digit”. This feature is common in ReA, but also in PsA and it is used to make a diagnosis of axial SpA using the ASAS criteria [36] or PsA using the ClASsification criteria for Psoriatic ARthritis (CASPAR) [37]. Enthesitis is an inflammation of the transitional zone where tendons and ligaments insert into the bone and sometimes it is the unique manifestation of this arthritis. Achilles tendonitis and plantar fasciitis are the most com- mon types of enthesitis in ReA, but another enthesis can be involved [38]. Extra-articular symptoms are frequently observed in ReA and include mucocutaneous, ocular and occasion- ally cardiac manifestations. Mucocutaneous lesions are very specific of ReA and more frequent in HLA-B27 positive patients. Circinate balanitis is the most common skin manifestation of this arthritis following by kerato- derma blennorrhagicum, occurring in almost 50% and 10% of the patients, respectively [39]. A well recognized complication of Yersinia-infection is erythema nodosum which is a painful rash predominantly on the extensor surfaces of the arms and legs [40]. Nails changes (nail dystrophy, subungual debris, and periungual pustules), hyperkeratosis and oral lesions also may occur [38]. One-third of patients with ReA suffer of conjunctivitis once it is established, being more common after a geni- tourinary infection or enteric infection by Shigella, Sal- monella and Campylobacter. The conjunctivitis is unilat- eral or bilateral with a mucopurulent discharge and its course is often mild and transient [41]. Acute anterior uveitis (AAU) may be observed in about a 5% patients with acute ReA and more than 50% patients with AAU are HLA-B27 positive. Th is manifestation is very painful and is characterized by sudden-onset, mostly unilateral [42-44]. Others less frequent ocular symptoms are kerati- tis [45], corneal ulceration, retrobulbar neutritis, scleritis and hypema which appear in persistent or chronic ReA [28]. The persistence of ocular inflammation may result in complications such as posterior synechiae, glaucoma, cystoids macular edema and cataract formation [46]. Cardiovascular manifestations in ReA and in other members of the SpA family have long been recognized and related to HLA-B27 positivity. Disturbances of the cardiac conduction system are found early [47,48] and cases with severe aortic insufficiency are found in late disease [49]. 4. Triggering Microbes Different bacteria species are associated with ReA de- velopment [15]. The classical enteric pathogens capable of triggering this arthritis belong to the genders Salmo- nella, Yersinia, Shigella and Campylobacter. On the other hand, C. trachomatis is the most common urogenital pathogen related to ReA [11,12]. Salmonella, Yersinia, Shigella and Campylobacter are Gram-negative bacteria with lipopolysaccharide (LPS) in their outer membrane. Furthermore, they are facultative or obligate intracellular, aerobic or microaerophilic bacteria. These characteristics probably account for their relation with ReA [31]. Epi- demiological studies support the high association be- tween infection with these bacteria and ReA develop- ment. Thus, in a study performed in different Rheuma- tology clinics in Berlin, Germany, from 52 patients with ReA a causative pathogen was identified in 29/52 (56%) [50]. In 17 (52%) of the patients with enteric ReA one of the enteric bacteria was identified: Salmonella in 11/33 (33%) and Yersinia in 6/33 (18%). C. trachomatis was the causative pathogen in 12/19 (63%) of the patients with urogenic ReA [50]. In 74 patients with the clinical picture of U-SpA, a specific triggering bacterium was also identified in 35/74 (47%) patients: Yersinia in 14/74 (19%), Salmonella in 9/74 (12%), and C. trachomatis in 12/74 (16%) [50]. Moreover, a 2-year epidemiological study on ReA and possible ReA in Oslo (Norway) re- ported an annual minimum incidence of Chlamydia-in- duced ReA (n = 25) of 4.6/100,000, and of enteric ReA (n = 27) of 5/100,000 individuals between 18 and 60 years [16]. In addition, a population-based cohort study (n = 71) in Southern Sweden showed in patients with a 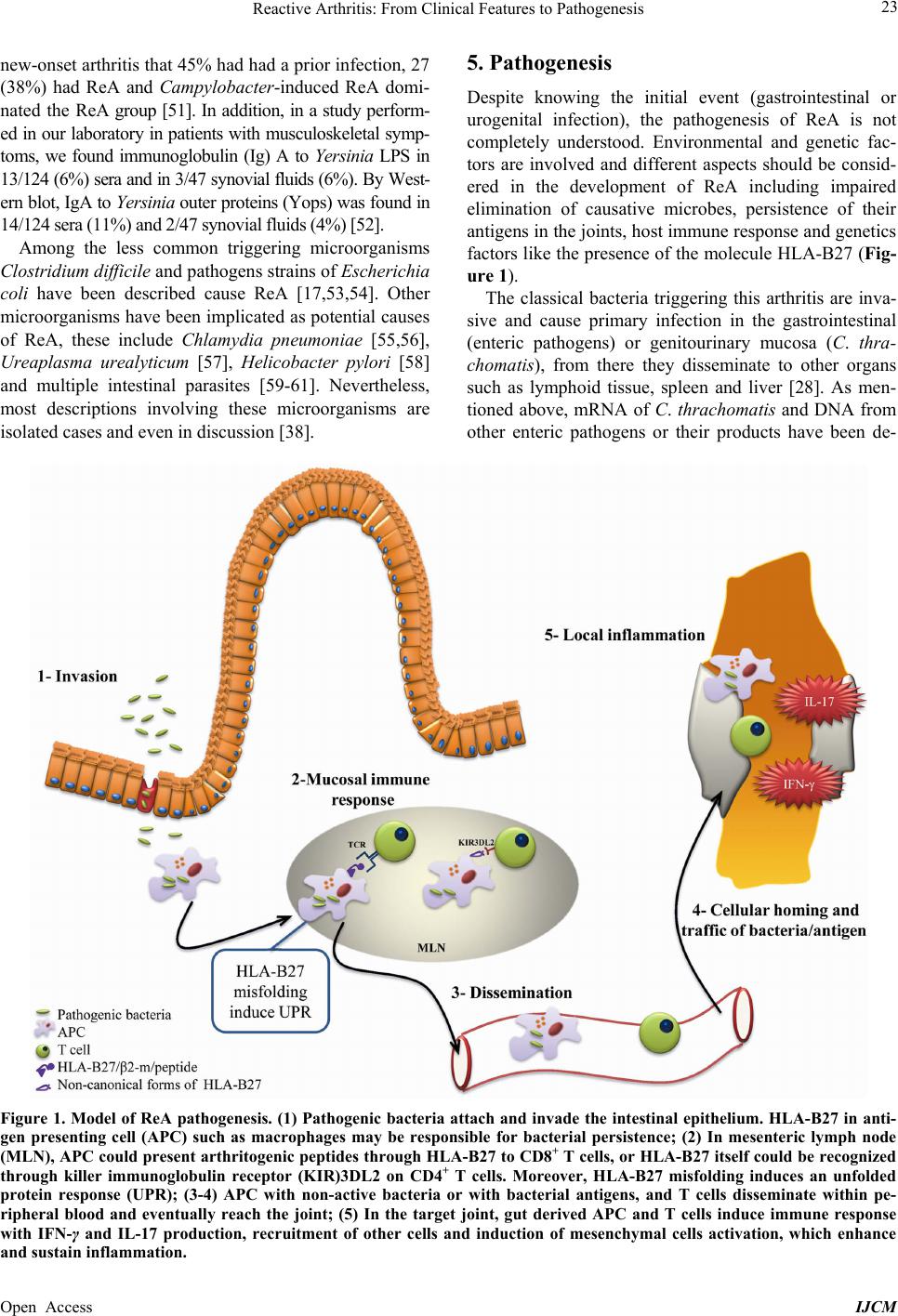 Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 23 new-onset arthritis that 45 % had had a prior infection, 27 (38%) had ReA and Campylobacter-induced ReA domi- na te d the R eA group [51]. In addition, in a study perform- ed in our laboratory in patients with musculoskeletal symp- toms, we found immunoglobulin (Ig) A to Yersinia LPS in 13/124 (6%) sera and in 3/47 synovial fluids (6%). By West- ern blot, IgA to Yersinia outer proteins (Yops) was found in 14/124 sera (11%) and 2/47 synovial fluids (4%) [52]. Among the less common triggering microorganisms Clostridium difficile and pathogens strains of Escherichia coli have been described cause ReA [17,53,54]. Other microorganisms have been implicated as potential causes of ReA, these include Chlam yd ia pneumoniae [55,56], Ureaplasma urealyticum [57], Helicobacter pylori [58] and multiple intestinal parasites [59-61]. Nevertheless, most descriptions involving these microorganisms are isolated cases and even in discussion [38]. 