Paper Menu >>
Journal Menu >>
 Advances in Chemical Engineering and Science, 2011, 1, 9-14 doi:10.4236/aces.2011.11002 Published Online January 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Investigation of Photocatalytic Degradation of Methyl Orange by Using Nano-Sized ZnO Catalysts Changchun Chen1*, Jiangfeng Liu1, Ping Liu2, Benhai Yu1 1College of Physics and Electronics Engineering, XinYang Normal University, XinYang, China 2College of Materials Science and Engineering, Nanjing University of Techno logy, Nanjing, China E-mail: changchunchen@hotmail.com Received December 30, 2010; revised January 19, 2011; accepted January 22, 2011 Abstract Nano-sized ZnO catalysts were prepared by a direct precipitation method under the optimal conditions (cal- cination of precursors at 550˚C for 120 min). The as-synthesized ZnO catalysts were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM) and UV-Vis spectroscopy. The photocatalytic prop- erties of ZnO nanoparticles were investigated via methyl orange (MO) as a model organic compound under UV light irradiation. The influence of operating parameters on MO degradation including the amount of ZnO catalysts, pH value of solutions, and the photodegradation temperature was thoroughly examined. In addition, the kinetic process of photocatalytic degradation of MO using nano-sized ZnO catalyst was also examined, and the degradation of MO follow the first order kinetics. Keywords: ZnO Nanoparticles, Photocatalytic Degradtion, Methyl Orange 1. Introduction Semiconductor photocatalysts such as TiO2 and ZnO nano-particles have attracted much attention in recent years due to their various applications to the photocata- lytic degradation of organic pollutants in water and air and dye sensitized photovoltaic solar cell [1-3]. Among these semiconductor photocatalysts, TiO2 is the most commonly used owing to its stable, harmless and inex- pensive properties. However, two typical defects includ- ing only exciting by high energy UV irradiation and a low quantum yield rate resulted from a low rate of elec- tron transfer to oxygen and a high rate of recombination between excited electron/hole pairs, limit the photo- oxidation rate of TiO2 nanoparticles. In order to improve the photocatalytic efficiency of TiO2 nanoparticles, most studies have been focused on the modification of TiO2 doped by metal ions, especially transition metal ions, which make it possible for TiO2 to absorb visible light by increasing the charge separation [4,5]. In addition, com- bination of different kinds of semiconductor photocata- lysts also is a promising way to improve the photocata- lytic efficiency [6]. Recently, ZnO nanoparticles appear to be a suitable alternative to TiO2 nanoparticles used for the photodegradation of pesticide carbetamide [7], herbi- cide triclopyr [8], pulp milling bleaching wasterwater [9], 2-phenylphenol [10], phenol [11], reactive blue 19 [12], and acid red 14 [13]. The substitution of TiO2 by ZnO used