 CellBio, 2013, 2, 248-256 Published Online December 2013 (http://www.scirp.org/journal/cellbio) http://dx.doi.org/10.4236/cellbio.2013.24027 Open Access CellBio Wound Closure on the Neonatal Rat Skin I. The Modulation of the Thickness of Epidermis at the Closing Incisional Wounds Mary Arai, Takashi Matsuzaki, Setsunosuke Ihara* Department of Biological Science, Faculty of Life and Environmental Science, Shimane University, Matsue, Japan Email: *ihara@life.shimane-u.ac.jp Received September 24, 2013; revised October 24, 2013; accepted November 1, 2013 Copyright © 2013 Mary Arai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Full-thickness incisional wounds were made on the dorsal skin of 1-day-old rats to elucidate the mechanism of the fluc- tuation of the epidermal thickness after the wound closure. The thickness of the epidermis covering the wound reached a peak around 96 h post-wounding (PW), and became thinner thereafter. The analyses of the cell proliferation and apoptosis at the epidermal wound regions revealed that the rate of TUNEL-positive cells that displays the cells under- going apoptosis increased as the epidermis became thinner around 120 h PW. Next, immunohistochemical analyses using antibodies against keratinocyte differentiation marker proteins indicated that the delay or interruption of the spinous to granular transition from 96 to 120 h PW might result in the epidermal thickening in the wound region. Third, the region undyed with anti-caspase-14 antibody extended downward in the thickened epidermis by 96 h PW, and in turn, it became intensely and widely stained with this antibody in the thinning epidermis by 120 h PW. Taken together, it is likely that the delay and acceleration of the terminal differentiation, including cornification of the epidermal kerati- nocytes may coordinately cause the fluctuation of the thickness of the epidermis at the wound site in rat neonates. Keywords: Wound Healing; Reepithelialization; Rat; Neonate; Epidermis; Terminal Differentiation; Cornification 1. Introduction Reepithelialization, a part of the process of the wound healing, is essential for organisms to survive. The pat- terns of reepithelialization are known to differ during the developmental stages [1-6]. First, the wounds in mam- malian adult skin are sealed with fibrin clot and then the wound closure is driven by epidermis crawling from the wound margin [7]. Meanwhile, the wounds in the neona- tal rat skin are covered rapidly with the extended wound- surrounding epidermis, and the abnormal expression pat- terns of keratins and cadherins at the wound site have been revealed by immunohistochemical analyses [6,8]. However, the late stages of reepithelialization after the wound closure have not yet been well investigated. In this study, we focused on the phenomenon that, once full- thickness incisional wounds made on the skin of neonatal rats were closed, the thickness of the covering epidermis increased and temporarily overran and, in turn, restored its normal level. First, we examined the possible fluctuation of the epi- dermal cell number at the wound site, and calculated the rate of the cell proliferation as well as that of the cell death detected by anti-BrdU immunostaining and TUNEL, respectively. Second, we analyzed the terminal differentiation of the epidermal keratinocytes. In order to identify the differentiation stage of the wound epidermis after the wound closure, the immunohistochemistry was performed using antibodies of involucrin, profilaggrin/ filaggrin, and loricrin as the epidermal differentiation markers [9-12]. Finally, we examined the distribution of CCAAT/enhancer-binding protein-α (C/EBP-α) and cas- pase-14. These two proteins are known to be endogenous factors regulating the terminal differentiation of the epi- dermal keratinocytes at the early or late stages, respec- tively [12-20]. The findings in the present study are summarized as follows. The delay or derangement in the terminal dif- ferentiation of keratinocyte occurs within the areas from the upper spinous to the granular layer in the thickening epidermis at the wound region. The thinning of the *Corresponding author.  M. ARAI ET AL. 249 wound epidermis may also be associated with the corni- fication probably accompanied by an enhancement of the cell death and the action of caspase-14. 2. Materials and Methods 2.1. Wounding Sprague-Dawley rats were anesthetized at 1 day after birth. Two incisional wounds 3 mm in length were made in the back skin on either side of the dorsal midline with a disposable scalpel (FEATHER). The wound sites were immediately covered with the cover agent Opsite (Smith & Nephew), and the wounded individuals were returned to the mother rats. The Opsite treatment had no effects on the wound healing except that it avoided unnecessary retardation of healing due to the infection that otherwise occasionally occurred (Koizumi et al., 2004). 