Paper Menu >>
Journal Menu >>
 Journal of Encapsulation and Adsorption Sciences, 2011, 1, 1-6 doi:10.4236/jeas.2011.11001 Published Online March 2011 (http://www.scirp.org/journal/jeas) Copyright © 2011 SciRes. JEAS Electron Confinement Effect of Laser Dyes within Dendritic Structures Francisco Márquez1,* María José Sabater2 1School of Science and Techn ol o gy , Turabo University, 00778PR, USA. 2Universidad P ol i técni c a de Val enci a -C .S.I.C., Av. Naranjos s/n, 46071-Valencia, Spain E-mail address: fmarquez@suagm.edu Received February 19, 2011; revised March 18, 2011; accepted March 25, 2011 Abstract Dendrimers are a novel class of nanometric-size macromolecules with a regular tree-dimensional like array of branch units [1,2]. Their synthetic availability in a wide range of sizes combined with their peculiar archi- tecture makes them versatile building blocks for a wide range of potential applications [3]. Some years ago, Meijer and co-workers reported that the modification of terminal amine functionalities of a fifth generation poly(propyleneimine) dendrimer (DAB-dendr-(NH2)64) with bulky substituents, (typically N-t-BOC pro- tected phenylalanine), results in the formation of the so-called “dendritic box” (DAB-dendr-(NH-t- BOC-L-Phe)64) [4]. Within this macromolecular structure it is possible to encapsulate a variety of guest mo- lecules due to the existence of internal cavities in the core. The photophysical properties of the guests can be modulated by the innovative electron confinement effect. In this respect, we wish to report that the emission frequency of organic dyes can be easily modulated by encapsulation in a dendritic box. The emission bands of dye molecules incorporated into a dendrimer can effectively be red shifted with respect to their emission in solution and contrary to other confined spaces of considerable hardness, the magnitude of this shifting can be regulated under appropriate experimental conditions. This peculiar effect could have unprecedented ap- plications in the development of supramolecular devices relating to the frequency tuning of organic laser dyes. Keywords: Dendrimer, Confinement, Dyes 1. Introduction The electron confinement effect, that has been experi- mentally observed in organic guests included within zeo- lite hosts, is based on the concept that the molecular or- bitals of the adsorbates inside the host cavities are not extended over all the space, as they are in the gas phase, but instead are forced to limit within a reduced and regu- lar space [5]. This confinement effect is stronger as the size of the confined guest approaches the cavity dimen- sions, producing an energy increase of all molecular or- bitals [6]. The HOMO has been predicted to be more sen- sitive that the LUMO, and the predicted effect is a reduc- tion of the HOMO-LUMO energy gap that is reflected in the optical properties of the guest, particularly the ba- thochromic shift of the fluorescence emission [6]. In principle, the polar or non-polar nature of the