5. Pathogenesis Despite knowing the initial event (gastrointestinal or urogenital infection), the pathogenesis of ReA is not completely understood. Environmental and genetic fac- tors are involved and different aspects should be consid- ered in the development of ReA including impaired elimination of causative microbes, persistence of their antigens in the joints, host immune response and g enetics factors like the presence of the molecule HLA-B27 (Fig- ure 1). The classical bacteria triggering this arthritis are inva- sive and cause primary infection in the gastrointestinal (enteric pathogens) or genitourinary mucosa (C. thra- chomatis), from there they disseminate to other organs such as lymphoid tissue, spleen and liver [28]. As men- tioned above, mRNA of C. thrachomatis and DNA from other enteric pathogens or their products have been de- Figure 1. Model of ReA pathogenesis. (1) Pathogenic bacteria attach and invade the intestinal epithelium. HLA-B27 in anti- gen presenting cell (APC) such as macrophages may be responsible for bacterial persistence; (2) In mesenteric lymph node (MLN), APC could present arthri togenic peptides through HLA-B27 to CD8+ T cells, or HLA-B27 itself could be recognized through killer immunoglobulin receptor (KIR)3DL2 on CD4+ T cells. Moreover, HLA-B27 misfolding induces an unfolded protein response (UPR); (3-4) APC with non-active bacteria or with bacterial antigens, and T cells disseminate within pe- ripheral blood and eventually reach the joint; (5) In the target joint, gut derived APC and T cells induce immune response with IFN-γ and IL-17 production, recruitment of other cells and induction of mesenchymal cells activation, which enhance and sustain inflammation.  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 24 tected in the synovial fluid or tissue of patients with ReA. These facts demonstrate that the entire bacteria or their products traffic from the initial site of infection to the joint. On the other hand, persistence of Y. enterocolitica has been informed in peripheral blood up to 4 year after initial infection in patients with ReA [62], as well in dif- ferent organs in ReA rat models [63]. Furthermore, per- sistence of Salmonella enteritidis has been demonstrated in human epithelial cells after 14 days of in vitro infec- tion [64]. The impaired elimination of causative microbes plus the traffic of their antigens to the joint could be re- sponsible of pathological immune response in the joint [28]. However, the detailed mechanisms by which those antigens reach the joint and induce inflammation remain to be fully elucidated. An imbalance in the cytokine levels may be responsi- ble for persistence of causative microbes and also reflect the pathological immune response in the joint. In this way, diminished levels of TNF produced by peripheral blood mononuclear cells (PBMC) of patients with ReA [65] and elevated amounts of IL-10 have been demon- strated at the beginning of the disease [66]. However, in patients at chronic stage of arthritis, elevated amounts of TNF produced by PBMC and CD3+IFN-γ+ cells from blood and synovial fluid have been reported [66]. An- other authors observed in patients with ReA enriched amounts of CD4+IL-17+ cells within synovial fluid sup- porting the hypothesis that IL-17 could contribute to pathogenesis of ReA [67]. In line with these results, we detected higher IL-17 and IFN-γ levels in regional lymph nodes of TNFRp55−/− mice with Y. enterocolitica -in- du ced ReA and significantly increased number of CD4+IL- 17+ cells in these mice compared to their counterpart wild-type [68]. In addition, in this animal model we ob- served decreased amounts of IL-10 and Treg cells at ar- thritis onset (day 14 after infection) in contrast with chro- nic stage of arthritis [69]. These works and others advo- cate the idea that in ReA, a specific cellular immune re- sponse take place in the joint and chronic stimuli allows to the cells maintain the inflammatory process for long periods [70]. The first genetic factor described to be related to ReA and SpA in general, is the molecule HLA-B27. The role of HLA-B27 in SpA is not completely known and several hypotheses try to explain it. Since HLA-B27 is a class I histocompatibility molecule, it h as been postulated that it presents arthritogenic bacterial peptides to CD8+T cells, thus stimulating an autoimmune response (molecular mimicry) [71-73]. However, in HLA-B27/human β2-mi- croglobulin (hβ2-m) transgenic rats, two different ap- proaches demonstrated that CD8+T cells are not neces- sary for development of SpA-like phenotype. Further- more, the depletion of CD8+T cells through antibodies [74] or the elimination of CD8α protein expression by chemical mutation of CD8α gene [75] does not prevent disease in this SpA rodent model. It was found that heavy chains of HLA-B27 have a tendency to misfold forming homodimers and hetero d i me r s due to aberrant disulfide bound formation by unpaired Cys residues at position 67 [76]. HLA-B27 misfolding causes a stress response in the endoplasmic reticulum and the cell activates multiple signaling pathways that orchestrate what is known as the unfolded protein re- sponse (UPR). One consequence of UPR activation is the polarization of cell to responding to patter recognition receptors (PRR) agonists (TLR 4, 2 and 3) toward the production of greater amount of IL-23 over IL-12, which in turns provide a stimulus for Th17 survival and activa- tion in individual with permissive IL-23R polymorphism [77]. Additionally, the non-can onical forms of HLA-B27 antigen expressed on cell surface are plausible to be rec- ognized by killer immunoglobulin receptors (KIR) such as KIR3DL2 on CD4+ T cells, and then, trigger inflam- mation [1,21,78]. Other genetic factors (e.g. IL-23R, IL-1R2, TNFRS1, TRADD, etc.) has been associated with AS, PsA and IBD-SpA [79]; therefore, it is possible that these factors may also have significance in ReA since even individu- als negative for HLA-B27 also develop ReA following mucosal infection. 