for photo-degradation is ascribed to the photo-degradation mechanism of ZnO being similar to that of TiO2 [3,14]. K. Gouvea et al. has confirmed that ZnO exhibits a better efficiency than TiO2 in photocata- lytic degradation of some reactive dyes in aqueous solu- tion [15]. As we known, ZnO nanoparticles can be syn- thesized by various approaches including sol-gel proc- essing, homogeneous precipitation, mechanical milling, organometallic synthesis, microwave method, spray py- rolysis, thermal evaporation and mechanochemical syn- thesis. However, ZnO nanoparticles fabricated by the abovementioned methods are prone to aggregate due to the large surface area and high surface energy. In order to improve the dispersion, it is necessary to modify the sur- face of ZnO nanoparticles. Some researches have re- vealed several physical and chemical methods for modi- fying the surface of ZnO nanoparticles. The chemical surface modification, which can be classified as surface grafting and esterification, is the most promising method because of the strong covalent bond between the surface modified particles and polymer chains. In previous re- searches, the ZnO nanoparticles were ever modified by SiO2 [16], PMMA [17] and PSt [18], and the influence of particles on the mechanical properties of polymer matrix  C. C. CHEN ET AL. Copyright © 2011 SciRes. ACES 10 was studied. In the present article, the nano-sized ZnO catalysts were prepared by a direct precipitation method under the optimal conditions (calcination of precursors at 550˚C for 120 min). The surfaces of ZnO nanoparticles fabri- cated by a direct precipitation method are not modified by SiO2, PMMA and PSt. The effect of various experi- ment parameters such as the amount of ZnO catalyst, pH of solutions, the photodegradation temperature, and the initial concentration of MO on the degradation of the MO has been thoro ughly examined with an aim to qua n- titatively probin g the regulation of photocatalytic activity of ZnO nano-sized particles fabricated by a direct pre- cipitation method. 2. Experimental 2.1. Preparation and Characterization Nano-sized ZnO particles in this study were prepared by a direct precipitation method. Zn(NO3)2, (NH4)2CO3, ethanol and de-ionized water were used in the experi- ments. All the reagents used in this study were the ana- lytical grade. The synthetic procedures of nano-sized ZnO particles were also thoroughly introduced elsewh ere [19]. As is reported in our recent study [20], the nano- sized ZnO particles fabricated by a direct precipitation method via the calcination of precursors at 550˚C for 120 min have the optimal photocatalytic activity. As a result, the nano-sized ZnO particles synthesized by the calcination of precursor at 550˚C for 120 min were used as catalysts in this study. The specific surface area of nano-sized ZnO particles synthesized by the calcination of precursor at 550˚C for 120 min was determined by nitrogen absorption Brunauer-Emett-Teller (BET) method. The BET measurements were performed on a Micromer- itics ASAP 200 instrument. The 26.58 m2/g of BET spe- cific surface area was obtained. The structural properties of these nano-sized ZnO particles were investigated by the -2 method of X-ray diffraction (XRD) with a Cu K1 ( = 0.154 nm) radiation at 40 kV and 30mA using a multipurpose XRD system (PANalytical). The morphol- ogy and particle size of these nano-sized ZnO particles were also analyzed by a scanning electron microscope (SEM, JXA840). SEM photographs for the nano-sized ZnO particles were recorded (LEO 435) at 30 kV from samples covered with a thin gold film. 2.2. Photocatalytic Degradation The MO solutions in concentrations varied from 5 to 50 mg/L (5, 10, 20, 30, 40 and 50) were prepared through dissolving MO powders in ultra pure water, respectively. The concentration of MO solution was determined by measuring the value at approximately 464 nm using a UV-Vis spectrophotometer 756PC (China). The reaction suspensions were prepared by adding nano-sized ZnO particles into the abovementioned MO solutions. The suspensions were ultrasonically sonicated for 20 min and magnetically stirred in dark for 45 min to ensure an ad- sorption/desorption equilibrium. The reaction suspensions containing MO and nano-sized ZnO photocatalyst were irradiated by a 300 W high-pressure mercury lamp with continuous stirring. In addition, the pH of MO solution adjusted by adding NaOH or HCl solutions was measured using Elico LI120. Absorbance measurements were also recorded in the range of 200-600 nm, using a UV-vis spectrophotometer. The photocatalytic degradation effi- ciency of the MO solutions was calculated with the fol- lowing formula: 0 0 100 AA % A , where A0 is the absorbance of MO dye solution before the illumination, A is the absorbance of MO solutions in suspension after time t. 3. Results and Discussion The XRD pattern of ZnO nanoparticles synthesized via the calcination of precursor at 550˚C for 120 min is showed in Figure 1. It could be seen that the diffraction peaks were more inten siv e and narr ower imp lyin g a good crystalline nature of the as-synthesized ZnO product, and all of the peaks can be well indexed to hexagonal phase ZnO reported in JCPDS card (NO.36-1451, a = 0.3249 nm, c = 0.5206 nm). Diffraction peaks rel ated to the im purities were not observed in the XRD pattern, confirming the high purity of the synthesized product. The average crystalline size (L) of the nano-sized ZnO particles can be calculated from the Debye-Scherrer formula [21]: 20 30 40 50 60 70 0 1000 2000 3000 4000 5000 6000 7000 (201) (112) (200) Intensity/(a.u.) 2/(o) (100) (002) (101) (102) (110) (103) Figure 1. XRD patterns of nano-sized ZnO particles synthesized by a direct precipitation method with the calcination of precursor at 550˚C for 120 minutes. 