2.2. BrdU Injection Two hours before sampling, 10 μl/gram body-weight 10 mM 5-bromo-2’-deoxyuridine (BrdU) were injected into the abdominal cavity of rats. 2.3. Histology and Immunohistochemistry The skin surrounding the wound was cut out at 0, 48, 72, 96, 120, or 144 h PW. Pieces of the skin were fixed with 4% paraformaldehyde in Ca2+, Mg2+-free phosphate-buff- ered saline [PBS(−)] for 1 h at room temperature, and embedded in paraffin. Sections (4 μm thickness) were stained with hematoxylin-eosin (HE) or analyzed immu- nohistochemically. For immunohistochemistry, deparaf- finized sections were pretreated with citric acid buffer (pH 6.0) at 95˚C, and rinsed with PBS(−). Alternatively, the sections were pretreated with 2N HCl, followed by digestion with 0.05% trypsin at 37˚C. The pretreated sections were incubated with 1% normal horse serum in PBS(−) for 20 min, and then reacted with primary anti- bodies at 4˚C overnight. They were washed with PBS(−), then incubated with secondary antibodies for 2 h. Sam- ples were washed with PBS(−), counterstained with 4’,6- diamino-2-phenylindole dihydrochloride (DAPI, Poly- sciences) at 0.1 μg/ml, and mounted with Fluoromount (Japan Tanner Corporation). The following primary anti- bodies were used: mouse anti-cytokeratin 14 monoclonal antibody (mAb) (Chemicon, 1:100); mouse anti-cy- tokeretin 10/13 mAb (Lab Vision, 1:100); mouse anti- cytokeratin 6 mAb (Progen, 1:50); rabbit anti-mouse involucrin poriclonal antibody (pAb) (Berkley antibody company, 1:500); rabbit anti-mouse loricrin pAb (abcam, 1:500); rabbit anti-rat profilaggrin/filaggrin pAb (466) (generously supplied by Dr. R. B. Presland, 1:250); and mouse anti-BrdU mAb (Dako Cytomation, 1:100). Alexa fluor 594 goat anti-mouse IgG1 (Molecular Probes, 1:1500); Alexa fluor 488 goat anti-mouse IgG2a (Mo- lecular Probes, 1:500); Alexa fluor 488 goat anti-mouse IgG1 (Molecular Probes, 1:500); and Alexa fluor 488 goat anti-rabbit IgG (Molecular Probes, 1:500) were used as secondary antibodies. The double staining of loricrin with involucrin and in- volucrin with filaggrin, and the staining of C/EBP-α and caspase 14 were performed using immunoenzyme tech- nique. Deparaffinized sections were treated with citric acid buffer (pH 6.0) at 95˚C or 10% TritonX-100 in PBS(−), and endogenous peroxidase was inactivated by H2O2 before blocking process. After incubation with HRP-conjugated anti-rabbit immunogrobulins (DakoCy- tomation, 1:100) as the secondary antibody, the samples were reacted with DAB for color reaction. The samples for the double staining were subjected to staining with the next primary antibodies, followed by staining the alkaline phosphatase-conjugated anti-rabbit immunogro- bulins (sigma-aldrich, 1:100) as the next secondary anti- body. The immunostained sections were finally counter- stained with hematoxylin. The following primary anti- bodies were used: anti-C/EBP-α rabbit pAb (Santa Cruz, 1:100); and anti-caspase 14 rabbit pAb (IMGENEX, 1:1000). 2.4. TUNEL The TUNEL reaction was performed using in situ cell death detection kit and TMR red (Roche Diagnotics) according to the manufacture’s instructions. 2.5. Numerical Applications of the Rate of BrdU and TUNEL Positive Cells The ratios of the numbers of BrdU- or TUNEL-positive cells to those of DAPI positive cells in the wound areas or the intact areas of epidermis were calculated. The epidermal regions between both wound edges and the normal interfollicular epidermal regions more than 5 mm away from the wound edges were defined as the wound areas and the intact areas of epidermis, respectively, in this study. 3. Results 3.1. Morphological and Immunohistochemical Changes in the Incisional Wound The standard technique using a scalpel No. 11 (FEATHER) in this study usually generated the full-thickness inci- sional wounds 1 mm in depth, as shown in Figure 1(A) (0 h PW). By 48 h PW, wound closure was completed by the epidermal sheets that crawled from the wound edge and contacted with each other at the wound center (Fig- ure 1(B)). From 72 to 120 h PW, the epidermis covering the wound was thickened downward the dermis as com- pared with the normal area of epidermis, and the thick- Open Access CellBio 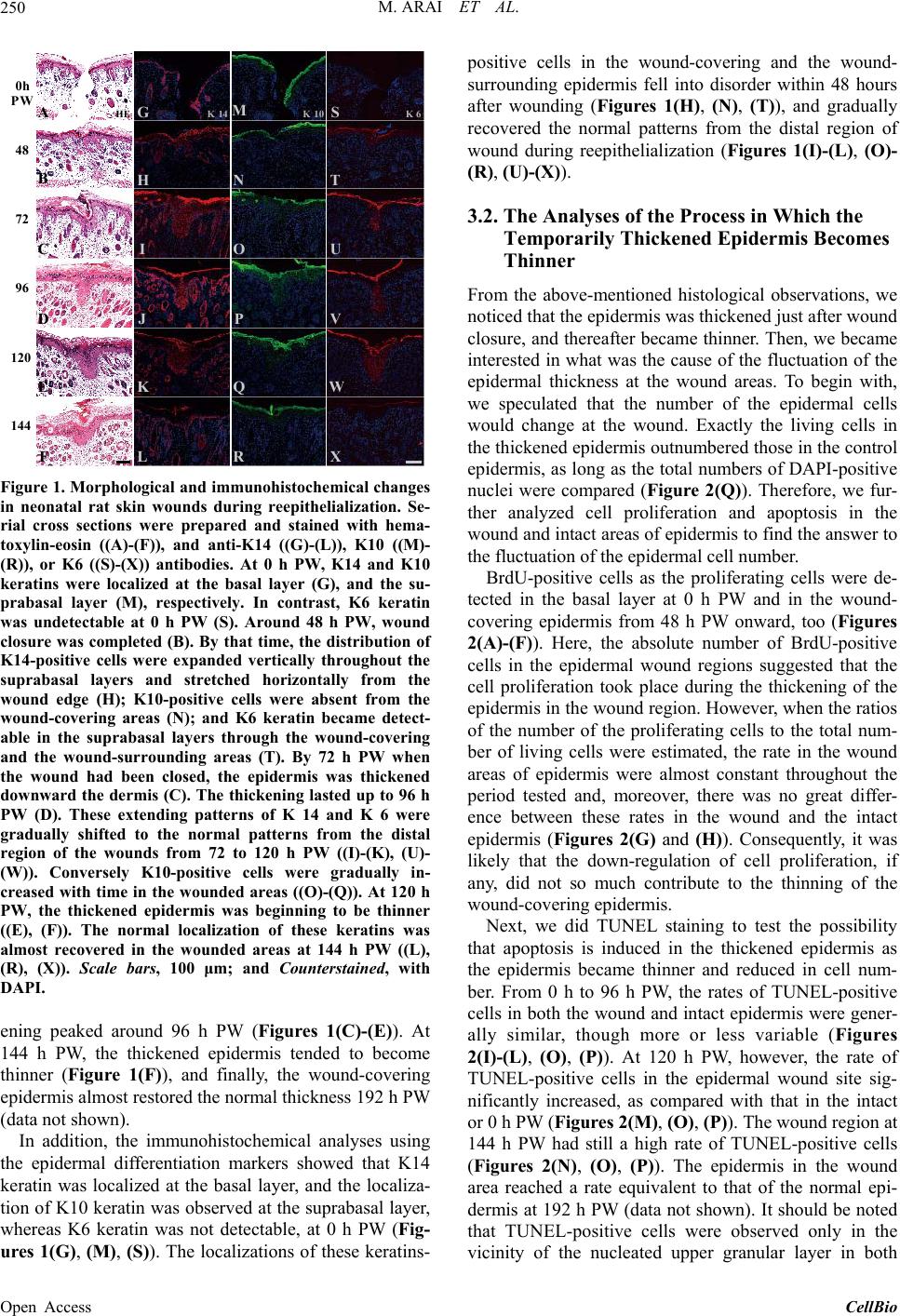 M. ARAI ET AL. 250 HE K 14K 10K 6 0h PW 48 72 96 120 144 A B C D E F G H I J K L M N O P Q R S T U V W X Figure 1. Morphological and immunohistochemical changes in neonatal rat skin wounds during reepithelialization. Se- rial cross sections were prepared and stained with hema- toxylin-eosin ((A)-(F)), and anti-K14 ((G)-(L)), K10 ((M)- (R)), or K6 ((S)-(X)) antibodies. At 0 h PW, K14 and K10 keratins were