6. Treatment Since infections trigger ReA, the use of antib iotic therapy in this arthropathy has been proposed and it is possible when the trigger bacterium has been isolated. However, the use of antibiotics is controversial probably because several studies have been conducted enrolling patients with ReA caused by heterogeneous pathogens. Moreover, other studies have often employed antibiotic monother- apy that may be not effective in the aberrant forms of bacteria causing ReA. In contrast, a clinical trial in 2010 enrolled only p atients with blood or synovial tissu e posi- tive for Chlamydia detected by PCR [80]. In this study, the patients were randomized to receive doxycycline + rifampin, azithromycin + rifampin or placebo. After six months, 63% of the patients with combination antibiotic therapy versus 20% of placebo group had clinical im- provement as measured by swollen joint count [80]. Therefore, this was the first trial that provide evidence supporting antibiotic therapy efficacy in Chlamydia-in- duced ReA. The current treatment of ReA is based on rest, non- steroidal anti-inflammatory drugs (NSAIDs) [81]. In case of NSAID-resistance or active disease for more than 4 weeks, intra-articular injection of corticosteroids is rec- ommended in patients with mono or oligoarthritis [82,83]. Topical corticosteroids are useful for ReA extra-articular symptoms such as uveitis, circinate balanitis and kerato-  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 25 derma blennorrhagicum [84]. In chronic and severe ReA, disease-modifying antir- heumatic drugs (DMARDs) are recommended and the most used is sulfasalazine (SSZ) [81] which shows lim- ited effectiveness in patients with ReA acute episodes [85]. Another DMARD is methotrexate, which may be used as an alternativ e to SSZ in patients who are allergic or intolerant to SSZ or who do not respond to this drug [84]. The DMARDs treatment is an alternative to anti- TNF therapy and may delay the swi tch to biol ogic agents. The TNF antagonists such as infliximab, etanercept and adalimumab showed impressive short-term improve- ments in AS [86], however, data on the use of this anti- TNF therapy in ReA are limited [87-92]. A largest recent experience with TNF antagonists in ReA supported the safety and efficacy of these agents in refractory ReA [89]. In this study, 10 patients with ReA previously refractory to NSAIDs and DMARDs received anti-TNF therapy within a median of 6 months (range 2 - 12 months) be- tween the onset of ReA and the initiation of th e treatment. After a follow-up of 20.6 months, no severe adverse events, including severe infection, were observed. Anti- TNF therapy was rapidly effective in 9 patients (90%), as shown by the rapid effect on a visual analog scale pain score, tender joint count, swollen joint count, and ex- tra-articular manifestations. Only mild infections were documented, none of which were associated with the triggering infection [89]. Our findings in Yersinia-induced ReA in TNFRp55−/− mice demonstrated that, in the absence of TNF signaling, redundant pathways, particularly Th17 and Th1 effec- tor cells, may act in concert to sustain inflammation in bacterial induced ReA [68]. Recently, we reported that TNFRp55 modulates macrophage functions in response to Yersinia LPS stimulation suggesting an essential r egu la- tory role of TNF via TNFRp55 signaling [93]. Further- more, we have reported that this pathway controlled the induction and function of Treg cells through differential regulation of cytokine production [69]. Our data support the concept that TNFRp55 signaling may participate in the modulation of immune response in ReA, suggesting caution in the use of TNF blockers in cases of chronic ReA. Treatment switch to a second anti-TNF agent can be an effective strategy in AS. There is a need for more long-term studies to examine the longitudinal efficacy in SpA of the newer biological therapies such as golimu- mab, a fully human antib od y anti-TNF, an d ritux imab, an antibody that induces B cell d epletion [86]. Ustekinumab, an anti-p40 antibody blocking both IL-23 and IL-12 has demonstrated clinical efficacy in PsA [94]. Secukinumab, an anti-IL-17A antibody, has been used in a randomized controlled trial with short duration of follow-up for AS treatment showing good efficacy [86]. A trend towards improvement was also demonstrated for secukinumab in PsA [95]. Until now, these newer biological agents have not been used in patie nts with Re A. 7. ReA Prognosis ReA usually has a self-limiting course since the most patients recover fully in 2 to 6 months. However, 15% - 30% of patients may develop chronic disease (>6 months with clinical symptoms) [15]. The prognosis of enteric ReA is best known being frequ ent recurrent acute attacks in patients with ReA triggered by Salmonella, Shigella and Yersinia [1,15]. In a Finnish study at mean of 11 years after Salmonella-induced ReA, 8/50 (16%) devel- oped chronic SpA and 5 (12%) of these patients fulfilled the criteria of AS [96]. In a similar study in 85 patients with acute Yersinia-induced ReA, half to the patients showed peripheral joint symptoms and on e-third of them had radiologic evidence of sacroiliitis [97]. A 20-year follow up study found that 32/100 of patients with Shig- ella-induced ReA had AS [98]. Only HLA-B27 positive patients ReA developed recurrent or chronic symptoms [96]. Therefore, the prognosis is less favorable in patients who are HLA-B27 positive [28]. 