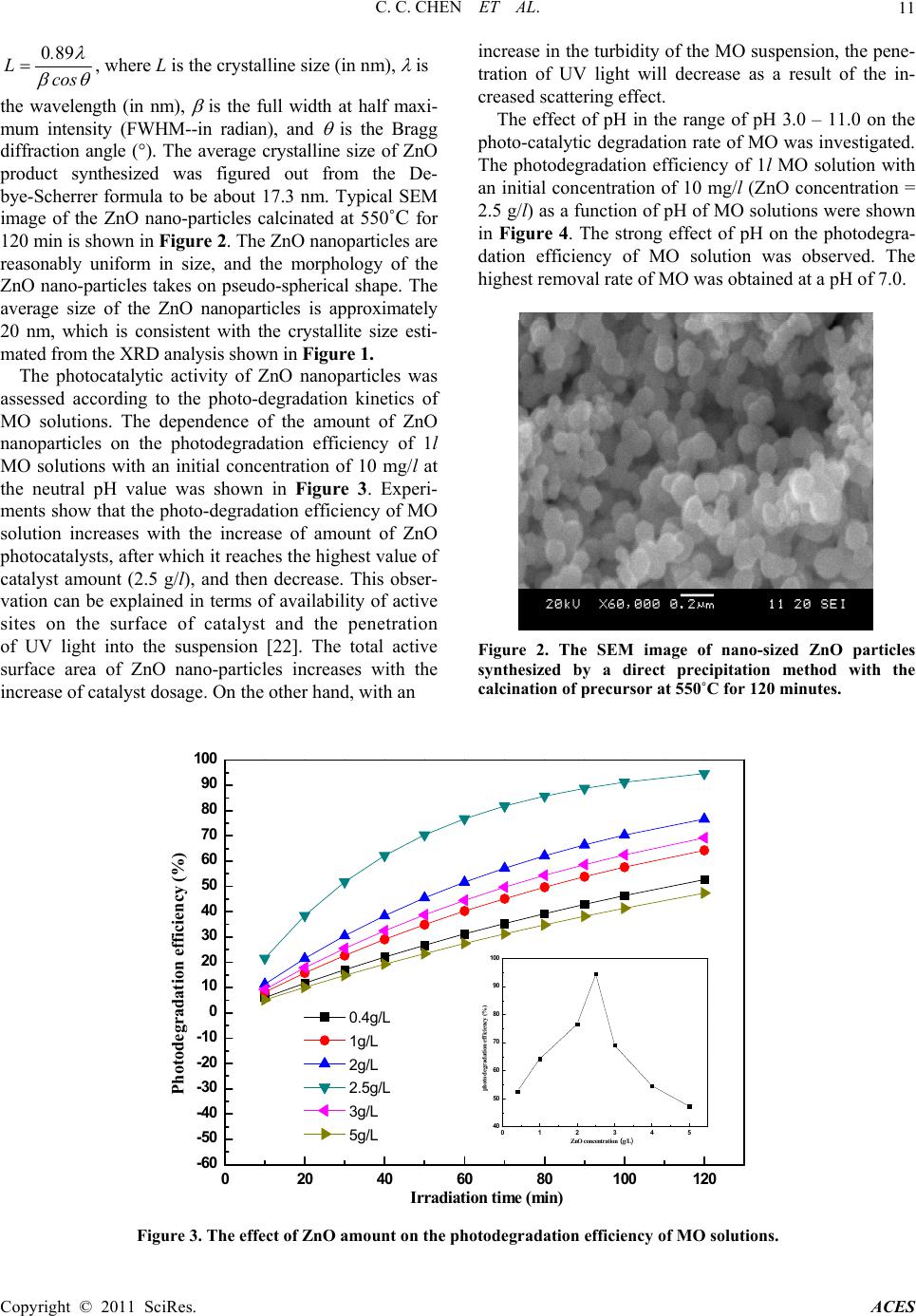 C. C. CHEN ET AL. Copyright © 2011 SciRes. ACES 11 089. Lcos , where L is the crystalline size (in nm), is the wavelength (in nm), is the full width at half maxi- mum intensity (FWHM--in radian), and is the Bragg diffraction angle (). The average crystalline size of ZnO product synthesized was figured out from the De- bye-Scherrer formula to be about 17.3 nm. Typical SEM image of the ZnO nano-particles calcinated at 550˚C for 120 min is shown in Figure 2. The ZnO nanoparticles are reasonably uniform in size, and the morphology of the ZnO nano-particles takes on pseudo-spherical shape. The average size of the ZnO nanoparticles is approximately 20 nm, which is consistent with the crystallite size esti- mated from t he XR D anal y si s shown in Figure 1. The photocatalytic activity of ZnO nanoparticles was assessed according to the photo-degradation kinetics of MO solutions. The dependence of the amount of ZnO nanoparticles on the photodegradation efficiency of 1l MO solutions with an initial concentration of 10 mg/l at the neutral pH value was shown in Figure 3. Experi- ments show that the photo-degradation efficiency of MO solution increases with the increase of amount of ZnO photocatalysts, after which it reaches the highest value of catalyst amount (2.5 g/l), and then decrease. This obser- vation can be explained in terms of availability of active sites on the surface of catalyst and the penetration of UV light into the suspension [22]. The total active surface area of ZnO nano-particles increases with the increase of catalyst dosage. On the other hand, with an increase in the turbidity of th e MO suspension, the pene- tration of UV light will decrease as a result of the in- creased scattering effect. The effect of pH in the range of pH 3.0 – 11.0 on the photo-catalytic degradation rate of MO was investigated. The photodegradation efficiency of 1l MO solution with an initial concentration of 10 mg/l (ZnO concentration = 2.5 g/l) as a function of pH of MO solutions were shown in Figure 4. The strong effect of pH on the photodegra- dation efficiency of MO solution was observed. The highest removal rate of MO was obtained at a pH of 7.0. Figure 2. The SEM image of nano-sized ZnO particles synthesized by a direct precipitation method with the calcination of precursor at 550˚C for 120 minutes. 0 20406080100120 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 60 70 80 90 100 012345 40 50 60 70 80 90 100 photodegradation efficiency (%) ZnO co ncentration (g/L) Photodegradation efficiency (%) Irradiation tim e (min) 0.4g /L 1g/L 2g/L 2.5g /L 3g/L 5g/L Figure 3. The effect of ZnO amount on the photodegradation efficiency of MO solutions.  