den- dritic shell can establish different interactions with sol- vents and this property is expected to alter the size of the dendritic box in a specific way. Thus, we envisaged that a chemical consequence of this is that solvation effects could lead to either an enlargement or a reduction of the original macromolecular dimensions by changing the solvent polarity. This would leave no alternative but to temporarily deform the size of the dendritic cavities in- side. As a consequence, guest molecules incorporated within the dendritic cavities might interact differently with the dendritic walls influenced by the solvent envi- ronment. When the dendritic cavity and the guest dimen- sions are closer, the interactions can be sufficiently im- portant as to experience strong repulsions. Such should be the case when solvents of opposite polarity to the dendritic system are employed. This stronger interaction may modify the HOMO-LUMO energy gap of the guest resulting in appreciable modifications of the photo- physical properties of the guest. This concept, which has been documentarily confirmed by theoretical predictions and recent experimental results, is a reflection of the con-  F. MARQUEZ ET AL Copyright © 2011 SciRes. JEAS 2 finement effect imposed by the host [5,6]. In order to test the validity of these assumptions, we studied the photophysics of several dye molecules in- corporated into a “dendritic box” of a fifth generation poly (propyleneimine) dendrimer (DAB-dendr-(NH-t- BOC-L-Phe)64). These experiments were conducted in solvents of pronounced different polarity and the photo- physical data were compared to those of dye molecules in neat solvents. 2. Experimental For the investigation presented here, three dye molecules (Bengal Rose 1, Rhodamine B 2 and Congo Red 3) were encapsulated in three different dendritic boxes con- structed from a fifth generation poly (propylene imine) dendrimer with 64 terminal amine groups [DAB- dendr-(NH-t-BOC-L-Phe)64] and a L-phenylalanine de- rivative. The dendritic box was synthesized according to a pre- viously reported procedure [4b]. Hence, in a typical pro- cedure 36 mg of Rose Bengal (3.5 x 10-5mol) or 24.5 mg of Red Congo (3.5 x 10-5 mol) and 16.9 mg of Rhoda- mine B (3.5 x 10-5 mol) was added to a solution of 50 mg of dendrimer DAB-dendr-(NH-t-BOC-L-Phe)64 (64 end amine groups) in 5 mL of CH2Cl2 with 1 mL of trie- thy- lamine and the solutions were stirred for 24 h at room temperature. Then 161 mg of N-tBOC-L-phenylalanine N-hydroxy-succinimide ester was added and the solu- tions were stirred overnight. Dilution to approx. 