8. Conclusion A gastrointestinal or urogenital infection may trigger ReA and genetic factors such as HLA-B27 which are associated with chronic and more severe arthritis. How- ever, the pathogenesis of this arthropathy is not com- pletely known. Therefore, there are no specific treatment for this disease. Anti-TNF therapy in ReA has been rec- ommended for refractory ReA suggesting an association with cytokine response and ReA development. Our ex- perimental evidence indicates caution in the use of TNF blockers in bacterial-triggered chronic arthritis. We be- lieve that further investigation in animal models should delineate the immunopathogenic mechanisms involved in ReA and contribute to more specific therapeutic inter- vention. 9. Sources of Funding This work was supported by grants from Agencia Na- cional de Promoción Científica y Tecnológica (PICT 2008-763; PICT 2011-0732), Universidad Nacional de San Luis (Project 0401), M.S.D.G. is member of the Scientific Career of National Council of Scientific and Technical Investigations; E. C. is National Council of Scientific and Technical Investigations fellow. REFERENCES [1] M. Dougados and D. Baeten, “Spondyloarthritis,” Lancet, Vol. 377, No. 9783, 2011, pp. 2127-2137.  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 26 http://dx.doi.org/10.1016/S0140-6736(11)60071-8 [2] D. Baeten, M. Breban, R. Lories, G. Schett and J. Sieper, “Are Spondylarthritides Related but Distinct Conditions or a Single Disease with a Heterogeneous Phenotype?” Arthritis & Rheumatism, Vol. 65, No. 1, 2013, pp. 12-20. http://dx.doi.org/10.1002/art.37829 [3] A. Van Tubergen and U. Weber, “Diagnosis and Classi- fication in Spondyloarthritis: Identifying a Chameleon,” Nature Reviews Rheumatology, Vol. 8, No. 5, 2012, pp. 253-261. http://dx.doi.org/10.1038/nrrheum.2012.33 [4] P. Ahvonen, K. Sievers and K. Aho, “Arthritis Associated with Yersinia enterocolitica Infection,” Acta Rheuma- tologica Scandinavica, Vol. 15, No. 3, 1969, pp. 232-253. [5] O. Laitenen, J. Tuuhea and P. Ahvonen, “Polyarthritis Associated with Yersinia enterocolitica Infection. Clini- cal Features and Laboratory Findings in Nine Cases with Severe Joint Symptoms,” Annals of the Rheumatic Dis- eases, Vol. 31, No. 1, 1972, pp. 34-39. http://dx.doi.org/10.1136/ard.31.1.34 [6] K. Aho, P. Ahvonen, T. Juvakoski, M. Kousa, M. Leiri- salo and O. Laitinen, “Immune Responses in Yersinia- Associated Reactive Arthritis,” Annals of the Rheumatic Diseases, Vol. 38, Suppl. 1, 1979, pp. 123-126. [7] C. J. Cox, K. E. Kempsell and J. S. Gaston, “Investigation of Infectious Agents Associated with Arthritis by Reverse Transcription PCR of Bacterial rRNA,” Arthritis Re- search & Therapy, Vol. 5, No. 1, 2003, pp. R1-R8. http://dx.doi.org/10.1186/ar602 [8] K. Granfors, S. Jalkanen, A. A. Lindberg, O. Maki-Ikola, R. Von Essen, R. Lahesmaa-Rantala, H. Isomaki, R. Saario, W. J. Arnold and A. Toivanen, “Salmonella Lipo- polysaccharide in Synovial Cells from Patients with Re- active Arthritis,” Lancet, Vol. 335, No. 8691, 1990, pp. 685-688. http://dx.doi.org/10.1016/0140-6736(90)90804-E [9] J. S. Hill Gaston, C. Cox and K. Granfors, “Clinical and Experimental Evidence for Persistent Yersinia Infection in Reactive Arthritis,” Arthritis & Rheumatism, Vol. 42, No. 10, 1999, pp. 2239-2242. http://dx.doi.org/10.1002/1529-0131(199910)42:10<2239 ::AID-ANR29>3.0.CO;2-L [10] R. Merilahti-Palo, K. O. Soderstrom, R. Lahesmaa-Ran- tala, K. Granfors and A. Toivanen, “Bacterial Antigens in Synovial Biopsy Specimens in Yersinia Triggered Reac- tive Arthritis,” Annals of the Rheumatic Diseases, Vol. 50, No. 2, 1991, pp. 87-90. http://dx.doi.org/10.1136/ard.50.2.87 [11] A. M. Beutler, J. A. Whittum-Hudson, R. Nanagara, H. R. Schumacher and A. P. Hudson, “Intracellular Location of Inapparently Infecting Chlamydia in Synovial Tissue from Patients with Reiter’s Syndrome,” Immunologic Research, Vol. 13, No. 2-3, 1994, pp. 163-171. http://dx.doi.org/10.1007/BF02918277 [12] H. C. Gerard, P. J. Branigan, H. R. Schumacher Jr. and A. P. Hudson, “Synovial Chlamydia trachomatis in Patients with Reactive Arthritis/Reiter’s Syndrome Are Viable but Show Aberrant Gene Expression,” The Journal of Rheu- matology, Vol. 25, No. 4, 1998, pp. 734-742. [13] J. M. Townes, “Reactive Arthritis after Enteric Infections in the United States: The Problem of Definition,” Clinical Infectious Diseases, Vol. 50, No. 2, 2010, pp. 247-254. http://dx.doi.org/10.1086/649540 [14] J. Braun, G. Kingsley, D. Van Der Heijde and J. Sieper, “On the Difficulties of Establishing a Consensus on the Definition of and Diagnostic Investigations for Reactive Arthritis. Results and Discussion of a Questionnaire Pre- pared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3-6, 1999,” The Journal of Rheumatology, Vol. 27, No. 9, 2000, pp. 2185-2192. [15] T. Hannu, “Reactive Arthritis,” Best Practice & Research Clinical Rheumatology, Vol. 25, No. 3, 2011, pp. 347- 357. http://dx.doi.org/10.1016/j.berh.2011.01.018 [16] T. K. Kvien, A. Glennas, K. Melby, K. Granfors, O. Andrup, B. Karstensen and J. E. Thoen, “Reactive Arthri- tis: Incidence, Triggering Agents and Clinical Presenta- tion,” The Journal of Rheumatology, Vol. 21, No. 1, 1994, pp. 115-122. [17] J. M. Townes, A. A. Deodhar, E. S. Laine, K. Smith, H. E. Krug, A. Barkhuizen, M. E. Thompson, P. R. Cieslak and J. Sobel, “Reactive Arthritis