C. C. CHEN ET AL. Copyright © 2011 SciRes. ACES 12 0 20406080100120 -30 -20 -10 0 10 20 30 40 50 60 70 80 90 100 24681012 65 70 75 80 85 90 95 100 Photod e gr adation efficien c y(%) pH of M O solution Photodegradation efficiency (%) Irradiation time (min) 3 5 6 7 9 11 Figure 4. Effect of pH on the photodegradation efficiency of methyl orange at different irradiation times. However, when TiO2 nanoparticles were utilized to catalyze the photo-degradation of MO, the higher re- moval rate of MO was obtained at lower pH values, which is reported in literature [23]. The effect of pH on the photodegradation of MO using ZnO catalysts can be explained as follows. As is pointed out by E. Topoglidis et al. [24], the point of zero charge (PZC) of nano-sized ZnO particle is about a pH of 9.30. Above the pH value, the surfaces of nano-sized ZnO particles are negatively charge. Below the pH value, the surfaces of nano-sized ZnO particles are positively charged. Methyl orange molecules have negative charges in a wide pH value range. Therefore, when the MO solution pH value is be- low the PZC, the MO anions should be readily adsorbed on the surfaces of nano-sized ZnO particles. As is described by H. Tian et al. [1], the photo- catalytic degradation efficiency of MO solutions with nano-sized TiO2 catalysts changes with the temperature variation of the MO solutions. Hence, the effect of MO solution temperature on the degradation efficiency of MO catalyzed by ZnO nano-particles was also discussed in the range from 20 to 70˚C at 10˚C intervals in this study. It can be seen in Figure 5 that at the first stage, the photo-degradation ratios of MO ascend with the in- crease of solution temperature from 20˚C to 50˚C, and begin to decrease at a temperature beyond 50˚C. How- ever, in many cases, the higher the temperature is, the quicker the chemical reaction rate does. The experimen- tal results shown in Figure 5 can be explained as follows. At an elevated temperature, the adsorbability of nano-sized ZnO particles to MO becomes low. The lower adsorbability 20 30 40 50 60 70 55 60 65 70 75 80 85 90 95 Photodegradation efficiency (%) photocatalytic degradation tmep eratu re ( 0C) Figure 5. Influence of solution temperature on photode- gradation efficiency of methyl orange( MO initial concen- tration of 10 mg/l, ZnO amount of 2.5 g/l, solution acidity of pH 7.0 and total volume of 1l. of MO will weaken the direct hole oxidation on the sur- face of nano-sized ZnO catalysts. The photocatalytic decomposition of MO organic pol- lutants on the surface of ZnO nano-particles also follow a pseudo first-order kinetic law, and can be expressed as 0 C ln kt C , where C and C0 are the reactant con- centration at time t = t and t = 0, respectively, k and t are the pseudo-first-order rate constant (reaction rate con- stant) and time, respectively [25]. The relationships be- tween –ln(C/C0) and irradiation time (Reaction time) are Photocatalytic degradation temperature (oC) 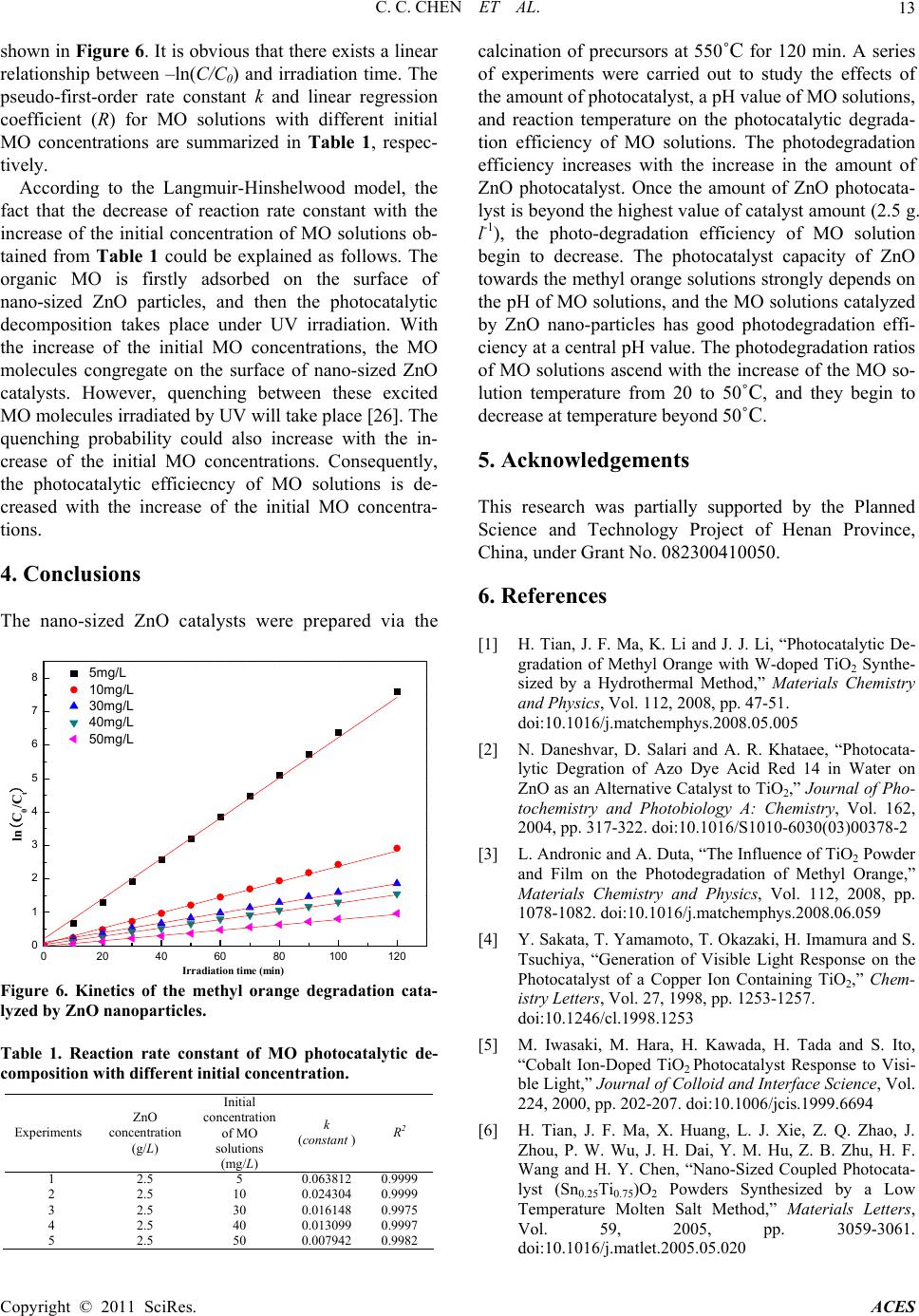 C. C. CHEN ET AL. Copyright © 2011 SciRes. ACES 13 shown in Figure 6. It is obviou s that there exists a linear relationship between –ln(C/C0) and irradiation time. The pseudo-first-order rate constant k and linear regression coefficient (R) for MO solutions with different initial MO concentrations are summarized in Table 1, respec- tively. According to the