localized at the basal layer (G), and the su- prabasal layer (M), respectively. In contrast, K6 keratin was undetectable at 0 h PW (S). Around 48 h PW, wound closure was completed (B). By that time, the distribution of K14-positive cells were expanded vertically throughout the suprabasal layers and stretched horizontally from the wound edge (H); K10-positive cells were absent from the wound-covering areas (N); and K6 keratin became detect- able in the suprabasal layers through the wound-covering and the wound-surrounding areas (T). By 72 h PW when the wound had been closed, the epidermis was thickened downward the dermis (C). The thickening lasted up to 96 h PW (D). These extending patterns of K 14 and K 6 were gradually shifted to the normal patterns from the distal region of the wounds from 72 to 120 h PW ((I)-(K), (U)- (W)). Conversely K10-positive cells were gradually in- creased with time in the wounded areas ((O)-(Q)). At 120 h PW, the thickened epidermis was beginning to be thinner ((E), (F)). The normal localization of these keratins was almost recovered in the wounded areas at 144 h PW ((L), (R), (X)). Scale bars, 100 μm; and Counterstained, with DAPI. ening peaked around 96 h PW (Figures 1(C)-(E)). At 144 h PW, the thickened epidermis tended to become thinner (Figure 1(F)), and finally, the wound-covering epidermis almost restored the normal thickness 192 h PW (data not shown). In addition, the immunohistochemical analyses using the epidermal differentiation markers showed that K14 keratin was localized at the basal layer, and the localiza- tion of K10 keratin was observed at the suprabasal layer, whereas K6 keratin was not detectable, at 0 h PW (Fig- ures 1(G), (M), (S)). The localizations of these keratins- positive cells in the wound-covering and the wound- surrounding epidermis fell into disorder within 48 hours after wounding (Figures 1(H), (N), (T)), and gradually recovered the normal patterns from the distal region of wound during reepithelialization (Figures 1(I)-(L), (O)- (R), (U)-(X)). 3.2. The Analyses of the Process in Which the Temporarily Thickened Epidermis Becomes Thinner From the above-mentioned histological observations, we noticed that the epidermis was thickened just after wound closure, and thereafter became thinner. Then, we became interested in what was the cause of the fluctuation of the epidermal thickness at the wound areas. To begin with, we speculated that the number of the epidermal cells would change at the wound. Exactly the living cells in the thickened epidermis outnumbered those in the control epidermis, as long as the total numbers of DAPI-positive nuclei were compared (Figure 2(Q)). Therefore, we fur- ther analyzed cell proliferation and apoptosis in the wound and intact areas of epidermis to find the answer to the fluctuation of the epidermal cell number. BrdU-positive cells as the proliferating cells were de- tected in the basal layer at 0 h PW and in the wound- covering epidermis from 48 h PW onward, too (Figures 2(A)-(F)). Here, the absolute number of BrdU-positive cells in the epidermal wound regions suggested that the cell proliferation took place during the thickening of the epidermis in the wound region. However, when the ratios of the number of the proliferating cells to the total num- ber of living cells were estimated, the rate in the wound areas of epidermis were almost constant throughout the period tested and, moreover, there was no great differ- ence between these rates in the wound and the intact epidermis (Figures 2(G) and (H)). Consequently, it was likely that the down-regulation of cell proliferation, if any, did not so much contribute to the thinning of the wound-covering epidermis. Next, we did TUNEL staining to test the possibility that apoptosis is induced in the thickened epidermis as the epidermis became thinner and reduced in cell num- ber. From 0 h to 96 h PW, the rates of TUNEL-positive cells in both the wound and intact epidermis were gener- ally similar, though more or less variable (Figures 2(I)-(L), (O), (P)). At 120 h PW, however, the rate of TUNEL-positive cells in the epidermal wound site sig- nificantly increased, as compared with that in the intact or 0 h PW (Figures 2(M), (O), (P)). The wound region at 144 h PW had still a high rate of TUNEL-positive cells (Figures 2(N), (O), (P)). The epidermis in the wound area reached a rate equivalent to that of the normal epi- dermis at 192 h PW (data not shown). It should be noted that TUNEL-positive cells were observed only in the vicinity of the nucleated upper granular layer in both Open Access CellBio 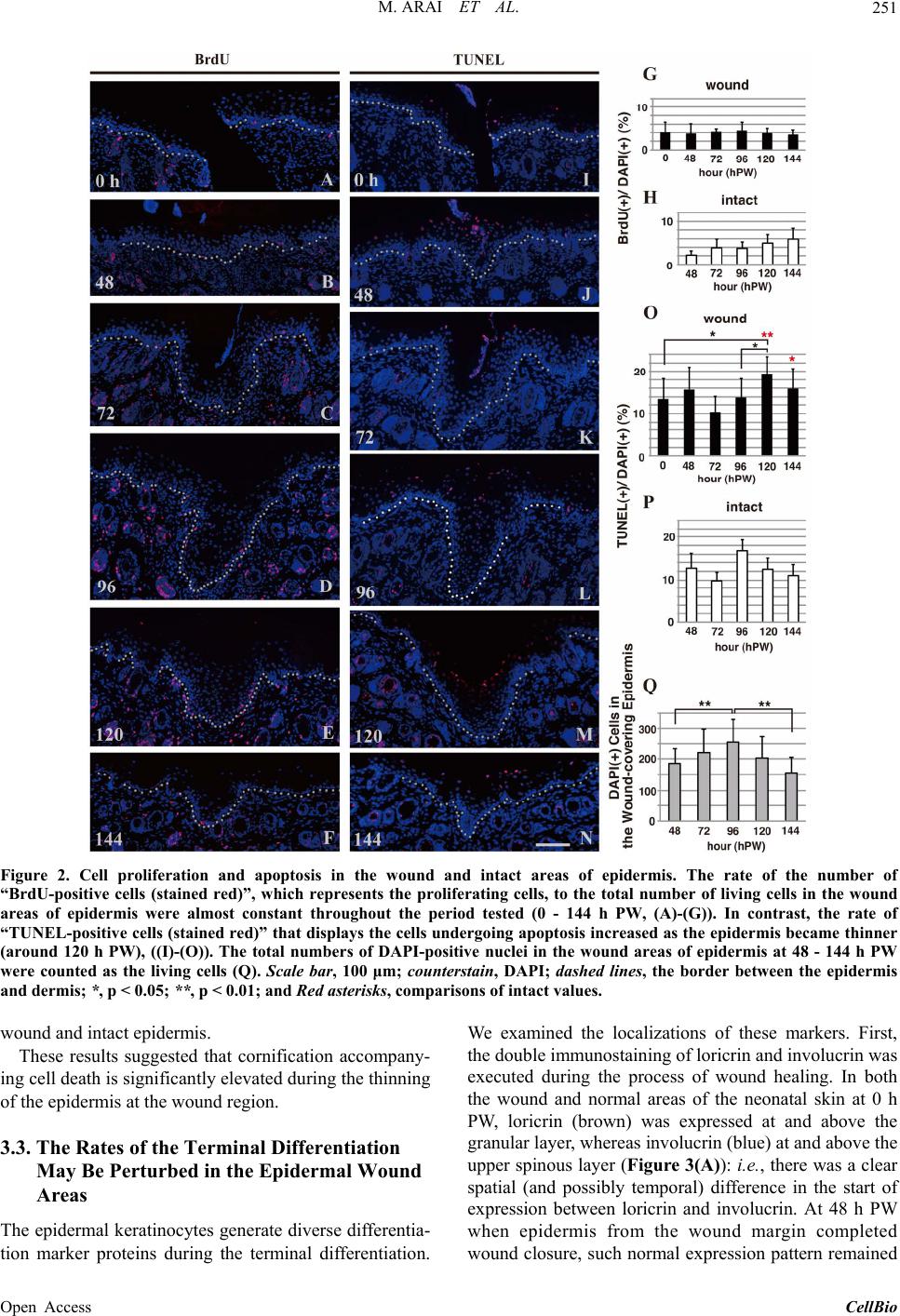 M. ARAI ET AL. Open Access CellBio 251 Figure 2. Cell proliferation and apoptosis in the wound and intact areas of epidermis. The rate of the number of “BrdU-positive cells (stained red)”, which represents the proliferating cells, to the total number of living cells in the wound areas of epidermis were almost constant throughout the period tested (0 - 144 h PW, (A)-(G)). In contrast, the rate of “TUNEL-positive cells (stained red)” that displays the cells undergoing apoptosis increased as the epidermis became thinner (around 120 h PW), ((I)-(O)). The total numbers of DAPI-positive nuclei in the wound areas of epidermis at 48 - 144 h PW were counted as the living cells (Q). Scale bar, 100 μm; counterstain, DAPI; dashed lines, the border between the epidermis and dermis; *, p < 0.05; **, p < 0.01; and Red asterisks, comparisons of intact values. We examined the localizations of these markers. First, the double immunostaining of loricrin and involucrin was executed during the process of wound healing. In both the wound and normal areas of the neonatal skin at 0 h PW, loricrin (brown) was expressed at and above the granular layer, whereas involucrin (blue) at and above the upper spinous layer (Figure 3(A)): i.e., there was a clear spatial (and possibly temporal) difference in the start of expression between loricrin and involucrin. At 48 h PW when epidermis from the wound margin completed wound closure, such normal expression pattern remained wound and intact epidermis. These results suggested that cornification accompany- ing cell death is significantly elevated during the thinning of the epidermis at the wound region. 3.3. The Rates of the Terminal Differentiation May Be Perturbed in the Epidermal Wound Areas The epidermal keratinocytes generate diverse differentia- tion marker proteins during the terminal differentiation.  M. ARAI ET AL. 252 FED CB wintactwoundint int w int wintwint w Lor Inv 0hPW 48 72 96120 144 Lor Inv LorInv Lor InvLor InvLorInv w Figure 3. The loricrin-positive lowermost epidermal layer became identical to the involucrin-positive one from the middle of reepithelialization. The upper and middle panels (the magnifications of wound (left) and intact (right) areas) show the expression of anti-loricrin (brown) and involucrin (blue) detected by double staining using the enzyme-labeled antibodies, and the lower panels show these proteins sin- gle-stained with fluorescence-labeled antibodies (green) in the adjacent sections. Loricrin (brown) was expressed at and above the granular layer, and involucrin (blue) ap- peared from the upper spinous layer in the neonatal skin at 0 h PW (A). It should be noted that these two proteins were apt to be stained from the same layer at 96 and 120 h PW in the epidermal thickening regions ((D) and (E) wound). Scale bars, 100 μm, 10 μm, and 100 μm from above; counterstain, hematoxylin and DAPI; and dashed lines, the border be- tween the epidermis and dermis. at the wound-covering epidermis (Figure 3(B)). On the contrary, at 96 and 120 h PW when the epidermis at the wound areas was thickened, these two proteins were ex- pressed from the identical cell layer in the epidermal thickening regions (Figures 3(D) and (E)), although it remained unclear which protein sifted. Later, the epider- mal wound area getting thinner inchmeal recovered the normal expression pattern (144 h PW, Figure 3(F)). Next, we carried out the double immunostaining of the epidermis of the neonates using the anti-involucrin and anti-profilaggrin/filaggrin antibodies. In both the wound and normal areas at 0 h PW, the expression of profilag- grin/filaggrin (blue) was observed at the zone lower than involucrin (brown), concretely, at and above the middle spinous cell layer (Figure 4(A)). In contrast, unlike the relationship of expression of loricrin and involucrin, the spatial difference in the expression of involucrin and pro- filaggrin/filaggrin were maintained in the same way as in the normal areas in the same thickening epidermis spe- cimens. Such an expression pattern remained unchanged by the thinning phase of the wound-covering epidermis (Figures 4(B)-(F)). FED C wintactwoundint int w int wintwint w Inv Fil 0hPW 48 72 96120 144 InvFil Inv Fil Inv FilInvFilInv Fil w Figure 4. The expression patterns of filaggrin remained unchanged during reepithelialization. The results of double staining of involucrin (brown) and filaggrin (blue) and sin- gle staining in the adjacent sections (green) are arranged in the same way as in Figure 3. Unlike the expression of in- volucrin (brown) that started from upper spinous layer, filaggrin (blue) was expressed at and above the middle spinous layer in the 0 h PW (A), and maintained this hier- archical relation until 144 h PW ((B)-(F)). Scale bars, 100 μm, 10 μm, and 100 μm from above; counterstain, hema- toxylin and DAPI; and dashed lines, the border between the epidermis and dermis. 