50ml with dichloromethane was followed by washing with water and saturated Na2CO3 solution. The organic layer was dried with MgSO4 and the solvent was evaporated under vacuum to afford the dye-doped dendrimer. In cases where the dyes were poorly soluble in water, the dendrimer was purified by dialysis until no traces of free dye were detected by HPLC. Dye-doped dendrimers were characterized by ma- trix-assisted laser desorption ionisation time-of-flight mass spectroscopy (MALDI-TOF-MS), 1H and 13C nu- clear magnetic resonance (NMR), ultraviolet and infrared spectroscopy and by cryo-Scanning Electron Micros- copy. 3. Results and Discussion The concentration of encapsulated guests was estimated by elemental analysis by determining the chlorine and sulfur content for compounds 1 and 2 respectively. The loading of compound 3 was estimated by UV/vis spec- troscopy. In all cases, the loading level of the dye incor- porated into the dendritic structure was ca. 3 dye mole- cules per dendrimer. The excitation and emission spectra of pure 1 in etha- nol were recorded at room temperature. The emission spectrum consisted of a sharp and featureless band around 574 nm and a small shoulder at ca. 620 nm. Sim- ilar spectra could be obtained when the spectra were reg- istered in the less polar solvents dichloromethane and n-hexane (see Figure 1). Interestingly, when the same dye molecule was en- capsulated within the dendrimer, a progressive red shift- ing of the band could be measured when the medium po- larity was reduced (from 8 nm in dichloromethane to 37 nm in n-hexane) (see Figure 2a). As for 1, common organic solvents such as ethanol, dichloromethane and n-hexane exerted hardly any no- ticeable influence on the emission spectra of pure dyes 2 and 3. Nevertheless, and as expected, the emission spec- tra of 2 and 3 when incorporated into the dendritic box underwent a remarkable red shifting (see Figure 2 b-c). The fluorescence band of the dye 2 incorporated into the dendritic structure displayed a maximum bathocromic shift of ca. 30 nm. However, the most spectacular red shift was observed in the case of the dye 3-doped den- drimer in which a red shift of ca. 200 nm was measured in n-hexane with respect to the emission spectrum re- corded in ethanol. The loading level of the dyes incorporated into the dendritic structures was similar in all cases and for this reason an explanation to these experimental results should be found in the increase of the spatial restrictions imposed to the dye, leading to an enhancement of the observed electron confinement effect. To rationalize these results it is necessary to consider that non polar solvents such as n-hexane can cause a dramatic shrink- age of the original size of the macromolecule. In addition, the non-polar character of the solvent may induce a much closer association of the dye-doped dendrimers or even its aggregation (Figure 3 displays a simulation of the dye 1-doped dendrimer indicating the possible equilibrium in polar and non-polar solvents). The combination of both phenomenons can paradigmatically lead to an effective reduction of the internal dendritic cavities where the guest molecules are located, hence inducing changes in the frontier orbitals of the dyes to an extent that will de- pend on the degree of interaction exerted by the branches of the macromolecule. This stronger confinement is re- flected by the notable reduction of the energy gap S0→S1* and