Following Culture-Con- firmed Infections with Bacterial Enteric Pathogens in Minnesota and Oregon: A Population-Based Study,” An- nals of the Rheumatic Diseases, Vol. 67, No. 12, 2008, pp. 1689-1696. http://dx.doi.org/10.1136/ard.2007.083451 [18] E. Collantes, P. Zarco, E. Munoz, X. Juanola, J. Mulero, J. L. Fernandez-Sueiro, J. C. Torre-Alonso, J. Gratacos, C. Gonzalez, E. Batlle, P. Fernandez, L. F. Linares, E. Brito and L. Carmona, “Disease Pattern of Spondyloarthro- pathies in Spain: Description of the First National Regis- try (REGISPONSER) Extended Report,” Rheumatology (Oxford), Vol. 46, No. 8, 2007, pp. 1309-1315. http://dx.doi.org/10.1093/rheumatology/kem084 [19] E. Buschiazzo, J. A. Maldonado-Cocco, P. Arturi, G. Ci- tera, A. Berman, A. Nitsche and O. L. Rillo, “Epidemiol- ogy of Spondyloarthritis in Argentina,” The American Journal of the Medical Sciences, Vol. 341, No. 4, 2011, pp. 289-292. http://dx.doi.org/10.1097/MAJ.0b013e31820f8cc3 [20] M. Vasala, S. Hallanvuo, P. Ruuska, R. Suokas, A. Siito- nen and M. Hakala, “High Frequency of Reactive Arthri- tis in Adults after Yersinia pseudotuberculosis O:1 Out- break Caused by Contaminated Grated Carrots,” Annals of the Rheumatic Diseases, 2013. http://dx.doi.org/10.1136/annrheumdis-2013-203431 [21] A. Mcmichael and P. Bowness, “HLA-B27: Natural Func- tion and Pathogenic Role in Spondyloarthritis,” Arthritis Research, Vol. 4, Suppl. 3, 2002, pp. S153-158. [22] A. Toivanen and P. Toivanen, “Reactive Arthritis,” Best Practice & Research Clinical Rheumatology, Vol. 18, No. 5, 2004, pp. 689-703. http://dx.doi.org/10.1016/j.berh.2004.05.008 [23] A. Chatzikyriakidou, P. V. Voulgari and A. A. Drosos, “What Is the Role of HLA-B27 in Spondyloarthropa- thies?” Autoimmunity Reviews, Vol. 10, No. 8, 2011, pp. 464-468. http://dx.doi.org/10.1016/j.autrev.2011.01.011 [24] G. P. Thomas and M. A. Brown, “Genetics and Genomics of Ankylosing Spondylitis,” Immunological Reviews, Vol. 233, No. 1, 2010, pp. 162-180.  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 27 http://dx.doi.org/10.1111/j.0105-2896.2009.00852.x [25] R. Bonfiglioli, R. A. Conde, P. D. Sampaio-Barros, P. Louzada-Junior, E. A. Donadi and M. B. Bertolo, “Fre- quency of HLA-B27 Alleles in Brazilian Patients with Psoriatic Arthritis,” Clinical Rheumatology, Vol. 27, No. 6, 2008, pp. 709-712. http://dx.doi.org/10.1007/s10067-007-0770-3 [26] A. Cipriani, S. Rivera, M. Hassanhi, G. Marquez, R. Hernandez, C. Villalobos and M. Montiel, “HLA-B27 Subtypes Determination in Patients with Ankylosing Spondylitis from Zulia, Venezuela,” Human Immunology, Vol. 64, No. 7, 2003, pp. 745-749. http://dx.doi.org/10.1016/S0198-8859(03)00085-5 [27] B. Martinez, L. Caraballo, M. Hernandez, R. Valle, M. Avila and A. Iglesias Gamarra, “HLA-B27 Subtypes in Patients with Ankylosing Spondylitis (as) in Colombia,” Revista de Investigación Clínica, Vol. 51, No. 4, 1999, pp. 221-226. [28] J. S. Hill Gaston and M. S. Lillicrap, “Arthritis Associ- ated with Enteric Infection,” Best Practice & Research Clinical Rheumatology, Vol. 17, No. 2, 2003, pp. 219- 239. http://dx.doi.org/10.1016/S1521-6942(02)00104-3 [29] V. Bellomio, A. Berman, R. Sueldo, M. J. Molina, A. Spindler, E. Lucero, H. Berman, A. Nitsche, C. Asnal, J. A. Maldonado Cocco, G. Citera, S. Paira, C. Sandoval, R. Wong, R. Gallo, O. Rillo, R. Chaparro, A. Alvarellos, J. A. Albiero, C. Graf, A. Zunino, C. G. Casado, C. B. Ro- meo, J. C. Barreira and E. Aroca Briones, “Respondia. Iberoamerican Spondyloarthritis Registry: Argentina,” Reumatología Clínica, Vol. 4, Suppl. 4, 2008, pp. S23-29. [30] S. Kobayashi and I. Kida, “Reactive Arthritis: Recent Advances and Clinical Manifestations,” Annals of Inter- nal Medicine, Vol. 44, No. 5, 2005, pp. 408-12. http://dx.doi.org/10.2169/internalmedicine.44.408 [31] M. Leirisalo-Repo, “Reactive Arthritis,” Scandinavian Journal of Rheumatology, Vol. 34, No. 4, 2005, pp. 251- 259. http://dx.doi.org/10.1080/03009740500202540 [32] W. F. Barth and K. Segal, “Reactive Arthritis (Reiter’s Syndrome),” American Family Physician, Vol. 60, No. 2, 1999, pp. 499-503, 507. [33] B. Kwiatkowska and A. Filipowicz-Sosnowska, “Reac- tive Arthritis,” Polskie Archiwum Medycyny Wewnętrznej, Vol. 119, No. 1-2, 2009, pp. 60-65. [34] T. Hannu, L. Mattila, H. Rautelin, P. Pelkonen, P. Lah- denne, A. Siitonen and M. Leirisalo-Repo, “Campylo- bacter-Triggered Reactive Arthritis: A Population-Based Study,” Rheumatology (Oxford), Vol. 41, No. 3, 2002, pp. 312-318. http://dx.doi.org/10.1093/rheumatology/41.3.312 [35] T. Rathod, A. Chandanwale, S. Chavan and M. Shah, “Po- lyarthritic, Symmetric Arthropathy in Reactive Arthritis,” Journal of Natural Science, Biology and Medicine, Vol. 2, No. 2, 2011, pp. 216-218. http://dx.doi.org/10.4103/0976-9668.92312 [36] M. Rudwaleit, D. Van Der Heijde, R. Landewe, J. Listing, N. Akkoc, J. Brandt, J. Braun, C. T. Chou, E. Collantes- Estevez, M. Dougados, F. Huang, J. Gu, M. A. Khan, Y. Kirazli, W. P. Maksymowych, H. Mielants, I. J. Sorensen, S. Ozgocmen, E. Roussou, R. Valle-Onate, U. Weber, J. Wei and J. Sieper, “The Development of Assessment of Spondyloarthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection,” Annals of the Rheumatic Diseases, Vol. 68, No. 6, 2009, pp. 777-783. http://dx.doi.org/10.1136/ard.2009.108233 [37] W. Taylor, D. Gladman, P. Helliwell, A. Marchesoni, P. Mease and H. Mielants, “Classification Criteria for Psori- atic Arthritis: Development of New Criteria from a Large International Study,” Arthritis & Rheumatism, Vol. 