G. ELDAR ET AL.
OPEN ACCESS OJINM
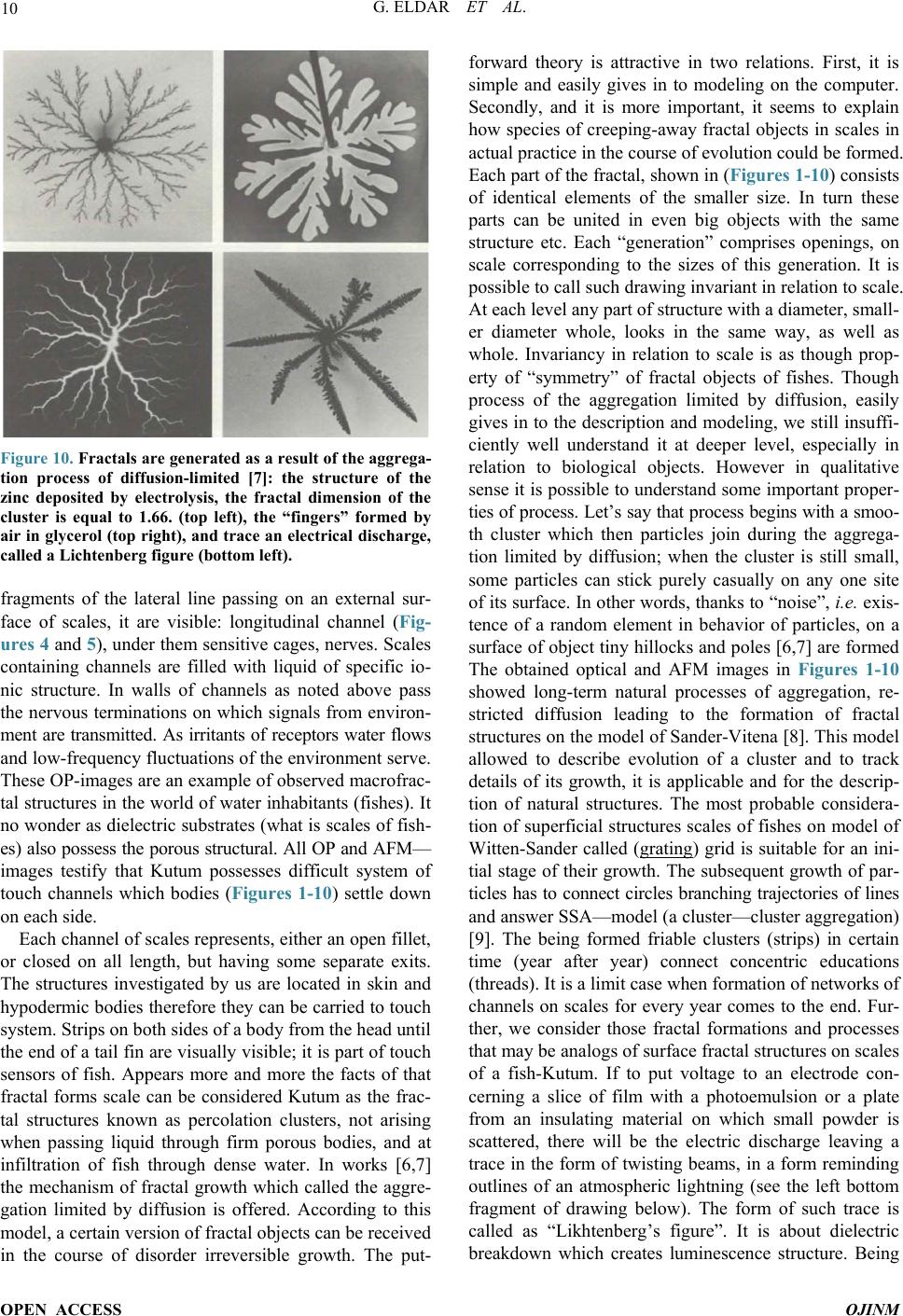
Figure 10. Fractals are generated as a result of the aggrega-
tion process of diffusion-limited [7]: the structure of the
zinc deposited by electrolysis, the fractal dimension of the
cluster is equal to 1.66. (top left), the “fingers” formed by
air in glycerol (top right), and trace an electrical discharge,
called a Lichtenberg figure (bottom left).
fragments of the lateral line passing on an external sur-
face of scales, it are visible: longitudinal channel (Fig-
ures 4 and 5), under them sensitive cages, nerves. Scales
containing channels are filled with liquid of specific io-
nic structure. In walls of channels as noted above pass
the nervous terminations on which signals from environ-
ment are transmitted. As irritants of receptors water flows
and low-frequency fluctuations of the environment serve.