3.4. The Localizations of Expression of Molecules Participating in the Epidermal Terminal Differentiation Fluctuated during Reepithelialization We immunohistochemically examined the expression of C/EBP-α and caspase-14, known to participate in the terminal differentiation of the epidermal keratinocytes at the early and late stages, respectively. At 0 h PW, C/EBP- α was intensely stained at both the nuclei and the cyto- plasm of the cells in the suprabasal layer, while caspase- 14 at the cytoplasm in the granular layer (Figures 5(A) and (H)). The staining intensities of both molecules at the leading edge of the epidermis were obviously fallen by 24 h PW (Figures 5(B) and (I)). At 48 h PW, these im- munostaining intensities were restored to the normal lev- els at the wound-covering epidermis. The epidermis at 72 h PW showed a similar tendency (Figures 5(C), (D), (J ), (K)). By 96 h PW when the thickness of the wound- covering epidermis reached the peak, the region intensely stained with anti-caspase-14 was confined to the upper layer at the thickening epidermis, and the unstained re- gion remarkably extended (Figure 5(L)). Subsequently, however, the region intensely stained with anti-caspase- 14 in turn enlarged downward by 120 h PW in the midst of the thinning phase (Figure 5(M)). From 96 to 120 h PW, the localization of C/EBP-α-positive cells was Open Access CellBio  M. ARAI ET AL. 253 Figure 5. The expression of C/EBP-α and caspase-14 in the wound areas. The localization of C/EBP-α and caspase-14 was immunohistochemically examined. In the neonatal epi- dermis at 0 h PW, C/EBP-α and caspase-14 were distrib- uted in the suprabasal layer (A) and granular layer (H), respectively. At 24 h PW, both of these molecules were di- minished at the wound edge ((B), (I)). After wound closure was completed, these immunostaining intensities were re- stored to the normal level at the wound-covering epidermis by 48 h PW ((C), (J)). After that, the localization of C/EBP- α-positive cells was hardly changed, although the thickness of wound epidermis was varied ((D)-(G)). In contrast, the region unstained with anti-caspase-14 extensively extended downward in the thickened epidermal region by 96 h PW (L). Subsequently, the region intensely stained with anti- caspase-14, in turn, became enlarged more downward in the thinning epidermis at 120 h PW (M). Scale bar, 100 μm; and counterstained, hematoxylin. hardly changed (Figures 5(E) and (F)). The epidermis at 144 h PW showed that both of these two molecules tended to restore the normal level of expression (Figures 5(G) and (N)). These results suggested that the modulation of the ex- pression of caspase-14 might be responsible for the epi- dermal thickness. 4. Discussion HE and immunostaining experiments in this study re- vealed the healing process of the incisional wounds on neonatal rat skin to be outlined as follows (Figure 1). The opened wounds were first closed by the epidermal cells that, keeping the cell-to-cell contact, rapidly ex- tended from the wound margin. Simultaneously, the wound- covering epidermis became thickened. Subsequently, the epidermis at the wound area became thinner up to the normal thickness, accompanying an elevation of the lev- els of the basal cells and the basement membrane. So, we have further continued morphological analyses of the incisional wounds, with the aim of pursuing the cause of above-stated fluctuation in the epidermal thickness. 4.1. The Phase of Thinning of Epidermis at the Wound Area and That of the Cell Death in the Upper Epidermis Overlap Each Other We estimated the rates of cell proliferation and cell death using anti-BrdU immunostaining and TUNEL staining, respectively, to verify the possible causal relationship between the epidermal cell numbers and the fluctuation of the epidermal thickness in the wound area. The analy- ses of cell proliferation revealed that there is no clear correlation between the fluctuation of thickness at the wound-covering epidermis and the rate of the proliferat- ing cells (Figures 2(A)-(H)). By contrast, the rate of TUNEL-positive cells rose shortly after peak (96 h PW) of the thickness (and the cell number) of the epidermis in the wound area, and reached the peak at 120 h PW, at which the thinning phase of epidermis was considered to start (Figures 2(M) and (O)). The peak value was 1.4 times as much as that at 96 h PW. It should be empha- sized here that the rates of cell death in the wound areas at 120 and 144 h PW were significantly higher than those in the intact areas at 120 and 144 h PW, respectively (Figures 2(O) and (P)). Thus, the fluctuation of the rate of cell death and that of the thickness of epidermis seemed to coincide with each other. In other words, an elevation of the apoptosis in some cell populations in the upper epidermis may partially