consequently by a bathocromic shift of the emission spectra of the guests. Obviously, this effect is stronger when a host-guest tight fit occurs (a requirement that can be achieved when non-polar solvents as n-hexane are used). Finally, the room temperature fluorescence lifetimes for the dyes incorporated into the dendritic structures 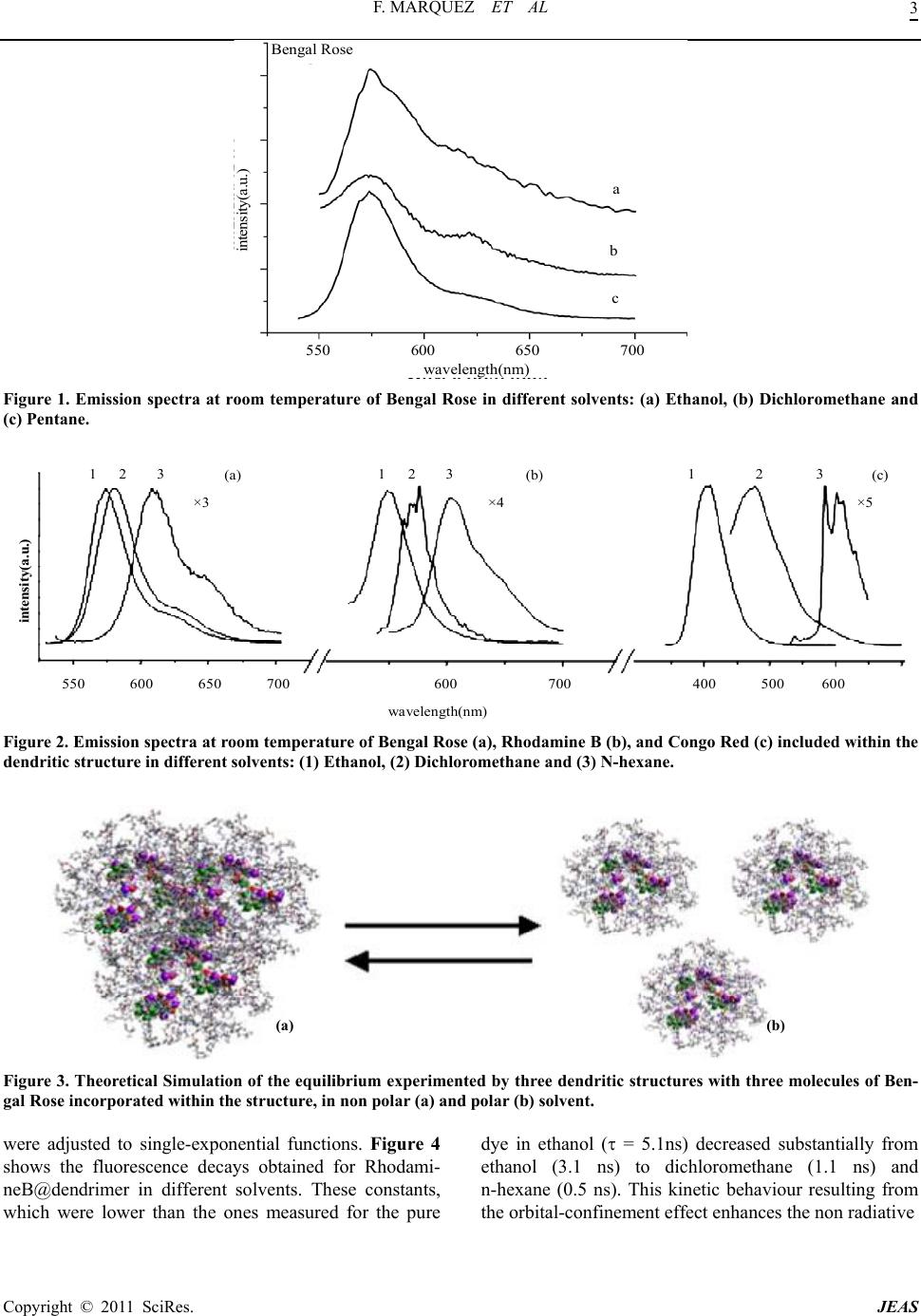 F. MARQUEZ ET AL Copyright © 2011 SciRes. JEAS 3 Bengal Rose intensity(a.u.) 550 600 650 700 wavelength(nm) a b c Figure 1. Emission spectra at room temperature of Bengal Rose in different solvents: (a) Ethanol, (b) Dichloromethane and (c) Pentane. wavelength(nm) 1 2 3 1 2 3 (c) ×5 550 600 650 700 600 700 400 500 600 1 2 3 (b) ×4 (a) ×3 intensity(a.u.) Figure 2. Emission spectra at room temperature of Bengal Rose (a), Rhodamine B (b), and Congo Red (c) included within the dendritic structure in different solvents: (1) Ethanol, (2) Dichloromethane and (3) N-hexane. (a) (b) Figure 3. Theoretical Simulation of the equilibrium experimented by three dendritic structures with three molecules of Ben- gal Rose incorporated within the structure, in non polar (a) and polar (b) solvent. were adjusted to single-exponential functions. Figure 4 shows the fluorescence decays obtained for Rhodami- neB@dendrimer in different solvents. These constants, which were lower than the ones measured for the pure dye in ethanol ( = 5.1ns) decreased substantially from ethanol (3.1 ns) to dichloromethane (1.1 ns) and n-hexane (0.5 ns). This kinetic behaviour resulting from the orbital-confinement effect enhances the non radiative 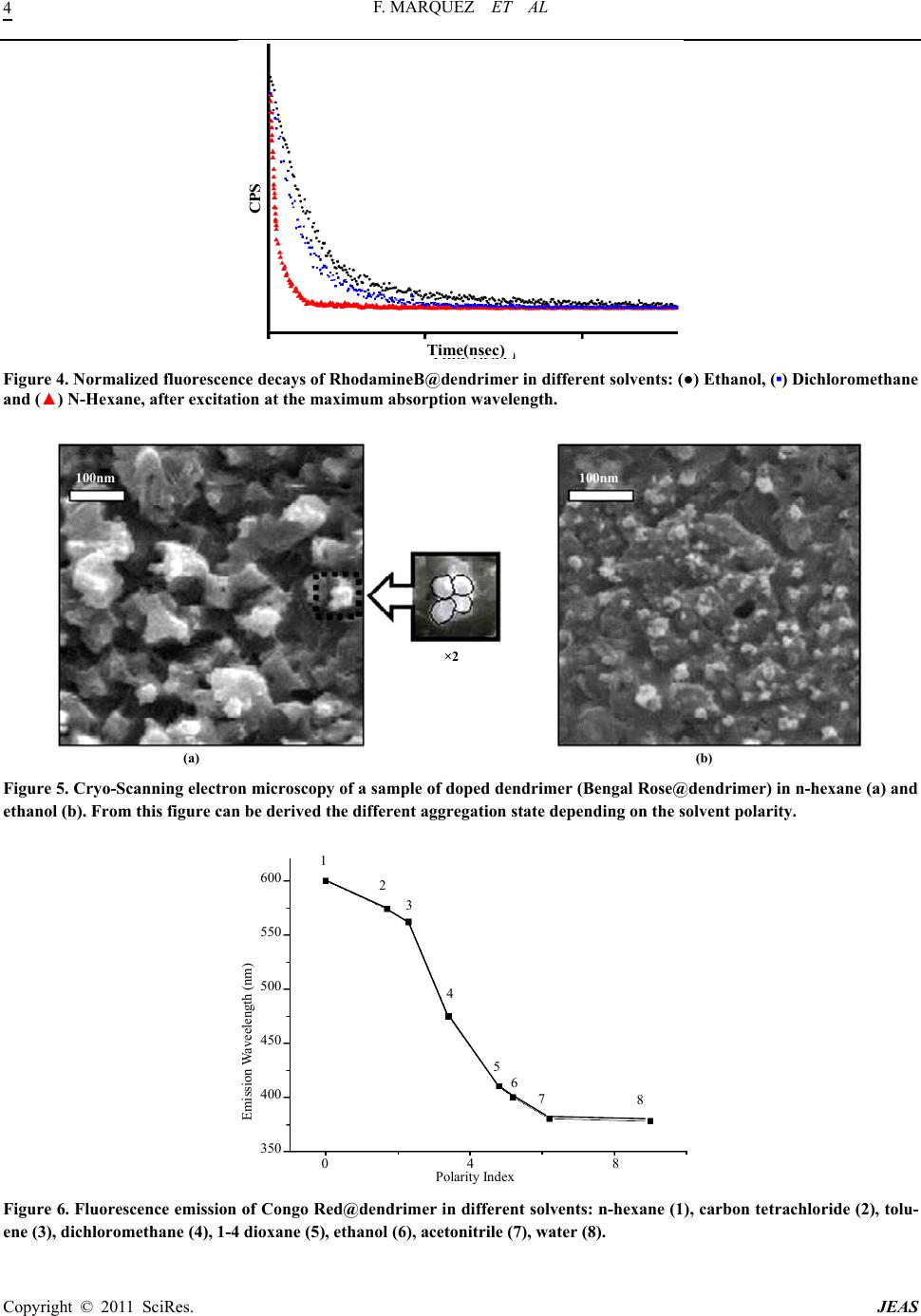 F. MARQUEZ ET AL Copyright © 2011 SciRes. JEAS 4 CPS Time ( nsec ) Figure 4. Normalized fluorescence decays of RhodamineB@dendrimer in different solvents: (●) Ethanol, (▪) Dichloromethane and (▲) N-Hexane, after excitation at the maximum absorption wavelength. ×2 (a) (b) 100nm 100nm Figure 5. Cryo-Scanning electron microscopy of a sample of doped dendrimer (Bengal Rose@dendrimer) in n-hexane (a) and ethanol (b). From this figure can be derived the different aggregation state depending on the solvent polarity. Emission Waveelength (nm) 600 550 500 450 400 350 0 4 8 Polarity Index 1 2 3 4 5 6 78 Figure 6. Fluorescence emission of Congo Red@dendrimer in different solvents: n-hexane (1), carbon tetrachloride (2), tolu- ene (3), dichloromethane (4), 1-4 dioxane (5), ethanol (6), acetonitrile (7), water (8).  F. MARQUEZ ET AL Copyright © 2011 SciRes. JEAS 5 deactivation pathway due to the closer proximity be- tween the lowest excited singlet states. Experimental evidence of this dynamic equilibrium observed in dendrimers exposed to different polarity environments has been obtained from cryo-SEM (cryo- scanning electron microscopy). Cryo-SEM was the method of choice for investigating the dendritic structures in different solvents because the samples were in solution. This technique can be a particularly useful method for studying new types and interaction modes among