These OP-images are an example of observed macrofrac-
tal structures in the world of water inhabitan ts (fishes). It
no wonder as dielectric substrates (what is scales of fish-
es) also possess the porous structural. All OP and AFM—
images testify that Kutum possesses difficult system of
touch channels which bodies (Figures 1-10) settle down
on each side.
Each channel of scales represents, either an open fillet,
or closed on all length, but having some separate exits.
The structures investigated by us are located in skin and
hypodermic bodies therefore they can be carried to touch
system. Strips on both sides of a body from the head until
the end of a tail fin are visually visib le; it is part of touch
sensors of fish. Appears more and more the facts of that
fractal forms scale can be considered Kutum as the frac-
tal structures known as percolation clusters, not arising
when passing liquid through firm porous bodies, and at
infiltration of fish through dense water. In works [6,7]
the mechanism of fractal growth which called the aggre-
gation limited by diffusion is offered. According to this
model, a certain version of fractal objects can be received
in the course of disorder irreversible growth. The put-
forward theory is attractive in two relations. First, it is
simple and easily gives in to modeling on the computer.
Secondly, and it is more important, it seems to explain
how species of creeping-away fractal objects in scales in
actual practice in the course of evolution could be formed.
Each part of the fractal, shown in (Figures 1-10) consists
of identical elements of the smaller size. In turn these
parts can be united in even big objects with the same
structure etc. Each “generation” comprises openings, on
scale corresponding to the sizes of this generation. It is
possible to call such drawing invariant in relation to scale.
At each level any part of structure with a diameter, small-
er diameter whole, looks in the same way, as well as
whole. Invariancy in relation to scale is as though prop-
erty of “symmetry” of fractal objects of fishes. Though
process of the aggregation limited by diffusion, easily
gives in to the description and modeling, we still insuffi-
ciently well understand it at deeper level, especially in
relation to biological objects. However in qualitative
sense it is possible to understand some important proper-
ties of process. Let’s say that process begins with a smoo-
th cluster which then particles join during the aggrega-
tion limited by diffusion; when the cluster is still small,
some particles can stick purely casually on any one site
of its surface. In other words, thanks to “noise”, i.e. exis-
tence of a random element in behavior of particles, on a
surface of object tiny hillocks and poles [6,7] are formed
The obtained optical and AFM images in Figures 1-10
showed long-term natural processes of aggregation, re-
stricted diffusion leading to the formation of fractal
structures on the model of Sander-Vitena [8]. This model
allowed to describe evolution of a cluster and to track
details of its growth, it is applicable and for the d escr ip-
tion of natural structures. The most probable considera-
tion of superficial structures scales of fishes on model of
Witten-Sander called (grating) grid is suitable for an ini-
tial stage of their growth. The subsequent growth of par-
ticles has to connect circles branching trajectories of lines
and answer SSA—model (a clu ster—cluster aggregation)
[9]. The being formed friable clusters (strips) in certain
time (year after year) connect concentric educations
(threads). It is a limit case when formation of networks of
channels on scales for every year comes to the end. Fur-
ther, we consider those fractal formations and processes
that may be analogs of surface fractal structures on scales
of a fish-Kutum. If to put voltage to an electrode con-
cerning a slice of film with a photoemulsion or a plate
from an insulating material on which small powder is
scattered, there will be the electric discharge leaving a
trace in the form of twisting beams, in a form reminding
outlines of an atmospheric lightning (see the left bottom
fragment of drawing below). The form of such trace is
called as “Likhtenberg’s figure”. It is about dielectric
breakdown which creates luminescence structure. Being