contribute to the reduction in the epidermal thickness. A previous study showed that TUNEL-positive cells were often observed at the granular layer just below the horny layer of epidermis [21]. However, it has recently been reported that apoptosis and the terminal differentia- tion of keratinocytes are two different independent path- ways [22,23]. Meanwhile, there is an article claiming that keratinocytes in the granular layer that express active caspase-14 display TUNEL-positive, being indicative of the physiological role of caspase-14 in relation to the DNA degradation [24]. Namely, an elevation of cell death (TUNEL-positive cells) at the upper part of the granular layer in the midst of thinning of the epidermis may result in an augmentation of cornification. 4.2. The Derangement Is Generated in the Terminal Differentiation on the Thickness-Fluctuating Epidermis at the Wound Area One of the two daughter cells that generates from an epidermal stem cell via the asymmetric cell division is known to have the developmental fate toward the termi- nal differentiation through which, following several cy- cles of proliferation, the cell differentiates into spinous, granular and horny cells in order [25]. Such a terminal differentiation process involves not only the shift of keratin expression from K5 and K14 (in the basal layer) to K1 and K10 (in the suprabasal layers) but also a se- quential switch-on of various differentiation markers such as involucrin, filaggrin, and loricrin in the supraba- sal zone [26]. We made the simultaneous comparison of the intraepidermal localization of these differentiation Open Access CellBio 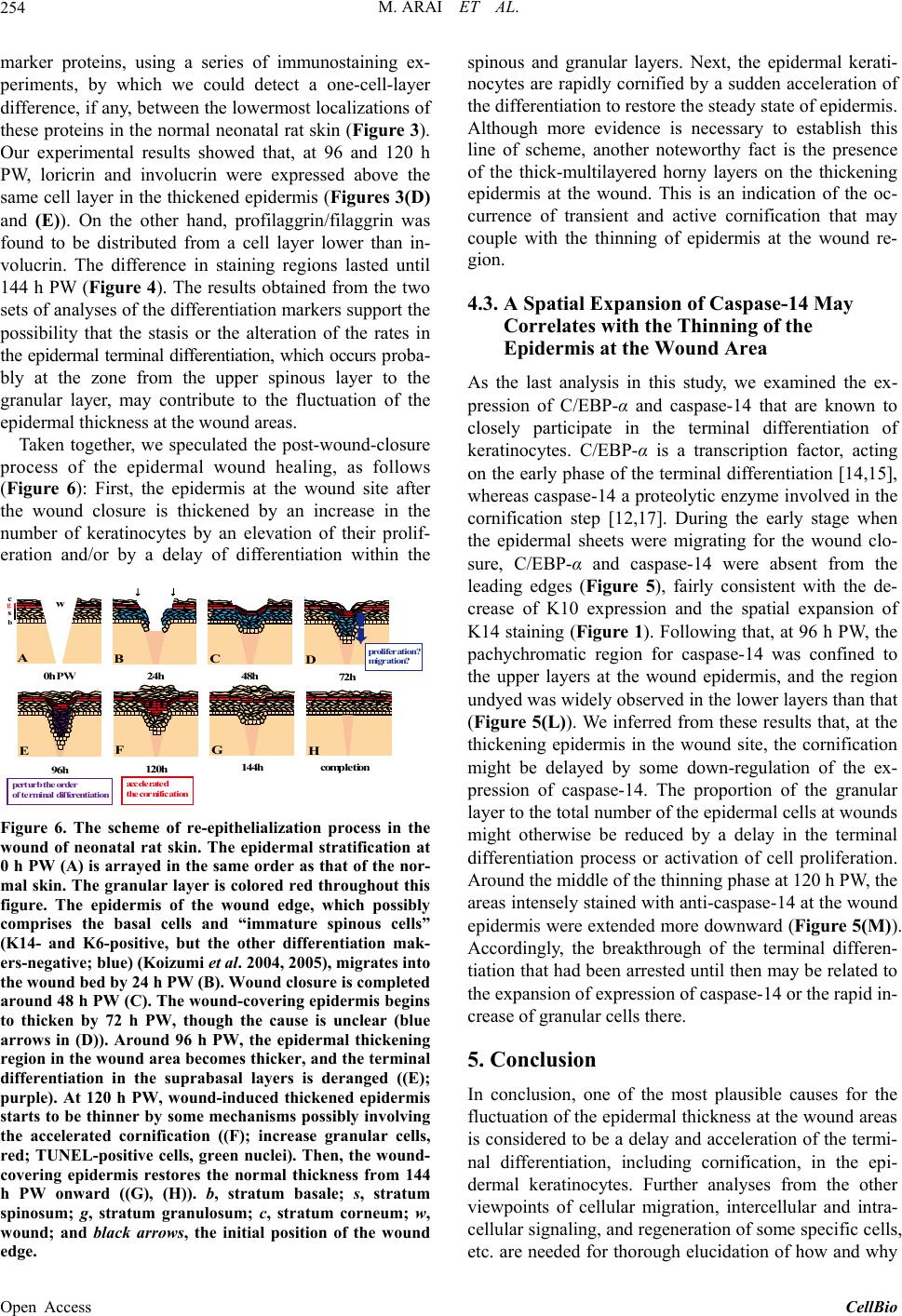 M. ARAI ET AL. 254 marker proteins, using a series of immunostaining ex- periments, by which we could detect a one-cell-layer difference, if any, between the lowermost localizations of these proteins in the normal neonatal rat skin (Figure 3). Our experimental results showed that, at 96 and 120 h PW, loricrin and involucrin were expressed above the same cell layer in the thickened epidermis (Figures 3(D) and (E)). On the other hand, profilaggrin/filaggrin was found to be distributed from a cell layer lower than in- volucrin. The difference in staining regions lasted until 144 h PW (Figure 4). The results obtained from the two sets of analyses of the differentiation markers support the possibility that the stasis or the alteration of the rates in the epidermal terminal differentiation, which occurs proba- bly at the zone from the upper spinous layer to the granular layer, may contribute to the fluctuation of the epidermal thickness at the wound areas. Taken together, we speculated the post-wound-closure process of the epidermal wound healing, as follows (Figure 6): First, the epidermis at the wound site after the wound closure is thickened by an increase in the number of keratinocytes by an elevation of their prolif- eration and/or by a delay of differentiation within the b s g c w A 24h B 96h pert urb the order of terminal differentiation E 48h C 72h prolifer ation? mi grat i o n? D 120h accel e ra te d the cornification F 144h G compl et ion H 0h PW Figure 6. The scheme of re-epithelialization process in the wound of neonatal rat skin. The epidermal stratification at 0 h PW (A) is arrayed in the same order as that of the nor- mal skin. The granular layer is colored red throughout this figure. The epidermis of the wound edge, which possibly comprises the basal cells and “immature spinous cells” (K14- and K6-positive, but the other differentiation mak- ers-negative; blue) (Koizumi et al. 2004, 2005), migrates into the wound bed by 24 h PW (B). Wound closure is completed around 48 h PW (C). The wound-covering epidermis begins to thicken by 72 h PW, though the cause is unclear (blue arrows in (D)). Around 96 h PW, the epidermal thickening region in the wound area becomes thicker, and the terminal differentiation in the suprabasal layers is deranged ((E); purple). At 120 h PW, wound-induced thickened epidermis starts to be thinner by some mechanisms possibly involving the accelerated cornification ((F); increase granular cells, red; TUNEL-positive cells, green nuclei). Then, the wound- covering epidermis restores the normal thickness from 144 h PW onward ((G), (H)). b, stratum basale; s, stratum spinosum; g, stratum granulosum; c, stratum corneum; w, wound; and black arrows, the initial position of the wound edge. spinous and granular layers. Next, the epidermal kerati- nocytes are rapidly cornified by a sudden acceleration of the differentiation to restore the steady state of epidermis. Although more evidence is necessary to establish this line of scheme, another noteworthy fact is the presence of the thick-multilayered horny layers on the thickening epidermis at the