Langmuir-Hinshelwood model, the fact that the decrease of reaction rate constant with the increase of the initial concentration of MO solutions ob- tained from Table 1 could be explained as follows. The organic MO is firstly adsorbed on the surface of nano-sized ZnO particles, and then the photocatalytic decomposition takes place under UV irradiation. With the increase of the initial MO concentrations, the MO molecules congregate on the surface of nano-sized ZnO catalysts. However, quenching between these excited MO molecules irradiated by UV will take place [26]. The quenching probability could also increase with the in- crease of the initial MO concentrations. Consequently, the photocatalytic efficiecncy of MO solutions is de- creased with the increase of the initial MO concentra- tions. 4. Conclusions The nano-sized ZnO catalysts were prepared via the 0 20406080100120 0 1 2 3 4 5 6 7 85mg/L 10mg/L 30mg/L 40mg/L 50mg/L ln(C0/Ct) Irradiation time (min) Figure 6. Kinetics of the methyl orange degradation cata- lyzed by ZnO nanoparticles. Table 1. Reaction rate constant of MO photocatalytic de- composition with different initial concentration. Experiments ZnO concentration (g/L) Initial concentration of MO solutions (mg/L) k (constant )R2 1 2.5 5 0.063812 0.9999 2 2.5 10 0.024304 0.9999 3 2.5 30 0.016148 0.9975 4 2.5 40 0.013099 0.9997 5 2.5 50 0.007942 0.9982 calcination of precursors at 550˚C for 120 min. A series of experiments were carried out to study the effects of the amount of photocat al y st , a pH value of M O soluti o ns, and reaction temperature on the photocatalytic degrada- tion efficiency of MO solutions. The photodegradation efficiency increases with the increase in the amount of ZnO photocatalyst. Once the amount of ZnO photocata- lyst is beyond the highest value of catalyst amount (2.5 g. l-1), the photo-degradation efficiency of MO solution begin to decrease. The photocatalyst capacity of ZnO towards the methyl or ange solutions strongly depend s on the pH of MO solutions, and the MO solu tions catalyzed by ZnO nano-particles has good photodegradation effi- ciency at a central pH value. The photodegradation ratios of MO solutions ascend with the increase of the MO so- lution temperature from 20 to 50˚C, and they begin to decrease at temperature beyond 50˚C. 5. Acknowledgements This research was partially supported by the Planned Science and Technology Project of Henan Province, China, under Grant No. 082300410050. 6. References [1] H. Tian, J. F. Ma, K. Li and J. J. Li, “Photocatalytic De- gradation of Methyl Orange with W-doped TiO2 Synthe- sized by a Hydrothermal Method,” Materials Chemistry and Physics, Vol. 112, 2008, pp. 47-51. doi:10.1016/j.matchemphys.2008.05.005 [2] N. Daneshvar, D. Salari and A. R. Khataee, “Photocata- lytic Degration of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2,” Journal of Pho- tochemistry and Photobiology A: Chemistry, Vol. 162, 2004, pp. 317-322. doi:10.1016/S1010-6030(03)00378-2 [3] L. Andronic and A. Duta, “The Influence of TiO2 Powder and Film on the Photodegradation of Methyl Orange,” Materials Chemistry and Physics, Vol. 112, 2008, pp. 1078-1082. doi:10.1016/j.matchemphys.2008.06.059 [4] Y. Sakata, T. Yamamoto, T. Okazaki, H. Imamura and S. Tsuchiya, “Generation of Visible Light Response on the Photocatalyst of a Copper Ion Containing TiO2,” Chem- istry Letters, Vol. 27, 1998, pp. 1253-1257. doi:10.1246/cl.1998.1253 [5] M. Iwasaki, M. Hara, H. Kawada, H. Tada and S. Ito, “Cobalt Ion-Doped TiO2 Photocatalyst Response to Visi- ble Light,” Journal of Colloid and Interface Science, Vol. 224, 2000, pp. 202-207. doi:10.1006/jcis.1999.6694 [6] H. Tian, J. F. Ma, X. Huang, L. J. Xie, Z. Q. Zhao, J. Zhou, P. W. Wu, J. H. Dai, Y. M. Hu, Z. B. Zhu, H. F. Wang and H. Y. Chen, “Nano-Sized Coupled Photocata- lyst (Sn0.25Ti0.75)O2 Powders Synthesized by a Low Temperature Molten Salt Method,” Materials Letters, Vol. 59, 2005, pp. 3059-3061. doi:10.1016/j.matlet.2005.05.020  C. C. CHEN ET AL. Copyright © 2011 SciRes. ACES 14 [7] I. Poulios, M. Kositzi and A. Kouras, “Photocatalytic Decomposition of Trichlopyr over Aqueous Semicon- ductor Suspensions,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 115, 1998, pp. 175-179. doi:10.1016/S1010-6030(98)00259-7 [8] J. P. Percherancier, R. Chapelion and B. Pouyet, “Semi- conductor Sensitized Photodegradation of Pestcides in Water: The Case of Carbetamide,” Journal of Photo- chemistry and Photobiology A: Chemistry, Vol. 87, 1995, pp. 261-265. doi:10.1016/1010-6030(94)03993-5 [9] M. C. Yeber, J. Rodriguez, J. Freer, J. Baeza, N. Duran and H. D. Mansilla, “Advanced Oxidation of a Pulp Mill Bleaching Wastewater,” Chemosphere, Vol. 39, 1999, pp. 1679-1683. doi:10.1016/S0045-6535(99)00068-5 [10] A. A. Khodja, T. Sehili, J. F. Pilichowski and P. Boule, “Photocatalytic Degradation of 2-phenylphenol on TiO2 and ZnO in Aqueous Suspensions,” Journal of Photo- chemistry and Photobiology A: Chemistry, Vol. 141, 2001, pp. 231-236. doi:10.1016/S1010-6030(01)00423-3 [11] C. Marci, V. Augugliaro, M. J. L. Munoz, C. Martin, L. Palmisano, V. Rives, M. Sehhiavello, R. J. D. Tilley and A. M. Venezia, “Preparation Characterization and Photo- catalytic Activity of Polycrystalline ZnO/TiO2 Systems,” The Journal of Physical Chemistry B, Vol. 105, No. 5, 2001, pp. 1026-1032. doi:10.1021/jp003172r [12] C. Lizama, J. Freer, J. Baeza and H. D. Mansilla, “Opti- mized Photodegradation of Reactive Blue 19 on TiO2 and ZnO Suspensions,” Catalysis Today, Vol. 76, 2002, pp. 235-239. doi:10.1016/S0920-5861(02)00222-5 [13] N. Daneshvar, D. Salari and A. R. Khataee, “Photocata- lytic Degradation of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2,” Journal of Pho- tochemistry and Photobiology A: Chemistry, Vol. 162, 2004, pp. 317-322. doi:10.1016/S1010-6030(03)00378-2 [14] D. L. Liao, C. A. Badour and B. Q. Liao, “Preparation of Nano-Sized TiO2/ZnO Composite Catalyst and Its Photocatalytic Activity for Degradation of Methyl Or- ange,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 194, 2008, pp. 11-19. doi:10.1016/jjphotochem. 