54, No. 8, 2006, pp. 2665-2673. http://dx.doi.org/10.1002/art.21972 [38] J. D. Carter and A. P. Hudson, “Reactive Arthritis: Clini- cal Aspects and Medical Management,” Rheumatic Dis- ease Clinics of North America, Vol. 35, No. 1, 2009, pp. 21-44. http://dx.doi.org/10.1016/j.rdc.2009.03.010 [39] I. B. Wu and R. A. Schwartz, “Reiter’s Syndrome: The Classic Triad and More,” Journal of the American Aca- demy of Dermatology, Vol. 59, No. 1, 2008, pp. 113-121. http://dx.doi.org/10.1016/j.jaad.2008.02.047 [40] B. M. Rosner, D. Werber, M. Hohle and K. Stark, “Cli- nical Aspects and Self-Reported Symptoms of Sequelae of Yersinia enterocolitica Infections in a Population- Based Study, Germany 2009-2010,” BMC Infectious Di- seases, Vol. 13, 2013, p. 236. [41] I. Colmegna, R. Cuchacovich and L. R. Espinoza, “HLA- B27-Associated Reactive Arthritis: Pathogenetic and Cli- nical Considerations,” Clinical Microbiology Reviews, Vol. 17, No. 2, 2004, pp. 348-369. http://dx.doi.org/10.1128/CMR.17.2.348-369.2004 [42] T. E. Feltkamp and J. H. Ringrose, “Acute Anterior Uvei- tis and Spondyloarthropathies,” Current Opinion in Rheu- matology, Vol. 10, No. 4, 1998, pp. 314-318. http://dx.doi.org/10.1097/00002281-199807000-00006 [43] M. Huhtinen, K. Laasila, K. Granfors, M. Puolakkainen, I. Seppala, L. Laasonen, H. Repo, A. Karma and M. Leirisalo- Repo, “Infectious Background of Patients with a History of Acute Anterior Uveitis,” Annals of the Rheumatic Dis- eases, Vol. 61, No. 11, 2002, pp. 1012-1016. http://dx.doi.org/10.1136/ard.61.11.1012 [44] D. Monnet, M. Breban, C. Hudry, M. Dougados and A. P. Brezin, “Ophthalmic Findings and Frequency of Extrao- cular Manifestations in Patients with HLA-B27 Uveitis: A Study of 175 Cases,” Ophthalmology, Vol. 111, No. 4, 2004, pp. 802-809. http://dx.doi.org/10.1016/j.ophtha.2003.07.011 [45] N. Kozeis, M. Trachana and S. Tyradellis, “Keratitis in Reactive Arthritis (Reiter Syndrome) in Childhood,” Cor- nea, Vol. 30, No. 8, 2011, pp. 924-925. http://dx.doi.org/10.1097/ICO.0b013e3182000916 [46] S. Kiss, E. Letko, S. Qamruddin, S. Baltatzis and C. S. Foster, “Long-Term Progression, Prognosis, and Treat- ment of Patients with Recurrent Ocular Manifestations of Reiter’s Syndrome,” Ophthalmol ogy, Vol. 110, No. 9, 2003, pp. 1764-1769. [47] L. Bergfeldt, “HLA B27-Associated Rheumatic Diseases with Severe Cardiac Bradyarrhythmias. Clinical Features and Prevalence in 223 Men with Permanent Pacemakers,”  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 28 American Journal of Medicine, Vol. 75, No. 2, 1983, pp. 210-215. http://dx.doi.org/10.1016/0002-9343(83)91193-2 [48] H. Nielsen, “Complete Heart Block in Reiter’s Syndrome,” Acta Cardiologica, Vol. 41, No. 6, 1986, pp. 451-455. [49] L. E. Brown, P. Forfia and J. A. Flynn, “Aortic Insuffi- ciency in a Patient with Reactive Arthritis: Case Report and Review of the Literature,” HSS Journal, Vol. 7, No. 2, 2011, pp. 187-189. http://dx.doi.org/10.1007/s11420-010-9184-x [50] C. Fendler, S. Laitko, H. Sorensen, C. Gripenberg-Lerche, A. Groh, J. Uksila, K. Granfors, J. Braun and J. Sieper, “Frequency of Triggering Bacteria in Patients with Reac- tive Arthritis and Undifferentiated Oligoarthritis and the Relative Importance of the Tests Used for Diagnosis,” Annals of the Rheumatic Diseases, Vol. 60, No. 4, 2001, pp. 337-343. http://dx.doi.org/10.1136/ard.60.4.337 [51] M. K. Söderlin, H. Kautiainen, M. Puolakkainen, K. Hed- man, M. Söderlund-Venermo, T. Skogh and M. Leirisalo- Repo, “Infections Preceding Early Arthritis in Southern Sweden: A Prospective Population-Based Study,” Jour- nal of Rheumatology, Vol. 30, No. 3, 2003, pp. 459-464. [52] M. G. Lacoste, H. Tamashiro, S. G. Correa, A. M. de Guz- mán and M. S. Di Genaro, “Correlation between Yersinia enterocolitica and Type I Collagen Reactivity in Patients with Arthropathies,” Rheumatology International, Vol. 27, No. 7, 2007, pp. 613-620. http://dx.doi.org/10.1007/s00296-006-0274-5 [53] J. Birnbaum, J. G. Bartlett and A. C. Gelber, “Clostridium difficile: An under-Recognized Cause of Reactive Arthri- tis?” Clinical Rheumatology, Vol. 27, No. 2, 2008, pp. 253-255. http://dx.doi.org/10.1007/s10067-007-0710-2 [54] P. Schiellerup, K. A. Krogfelt and H. Locht, “A Com- parison of Self-Reported Joint Symptoms Following In- fection with Different Enteric Pathogens: Effect of HLA- B27,” Journal of Rheumatology, Vol. 35, No. 3, 2008, pp. 480-487. [55] T. Hannu, M. Puolakkainen and M. Leirisalo-Repo, “Chla- mydia pneumoniae as a Triggering Infection in Reactive Arthritis,” Rheumatology, Vol. 38, No. 5, 1999, pp. 411- 414. [56] A. Rizzo, M. D. Domenico, C. R. Carratelli and R. Pao- lillo, “The Role of Chlamydia and Chlamydophila Infec- tions in Reactive Arthritis,” Internal Medicine, Vol. 51, No. 1, 2012, pp. 113-117. http://dx.doi.org/10.2169/internalmedicine.51.6228 [57] I. Galadari and H. Galadari, “Nonspecific Urethritis and Reactive Arthritis,” Clinics in Dermatology, Vol. 22, No. 6, 2004, pp. 469-475. http://dx.doi.org/10.1016/j.clindermatol.2004.07.010 [58] M. K. Soderlin, E. Alasaarela and M. Hakala, “Reactive Arthritis Induced by Clostridium difficile Enteritis as a Complication of Helicobacter pylori Eradication,” Clini- cal Rheumatology, Vol. 18, No. 4, 1999, pp. 337-338. http://dx.doi.org/10.1007/s100670050113 [59] D. W. Carlson and D. R. Finger, “Beaver Fever Arthri- tis,” Journal of Clinical Rheumatology, Vol. 10, No. 2, 2004, pp. 86-88. http://dx.doi.org/10.1097/01.rhu.0000120979.11380.16 [60] A. Sing, S. Bechtold, J. Heesemann, B. H. Belohradsky and H. Schmidt, “Reactive Arthritis Associated with Pro- longed Cryptosporidial Infection,” Journal of Infection, Vol. 47, No. 2, 2003, pp. 181-184. http://dx.doi.org/10.1016/S0163-4453(03)00035-5 [61] B. Tejera, D. Grados, M. Martinez-Morillo and S. Roure, “Reactive Arthritis Caused by Blastocystis hominis,” Reumatología Clinica, Vol. 8, No. 1, 2012, pp. 50-51. [62] K. Granfors, R. Merilahti-Palo, R. Luukkainen, T. Mot- tonen, R. Lahesmaa, P. Probst, E. Marker-Hermann and P. Toivanen, “Persistence of Yersinia Antigens in Peripheral Blood Cells from Patients with Yersinia enterocolitica O:3 Infection with or without Reactive Arthritis,” Arthritis & Rheumatism, Vol. 41, No. 5, 1998, pp. 855-862. http://dx.doi.org/10.1002/1529-0131(199805)41:5<855:: AID-ART12>3.0.CO;2-J [63] J. A. Curfs, J. G. Meis, H. L. Van Der Lee, J. Mulder, W. G. Kraak and J. A. Hoogkamp-Korstanje, “Persistent Yer- sinia enterocolitica Infection in Three Rat Strains,” Micro- bial Pathogenesis, Vol. 19, No. 1, 1995, pp. 57-63. http://dx.doi.org/10.1006/mpat.1995.0045 [64] M. Saarinen, L. J. Pelliniemi and K. Granfors, “Survival and Degradation of Salmonella enterica Serotype Enteri- tidis in Intestinal Epithelial Cells in Vitro,” Journal of Medical Microbiology, Vol. 45, No. 6, 1996, pp. 463-471. http://dx.doi.org/10.1099/00222615-45-6-463 [65] J. Braun, Z. Yin, I. Spiller, S. Siegert, M. Rudwaleit, L. Liu, A. Radbruch and J. Sieper, “Low Secretion of Tumor Necrosis Factor Alpha, but No Other Th1 or Th2 Cyto- kines, by Peripheral Blood Mononuclear Cells Correlates with Chronicity in Reactive Arthritis,” Arthritis & Rheu- matism, Vol. 42, No. 10, 1999, pp. 2039-2044. http://dx.doi.org/10.1002/1529-0131(199910)42:10<2039 ::AID-ANR3>3.0.CO;2-6 [66] I. Butrimiene, S. Jarmalaite, J. Ranceva, A. Venalis, L. Jasiuleviciute and A. Zvirbliene, “Different Cytokine Pro- files in Patients with Chronic and Acute Reactive Arthri- tis,” Rheumatology, Vol. 43, No. 10, 2004, pp. 1300-1304. http://dx.doi.org/10.1093/rheumatology/keh323 [67] H. Shen, J. C. Goodall and J. S. Gaston, “Frequency and Phenotype of T Helper 17 Cells in Peripheral Blood and Synovial Fluid of Patients with Reactive Arthritis,” Jour- nal of Rheumatology, Vol. 37, No. 10, 2010, pp. 2096- 2099. http://dx.doi.org/10.3899/jrheum.100146 [68] R. J. Eliçabe, E. Cargnelutti, M. I. Serer, P. W. Stege, S. R. Valdez, M. A. Toscano, G. A. Rabinovich and M. S. Di Genaro, “Lack of TNFR p55 Results in Heightened Expression of IFN-γ and Il-17 During the Development of Reactive Arthritis,” Journal of Immunology, Vol. 185, No. 7, 2010, pp. 4485-4495. http://dx.doi.org/10.4049/jimmunol.0902245 [69] E. Cargnelutti, J. L. Arias, S. R. Valdez, G. A. Rabino- vich and M. S. Di Genaro, “TNFR p55 Controls Regula- tory T Cell Responses in Yersinia-Induced Reactive Ar- thritis,” Immunology and Cell Biology, Vol. 91, No. 2, 2013, pp. 159-166. http://dx.doi.org/10.1038/icb.2012.65 [70] J. Sieper, J. Braun, P. Wu and G. Kingsley, “T Cells Are  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 29 Responsible for the Enhanced Synovial Cellular Immune Response to Triggering Antigen in Reactive Arthritis,” Clinical & Experimental Immunology, Vol. 91, No. 1, 1993, pp. 96-102. http://dx.doi.org/10.1111/j.1365-2249.1993.tb03361.x [71] C. Alvarez-Navarro, J. J. Cragnolini, H. G. Dos Santos, E. Barnea, A. Admon, A. Morreale and J. A. López De Cas- tro, “Novel HLA-B27-Restricted Epitopes from Chlamy- dia trachomatis Generated Upon Endogenous Processing of Bacterial Proteins Suggest a Role of Molecular Mimi- cry in Reactive Arthritis,” Journal of Biological Chemis- try, Vol. 288, No. 36, 2013, pp. 25810-25825. http://dx.doi.org/10.1074/jbc.M113.493247 [72] R. Benjamin and P. Parham, “Guilt by Association: HLA- B27 and Ankylosing Spondylitis,” Immunology Today, Vol. 11, No. 4, 1990, pp. 137-142. http://dx.doi.org/10.1016/0167-5699(90)90051-A [73] W. Kuon, H. G. Holzhutter, H. Appel, M. Grolms, S. Kollnberger, A. Traeder, P. Henklein, E. Weiss, A. Thiel, R. Lauster, P. Bowness, A. Radbruch, P. M. Kloetzel and J. Sieper, “Identification of HLA-B27-Restricted Peptides from the Chlamydia trachomatis Proteome with Possible Relevance to HLA-B27-Associated Diseases,” Journal of Immunology, Vol. 167, No. 8, 2001, pp. 4738-4746. [74] E. May, M. L. Dorris, N. Satumtira, I. Iqbal, M. I. Reh- man, E. Lightfoot and J. D. Taurog, “CD8 Alpha Beta T Cells Are Not Essential to the Pathogenesis of Arthritis or Colitis in HLA-B27 Transgenic Rats,” Journal of Immu- nology, Vol. 170, No. 2, 2003, pp. 1099-1105. [75] J. D. Taurog, M. L. Dorris, N. Satumtira, T. M. Tran, R. Sharma, R. Dressel, J. Van Den Brandt a nd H. M. Reich- ardt, “Spondylarthritis in HLA-B27/Human Beta2-Micro- globulin-Transgenic Rats Is Not Prevented by Lack of CD8,” Arthritis & Rheumatism, Vol. 60, No. 7, 2009, pp. 1977-1984. http://dx.doi.org/10.1002/art.24599 [76] M. A. Whelan and J. R. Archer, “Chemical Reactivity of an HLA-B27 Thiol Group,” European Journal of Immu- nology, Vol. 23, No. 12, 1993, pp. 3278-3285. http://dx.doi.org/10.1002/eji.1830231233 [77] R. A. Colbert, T. M. Tran and G. Layh-Schmitt, “HLA- B27 Misfolding and Ankylosing Spondylitis,” Molecular Immunology, Vol. 57, No. 1, 2014, pp. 44-51. http://dx.doi.org/10.1016/j.molimm.2013.07.013 [78] L. H. Boyle, J. C. Goodall, S. S. Opat and J. S. Gaston, “The Recognition of HLA-B27 by Human CD4(+) T Lymphocytes,” Journal of Immunology, Vol. 167, No. 5, 2001, pp. 2619-2624. [79] J. D. Reveille, “Genetics