wound. This is an indication of the oc- currence of transient and active cornification that may couple with the thinning of epidermis at the wound re- gion. 4.3. A Spatial Expansion of Caspase-14 May Correlates with the Thinning of the Epidermis at the Wound Area As the last analysis in this study, we examined the ex- pression of C/EBP-α and caspase-14 that are known to closely participate in the terminal differentiation of keratinocytes. C/EBP-α is a transcription factor, acting on the early phase of the terminal differentiation [14,15], whereas caspase-14 a proteolytic enzyme involved in the cornification step [12,17]. During the early stage when the epidermal sheets were migrating for the wound clo- sure, C/EBP-α and caspase-14 were absent from the leading edges (Figure 5), fairly consistent with the de- crease of K10 expression and the spatial expansion of K14 staining (Figure 1). Following that, at 96 h PW, the pachychromatic region for caspase-14 was confined to the upper layers at the wound epidermis, and the region undyed was widely observed in the lower layers than that (Figure 5(L)). We inferred from these results that, at the thickening epidermis in the wound site, the cornification might be delayed by some down-regulation of the ex- pression of caspase-14. The proportion of the granular layer to the total number of the epidermal cells at wounds might otherwise be reduced by a delay in the terminal differentiation process or activation of cell proliferation. Around the middle of the thinning phase at 120 h PW, the areas intensely stained with anti-caspase-14 at the wound epidermis were extended more downward (Figure 5(M)). Accordingly, the breakthrough of the terminal differen- tiation that had been arrested until then may be related to the expansion of expression of caspase-14 or the rapid in- crease of granular cells there. 5. Conclusion In conclusion, one of the most plausible causes for the fluctuation of the epidermal thickness at the wound areas is considered to be a delay and acceleration of the termi- nal differentiation, including cornification, in the epi- dermal keratinocytes. Further analyses from the other viewpoints of cellular migration, intercellular and intra- cellular signaling, and regeneration of some specific cells, etc. are needed for thorough elucidation of how and why Open Access CellBio  M. ARAI ET AL. 255 the thickness of the wound-covering epidermis fluctu- ates. 6. Acknowledgements We thank the members of our Morphogenesis Laborato- ries for their support and helpful discussions in weekly seminars for furthering this study. REFERENCES [1] J. D. Burrington, “Wound Healing in the Fetal Lamb,” Journal of Pediatric Surgery, Vol. 6, No. 5, 1971, pp. 523-528. http://dx.doi.org/10.1016/0022-3468(71)90373-3 [2] J. B. Dixon, “Inflammation in the Foetal and Neonatal Rat: the Local Reactions to Skin Burns,” The Journal of Pathology and Bacteriology, Vol. 80, No. 1, 1960, pp. 73-82. http://dx.doi.org/10.1002/path.1700800109 [3] S. Ihara, Y. Motobayashi, E. Nagao and A. Kistler, “On- togenetic Transition of Wound Healing Pattern in Rat Skin Occurring at the Fetal Stage,” Development, Vol. 110, No. 3, 1990, pp. 671-680. [4] M. T. Longaker, D. J. Whitby, N. S. Adzick, T. M. Crombleholme, J. C. Langer, B. W. Duncan, S. M. Brad- ley, R. Stern, M. W. Ferguson and M. R. Harrison, “Studies in Fetal Wound Healing, VI. Second and Early Third Trimester Fetal Wounds Demonstrate Rapid Colla- gen Deposition without Scar Formation,” Journal of Pe- diatric Surgery, Vol. 25, No. 1, 1990, pp. 63-68, Discus- sion 8-9. [5] P. Martin, “Mechanisms of Wound Healing in the Em- bryo and Fetus,” Current Topics in Developmental Biol- ogy, Vol. 32, 1996, pp. 175-203. http://dx.doi.org/10.1016/S0070-2153(08)60428-7 [6] M. Koizumi, T. Matsuzaki and S. Ihara, “The Subsets of Keratinocytes Responsible for Covering Open Wounds in Neonatal Rat Skin,” Cell and Tissue Research, Vol. 315, No. 2, 2004, pp. 187-195. http://dx.doi.org/10.1007/s00441-003-0823-0 [7] P. Martin and S. M. Parkhurst, “Parallels between Tissue Repair and Embryo Morphogenesis,” Development, Vol. 131, No. 13, 2004, pp. 3021-3034. http://dx.doi.org/10.1242/dev.01253 [8] M. Koizumi, T. Matsuzaki and S. Ihara, “Expression of P-Cadherin Distinct from That of E-Cadherin in Re-Epi- thelialization in Neonatal Rat Skin,” Development, Growth & Differentiation, Vol. 47, No. 2, 2005, pp. 75-85. http://dx.doi.org/10.1111/j.1440-169x.2004.00784.x [9] C. Byrne, M. Tainsky and E. Fuchs, “Programming Gene Expression in Developing Epidermis,” Development, Vol. 120, No. 9, 1994, pp. 2369-2383. [10] E. R. Li, D. M. Owens, P. Djian and F. M. Watt, “Ex- pression of Involucrin in Normal, Hyperproliferative and Neoplastic Mouse Keratinocytes,” Experimental Derma- tology, Vol. 