2007.07.008 [15] K. Gouvea, F. Wypych, S. G. Moraes, N. Duran, N. Na- gata and P. Peralta-Zamora, “Semiconductor-Assisted Photocatalytic Degradation of Reactive Dyes in Aqueous Solution,” Chemosphere, Vol. 40, 2000, pp. 433-440. doi: 10.1016/S0045-6535(99)00313-6 [16] R. Y. Hong, T. T. Pan, J. Z. Qian and H. Z. Li, “Synthesis and Surface Modification of ZnO Nanoparticles,” Chemical Engineering Journal, Vol. 119, 2006, pp.71-81. doi:10.1016/j.cej.2006.03.003 [17] R. Y. Hong, J. Z. Qian and J. X. Cao, “Synthesis and Characterization of PMMA Grafted ZnO Nanoparticles,” Powder Technology, Vol. 163, 2006, pp. 160-168. doi: 10.1016/j.powtec.2006.01.015 [18] R. Y. Hong, L. L. Chen, J. H. Li, H. Z. Li, Y. Zheng and J. Ding, “Preparation and Application of Polysty- rene-Grafted ZnO Nanoparticles,” Polymers for Ad- vanced Technologies, Vol. 18, No. 11, 2007, pp. 901-909. doi:10.1002/pat.926 [19] C. C. Chen, P. Liu and C. H. Lu, “Synthesis and Charac- terization of Nano-Sized ZnO Powders by Direct Pre- cipitation Method,” Chemical Engineering Journal, Vol. 144, 2008, pp. 509-513. doi:10.1016/j.cej.2008.07.047 [20] C. C. Chen, P. Liu, J. F. Liu and B. H. Yu, “The Investi- gation of Photocatalytic Activity of Nano-Sized ZnO Par- ticles Synthesized by a Direct Precipitation Method for Degradation of Methyl Orange,” (submitted to Journal of Photochemistry and Photobiology A: Chemistry). [21] C. Chen, B. Yu, J. Liu, Q. Dai, Y. Zhu, “Investigation of ZnO Films on Si(111) Substrate Grown by Low Energy O+ Assisted Pulse Laser Deposited Technology,” Materi- als Letters, Vol. 61, 2007, pp. 2961-2964. doi:10.1016 /j.matlet.2006.10.047 [22] M. S. T. Goncalves, A. M. F. Oliveira-Campos, E. M. M. S. Pinto, P. M. S. Plasencia and M. J. R. P. Queiroz, “Photochemical Treatment of Solutions of Azo Dyes Containing TiO2,” Chemosphere, Vol. 39, 1999, pp. 781-786. doi:10.1016/S0045-6535(99)00013-2 [23] W. Nam, J. Kim and G. Y. Han, “Photocatalytic Oxida- tion of Methul Orange in a Three-Phase Fluidized Bed Reactor,” Chemosphere, Vol. 47, 2002, pp. 1019-1024. doi:10.1016/S0045-6535(01)00327-7 [24] E. Topoglidis, A. E. G. Cass, B. O’Regan and J. R. Dur- rant, “Immobilisation and Bioelectrochemistry of Pro- teins on Nanoporous TiO2 and ZnO Films,” Journal of Electroanalytical Chemistry, Vol. 517, 2001, pp. 20-27. doi:10.1016/S0022-0728(01)00673-8 [25] A. Fujishima, T. N. Rao and D. A. Tryk, “Titanium Di- oxide Photocatalysis,” Journal of Photochemistry and Photobiology C: Photochemistry Reviews, Vol. 1, 2000, pp. 1-21. doi:10.1016/S1389-5567(00)00002-2 [26] A. V. Emeline, W. Ryabchuk and N. Serpone, “Factors Affecting the Efficiency of a Photocatalysed Process in Aqueous Metal-Oxide Dispersions, Prospect of Distin- guishing Between Two Kinetic Models,” Journal of Photobiology A: Chemistry, Vol. 133, 2000, pp. 89-97. doi:10.1016/S1010-6030(00)00225-2 |