solvents and dendrimers. This study was performed on the dye 1-doped dendrimer in ethanol and n-hexane. Samples were cooled to -170˚C and subsequently analysed (see Supplementary Infor- mation for experimental details). Figure 5 displays the results obtained by cryo-SEM indicating that increas- ing the polarity (in going from n-hexane to ethanol) substantially modifies both the molecular dimension and aggregation state of the doped dendrimers. Other confined spaces that can affect the guest-host relationships similarly, in this case through the hardness of their walls, are the zeolite framework [6]. In fact, it has been reported that the photophysical properties of several probe molecules such as naphthalene or anthra- cene within pure silica zeolites are strongly affected by the zeolite host [6]. The magnitude of this shifting ap- pears in theory to be lower than the one attained in this case for our dye-doped dendrimer. Even more, the ab- sorption of a guest included in a dendritic box does not involve the disadvantage of light scattering typical of in- organic opaque solids, thus leading to much more effi- cient photochemical and photophysical processes. To sum up, the ability of the dendrimers to create con- fined microenvironments in determined experimental conditions can be taken as an easy way to modify the emission spectrum of dye molecules trapped in their cav- ities. This may have important implications from the technological point of view since dye-doped dendrimers can serve to enlarge the frequency range of laser dyes simply by choosing a solvent or a mixture of solvents of suitable polarity. What is more, the ability of these dye-doped dendrimers to modify the emission frequency range simply by changing the medium polarity may have interesting applications in the development of new su- pramolecular devices. This is clearly exemplified in Figure 6, where the fluorescence emission wavelength of the dye 3-doped dendrimer in different solvents is plotted. As can be seen there, the fluorescence emission is clearly correlated with the polarity of the solvents and this effect may only be justified as due to the confine- ment effect of the dye. 4. Acknowledgements Generous financial support from NASA (Grant NAG10- 335) is gratefully acknowledged. Financial support from US Department of Energy through the Massey Chair project at University of Turabo is also acknowledged. 5. References [1] J. M. J. Fréchet, “Functional Polymers and Dendrimers: Reactivity, Molecular Architecture, and Interfacial En- ergy,” Science, Vol. 263, 1994, pp. 1710-1715. doi:10.1126/science.8134834 [2] G. R. Newkome, C. N. Moorefield and F. Vögtle, “Den- dritic Molecules: Concepts, Synthesis, Perspectives; VCH: Weinheim, Germany, 1996. A. W. Bosman, H. M. Janssen and E. W. Meijer, “About Dendrimers: Structure, Physical Properties, and Applica- tions,” Chemical Reviews, Vol. 99, 1999, pp. 1665-1688. doi:10.1021/cr970069y D. A. Tomalia, “Starburst/Cascade Dendrimers: Funda- mental Building Blocks for a New