of Spondyloarthritis-Beyond the MHC,” Nature Reviews Rheumatology, Vol. 8, No. 5, 2012, pp. 296-304. http://dx.doi.org/10.1038/nrrheum.2012.41 [80] J. D. Carter, H. C. Gerard, J. A. Whittum-Hudson and A. P. Hudson, “Combination Antibiotics for the Treatment of Chlamydia-Induced Reactive Arthritis: Is a Cure in Sight?” International Journal of Clinical Rheumatology, Vol. 6, No. 3, 2011, pp. 333-345. http://dx.doi.org/10.2217/ijr.11.20 [81] J. Sieper, “Developments in Therapies for Spondyloar- thritis,” Nature Reviews Rheumatology, Vol. 8, No. 5, 2012, pp. 280-287. http://dx.doi.org/10.1038/nrrheum.2012.40 [82] A. Toivanen, “Managing Reactive Arthritis,” Rheumato- logy, Vol. 39, No. 2, 2000, pp. 117-119. http://dx.doi.org/10.1093/rheumatology/39.2.117 [83] M. Dougados, “Current Therapy for Seronegative Arth- ritides (Spondyloarthritis),” Bulletin of the NYU Hospital for Joint Diseases, Vol. 69, No. 3, 2011, pp. 250-252. [84] D. Flores, J. Marquez, M. Garza and L. R. Espinoza, “Reactive Arthritis: Newer Developments,” Rheumatic Disease Clinics of North America, Vol. 29, No. 1, 2003, pp. 37-59. http://dx.doi.org/10.1016/S0889-857X(02)00081-9 [85] D. O. Clegg, D. J. Reda, M. H. Weisman, J. J. Cush, F. B. Vasey, H. R. Schumacher Jr., E. Budiman-Mak, D. J. Balestra, W. D. Blackburn, G. W. Cannon, R. D. Inman, F. P. Alepa, E. Mejias, M. R. Cohen, R. Makkena, M. L. Mahowald, J. Higashida, S. L. Silverman, N. Parhami, J. Buxbaum, C. M. Haakenson, R. H. Ward, B. J. Manaster, R. J. Anderson, W. G. Henderson, et al., “Comparison of Sulfasalazine and Placebo in the Treatment of Reactive Arthritis (Reiter’s Syndrome). A Department of Veterans Affairs Cooperative Study,” Arthritis & Rheumatism, Vol. 39, No. 12, 1996, pp. 2021-2027. http://dx.doi.org/10.1002/art.1780391211 [86] L. Goh and A. Samanta, “Update on Biologic Therapies in Ankylosing Spondylitis: A Literature Review,” Inter- national Journal of Rheumatic Diseases, Vol. 15, No. 5, 2012, pp. 445-454. http://dx.doi.org/10.1111/j.1756-185X.2012.01765.x [87] S. D. Flagg, R. Meador, E. Hsia, T. Kitumnuaypong and H. R. Schumacher, “Decreased Pain and Synovial Inflam- mation after Etanercept Therapy in Patients with Reactive and Undifferentiated Arthritis: An Open-Label Trial,” Arthritis Care & Research, Vol. 53, No. 4, 2005, pp. 613- 617. http://dx.doi.org/10.1002/art.21323 [88] R. Meador, E. Hsia, T. Kitumnuaypong and H. R. Schu- macher, “TNF Involvement and Anti-TNF Therapy of Re- active and Unclassified Arthritis,” Clinical and Experi- mental Rheumatology, Vol. 20, No. 6, 2002, pp. S130- S134. [89] A. Meyer, E. Chatelus, D. Wendling, J. M. Berthelot, E. Dernis, E. Houvenagel, J. Morel, O. Richer, T. Schaever- beke, J. E. Gottenberg and J. Sibilia, “Safety and Efficacy of Anti-Tumor Necrosis Factor Alpha Therapy in Ten Pa- tients with Recent-Onset Refractory Reactive Arthritis,” Arthritis & Rheumatism, Vol. 63, No. 5, 2011, pp. 1274- 1280. http://dx.doi.org/10.1002/art.30272 [90] K. S. Oili, H. Niinisalo, T. Korpilahde and J. Virolainen, “Treatment of Reactive Arthritis with Infliximab,” Scan- dinavian Journal of Rheumatology, Vol. 32, No. 2, 2003, pp. 122-124. http://dx.doi.org/10.1080/03009740310000157 [91] M. Rihl, A. Klos, L. Kohler and J. G. Kuipers, “Infection and Musculoskeletal Conditions: Reactive Arthritis,” Best Practice & Research Clinical Rheumatology, Vol. 20, No. 6, 2006, pp. 1119-1137. http://dx.doi.org/10.1016/j.berh.2006.08.008 [92] M. D. Schafranski, “Infliximab for Reactive Arthritis Se- condary to Chlamydia trachomatis Infection,” Rheuma-  Reactive Arthritis: From Clinical Features to Pathogenesis Open Access IJCM 30 tology International, Vol. 30, No. 5, 2010, pp. 679-680. http://dx.doi.org/10.1007/s00296-009-0965-9 [93] R. J. Eliçabe, J. L. Arias, G. A. Rabinovich and M. S. Di Genaro, “TNFRp55 Modulates IL-6 and Nitric Oxide Re- sponses Following Yersinia Lipopolysaccharide Stimu- lation in Peritoneal Macrophages,” Immunobiology, Vol. 216, No. 12, 2011, pp. 1322-1330. http://dx.doi.org/10.1016/j.imbio.2011.05.009 [94] A. Gottlieb, A. Menter, A. Mendelsohn, Y. K. Shen, S. Li, C. Guzzo, S. Fretzin, R. Kunynetz and A. Kavanaugh, “Ustekinumab, a Human Interleukin 12/23 Monoclonal Antibody, for Psoriatic Arthritis: Randomised, Double- Blind, Placebo-Controlled, Crossover Trial,” Lancet, Vol. 373, No. 9664, 2009, pp. 633-640. http://dx.doi.org/10.1016/S0140-6736(09)60140-9 [95] I. B. Mcinnes, J. Sieper, J. Braun, P. Emery, D. Van Der Heijde, J. D. Isaacs, G. Dahmen, J. Wollenhaupt, H. Schulze-Koops, J. Kogan, S. Ma, M. M. Schumacher, A. P. Bertolino, W. Hueber and P. P. Tak, “Efficacy and Safety of Secukinumab, a Fully Human Anti-Interleukin- 17A Monoclonal Antibody, in Patients with Moderate- to-Severe Psoriatic Arthritis: A 24-Week, Randomised, Double-Blind, Placebo-Controlled, Phase II Proof-of-Con- cept Trial ,” Annals of the Rheumatic Diseases, 2013. [96] M. Leirisalo-Repo, P. Helenius, T. Hannu, A. Lehtinen, J. Kreula, M. Taavitsainen and S. Koskimies, “Long-Term Prognosis of Reactive Salmonella Arthritis,” Annals of the Rheumatic Diseases, Vol. 56, No. 9, 1997, pp. 516- 520. http://dx.doi.org/10.1136/ard.56.9.516 [97] M. Leirisalo-Repo and H. Suoranta, “Ten-Year Follow-up Study of Patients with Yersinia Arthritis,” Arthritis & Rheumatism, Vol. 31, No. 4, 1988, pp. 533-537. http://dx.doi.org/10.1002/art.1780310410 [98] E. Sairanen, I. Paronen and H. Mähönen, “Reiter’s Syn- drome: A Follow-up Study,” Acta Medica Scandinavica, Vol. 185, No. 1-6, 1969, pp. 57-63.
|