9, No. 6, 2000, pp. 431-438. http://dx.doi.org/10.1034/j.1600-0625.2000.009006431.x [11] R. B. Presland, M. K. Kuechle, S. P. Lewis, P. Fleckman and B. A. Dale, “Regulated Expression of Human Filag- grin in Keratinocytes Results in Cytoskeletal Disruption, Loss of Cell-Cell Adhesion, and Cell Cycle Arrest,” Ex- perimental Cell Research, Vol. 270, No. 2, 2001, pp. 199- 213. http://dx.doi.org/10.1006/excr.2001.5348 [12] G. Denecker, P. Ovaere, P. Vandenabeele and W. De- clercq, “Caspase-14 Reveals Its Secrets,” The Journal of Cell Biology, Vol. 180, No. 3, 2008, pp. 451-458. http://dx.doi.org/10.1083/jcb.200709098 [13] P. F. Jhonson, “Molecular Stop Signs: Regulation of Cell- Cycle Arrest by C/EBP Transcription Factors,” Journal of Cell Science, Vol. 118, 2005, pp. 2545-2555. http://dx.doi.org/10.1242/jcs.02459 [14] E. V. Maytin, J. C. Lin, R. Krishnamurthy, N. Batchva- rova, D. Ron, P. J. Mitchell and J. F. Habener, “Keratin 10 Gene Expression during Differentiation of Mouse Epidermis Requires Transcription Factors C/EBP and AP-2,” Developmental Biology, Vol. 216, No. 1, 1999, pp. 164-181. http://dx.doi.org/10.1006/dbio.1999.9460 [15] H. S. Oh and R. C. Smart, “Expression of CCAAT/En- hancer Binding Proteins (C/EBP) Is Associated with Squamous Differentiation in Epidermis and Isolated Pri- mary Keratinocytes and Is Altered in Skin Neoplasms,” The Journal of Investigative Dermatology, Vol. 110, No. 6, 1998, pp. 939-945. http://dx.doi.org/10.1046/j.1523-1747.1998.00199.x [16] M. Van de Craen, G. Van Loo, S. Pype, W. Van Criek- inge, I. Van den brande, F. Molemans, W. Fiers, W. De- clercq and P. Vandenabeele, “Identification of a New Caspase Homologue: Caspase-14,” Cell Death and Dif- ferentiation, Vol. 5, No. 10, 1998, pp. 838-846. http://dx.doi.org/10.1038/sj.cdd.4400444 [17] S. Lippens, M. Kockx, M. Knaapen, L. Mortier, R. Pola- kowska, A. Verheyen, M. Garmyn, A. Zwijsen, P. Form- stecher, D. Huylebroeck, P. Vandenabeele and W. De- clercq, “Epidermal Differentiation Does Not Involve the Pro-Apoptotic Executioner Caspases, but Is Associated with Caspase-14 Induction and Processing,” Cell Death and Differentiation, Vol. 7, No. 12, 2000, pp. 1218-1224. http://dx.doi.org/10.1038/sj.cdd.4400785 [18] L. Alibardi, E. Tschachler and L. Eckhart, “Distribution of Caspase-14 in Epidermis and Hair Follicles Is Evolu- tionarily Conserved among Mammals,” The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, Vol. 286, No. 2, 2005, pp. 962- 973. http://dx.doi.org/10.1002/ar.a.20234 [19] E. Hoste, P. Kemperman, M. Devos, G. Denecker, S. Kezic, N. Yau, B. Gilbert, S. Lippens, P. De Groote, R. Roelandt, P. Van Damme, K. Gevaert, R. B. Presland, H. Takahara, G. Puppels, P. Caspers, P. Vandenabeele and W. Declercq, “Caspase-14 Is Required for Filaggrin Degradation to Natural Moisturizing Factors in the Skin,” The Journal of Investigative Dermatology, Vol. 131, No. 11, 2011, pp. 2233-2241. http://dx.doi.org/10.1038/jid.2011.153 [20] L. Eckhart and E. Tschachler, “Cuts by Caspase-14 Con- trol the Proteolysis of Filaggrin,” The Journal of Investi- gative Dermatology, Vol. 131, No. 11, 2011, pp. 2173- 2175. http://dx.doi.org/10.1038/jid.2011.282 [21] C. A. McCall and J. J. Cohen, “Programmed Cell Death Open Access CellBio  M. ARAI ET AL. Open Access CellBio 256 in Terminally Differentiating Keratinocytes: Role of En- dogenous Endonuclease,” The Journal of Investigative Dermatology, Vol. 97, No. 1, 1991, pp. 111-114. http://dx.doi.org/10.1111/1523-1747.ep12478519 [22] M. Rendl, J. Ban, P. Mrass, C. Mayer, B. Lengauer, L. Eckhart, W. Declercq and E. Tschachler, “Caspase-14 Expression by Epidermal Keratinocytes is Regulated by Retinoids in a Differentiation-Associated Manner,” The Journal of Investigative Dermatology, Vol. 119, No. 5, 2002, pp. 1150-1155. http://dx.doi.org/10.1046/j.1523-1747.2002.19532.x [23] S. Lippense, G. Denecker, P. Ovaere, P. Vandenabeele and W. Declercq, “Death Penalty for Keratinocytes: Apop- tosis versus Cornification,” Cell Death and Differentia- tion, Vol. 12, Suppl. 2, 2005, pp. 1497-1508. http://dx.doi.org/10.1038/sj.cdd.4401722 [24] T. Hibino, E. Fujita, Y. Tsuji, J. Nakanishi, H. Iwaki, C. Katagiri and T. Momoi, “Purification and Characteriza- tion of Active Caspase-14 from Human Epidermis and Development of the Cleavage Site-Directed Antibody,” Journal of Cellular Biochemistry, Vol. 109, No. 3, 2010, pp. 487-497. [25] E. Fuchs, “Skin Stem Cells: Rising to the Surface,” The Journal Cell Biology, Vol. 180, No. 2, 2008, pp. 273-284. http://dx.doi.org/10.1083/jcb.200708185 [26] H. Tseng and H. Green, “Association of Basonuclin with Ability of Keratinocytes to Multiply and with Absence of Terminal Differentiation,” The Journal Cell Biology, Vol. 126, No. 2, 1994, pp. 495-506. http://dx.doi.org/10.1083/jcb.126.2.495
|