Nanoscopic Chemistry Set,” Advanced Materials, Vol. 6, 1994, p. 529. doi:10.1002/adma.19940060703 H. F. Chow, T. K. K. Mong, M. F. Nongrum and C. W. Wan, “The Synthesis and Properties of Novel Functional Dendritic Molecules,” Tetrahedron, Vol. 54, 1998, pp. 8543-8660. doi:10.1016/S0040-4020(98)00409-8 S. M. Grayson and J. M. J. Fréchet, “Convergent Den- drons and Dendrimers: From Synthesis to Applications,” Chemical Reviews, Vol. 101, 2001, pp. 3819-3868. doi:10.1021/cr990116h [3] O. A. Matthews, A. N. Shipway and J. F. Stoddart, “Den- drimers—Branching out from Curiosities into New Tech- nologies,” Progress in Polymer Science, Vol. 23, No. 1, 1998, pp. 1-58. doi:10.1016/S0079-6700(97)00025-7 M. Fisher and F. Vögtle, “Dendrimers: From Design to Application—A Progress Report,” Angewandte Chemie International Edition, Vol. 38, No. 7, 1999, pp. 884-905. doi:10.1002/(SICI)1521-3773(19990401)38:7<884::AID- ANIE884>3.0.CO;2-K S. Hecht and J. M. J. Fréchet, “Dendritic Encapsulation of Function: Applying Nature’s Site Isolation Principle from Biomimetics to Materials Science,” Angewandte Chemie International Edition, Vol. 40, No. 1, 2001, pp. 74-91. doi:10.1002/1521-3773(20010105)40:1<74::AID-ANIE7 4>3.0.CO;2-C D. Astruc and F. Chardac, “Dendritic Catalysts and Den- drimers in Catalysis,” Chemical Reviews, Vol. 101, No. 9, 2001, pp. 2991-3024. doi:10.1021/cr010323t [4] E. M. M. de Brabander-van der Berg and E. W. Meijer, “Poly (propylene imine) Dendrimers: Large-Scale Syn- thesis by Hetereogeneously Catalyzed Hydrogenations,” Angewandte Chemie International Edition, Vol. 32, No. 9, 1993, pp. 1308-1311. doi:10.1002/anie.199313081 J. F. G. A. Jansen, E. M. M. de Brabander-van der Berg and E. W. Meijer, “Encapsulation of Guest Molecules into a Dendritic Box,” Science, Vol. 266, No. 5188, 1994, pp. 1226-1229. doi:10.1126/science.266.5188.1226 [5] C. M. Zicovich-Wilson, P. Viruela and A. Corma, “Elec-  F. MARQUEZ ET AL Copyright © 2011 SciRes. JEAS 6 tronic Confinement of Molecules in Microscopic Pores. A New Concept Which Contributes to the Explanation of the Catalytic Activity of Zeolites,” Journal of Physical Chemistry, Vol. 98, No. 42, 1994, pp. 10863-10870. doi:10.1021/j100093a030 [6] F. Márquez, C. M. Zicovich-Wilson, A. Corma, E. Palo- mares, H. García, “Naphthalene Included within All-Sil- ica Zeolites: Influence of the Host on the Naphthalene Photophysics,” Journal of Physical Chemistry, Vol. 105, No. 4, 2001, pp. 9973-9979. doi:10.1021/jp012095c F. Márquez, H. García, E. Palomares, A. Corma and L. Fernandez, “Spectroscopic Evidence in Support of the Molecular Orbital Confinement Concept: Case of An- thracene Incorporated in Zeolites,” Journal of American Chemical Society, Vol. 122, No. 27, 2000, pp. 6520-6521. doi:10.1021/ja0003066 F. Márquez, V. Martí, E. Palomares, A. García and W. Adam, Journal of American Chemical Society, Vol. 124, 2002, p. 7264 B. Menaa, M. Takahashi, Y. Tokuda and T. Yoko, Jour- nal of Photochemistry and Photobiology A: Chemistry, Vol. 194, 2008, p. 362. |

