 Modern Plastic Surgery, 2014, 4, 5-10 Published Online January 2014 (http://www.scirp.org/journal/mps) http://dx.doi.org/10.4236/mps.2014.41002 OPEN ACCESS MPS The Effect of Neuro Gen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release Michael J. Fitzmaurice Fitzmaurice Hand Institute, Phoenix, USA. Email: mfitz70@me.com Received June 14th, 2013; revised July 15th, 2013; accepted July 24th, 2014 Copyright © 2014 Michael J. Fitzmaurice. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Michael J . Fitzmaurice. All Copyright © 2014 are guarded by law and by SCIRP as a guardian. ABSTRACT 61 patients with clinically diagnosed and electromyographically confirmed carpal tunnel syndrome were enrolled in a prospective study to evaluate the effectiveness of a nerve supplement on pillar pain after carpal tunnel sur- gery. All of the patients underwent endoscopic carpal tunnel release. 15 of the patients also took the nerve sup- port supplement NeuroGen® as part of their perioperative treatment. The supplement group demonstrated a significantly lower amount of pillar pain (VAS) at initial follow up compared to the control group (1.13 and 4.05 respectively). 46% (7/15) of the supplement group were completely free of pillar pain compared to only 9% (4/46) of the control group at the first follow up. 53% (8/15) of the NeuroGen® group did not require any pain medica- tions compared to 35% (16/46) of the control group. The Nerve supplement NeuroGen® significantly reduces pain after carpal tunnel surgery. KEYWORDS Carpal Tunnel; Endoscopic; ECTR; Nerve Supplement; Nerve; Supplement 1. Introduction Pillar pain is one of the most common complications after carpal tunnel surgery and has an occurrence from 7% - 61% [1-8 ]. This pain is located in the palm and can li m- it strength and delay return to daily activities and work. Endoscopic carpal tunnel release (ECTR) has demon- strated less pain than the traditional open procedures, however some authors have reported rates of pillar pain up to 50% with the endoscopic technique [3,6]. The true incidence of pillar pain is difficult to report due to the lack of a standardized assessment or grading system. Several authors have used numerical (0 - 4) or nominal (nil, mild, moderate, etc.) scales to grade the severity. The etiology of pillar pain remains elusive, however, four main theories are well described by Ludlow et al . [9] These include ligamentous or muscular, altered structure of the carpal arch, neurogenic causes and edematous causes. The ligamentous and muscular theory includes detachment of the hypothenar and thenar muscles from the fascia, pulling on forming scar tissue from these muscles and the raw edge of the slightly migrated liga- ment as possible causes. This theory proposes that the reason why endoscopic release causes less pillar pain is the intact palmar aponeurosis, which is cut during open procedures. Release of the transverse carpal ligament alters the structures in the carpal arch. The increased vo- lume may cause pillar pain and open techniques often cause slightly more widening which may account for the increased pain compared to the endoscopic release. The altered forces over the pisotriquetral joint may cause hy- pothenar pain and authors have suggested excision of the pisiform as treatment for persistent pain. The neurogenic theory suggests that pain develops when many small transverse subcutaneous nerve branches are cut during the procedure and the pain is more likely with the tradi- tional single incision open procedure. The edematous theory suggests that it is the swelling at the base of the palm responsible for the pain, which resolves when the swelling is reduced. NeuroGen® is a nerve support supplement which con- 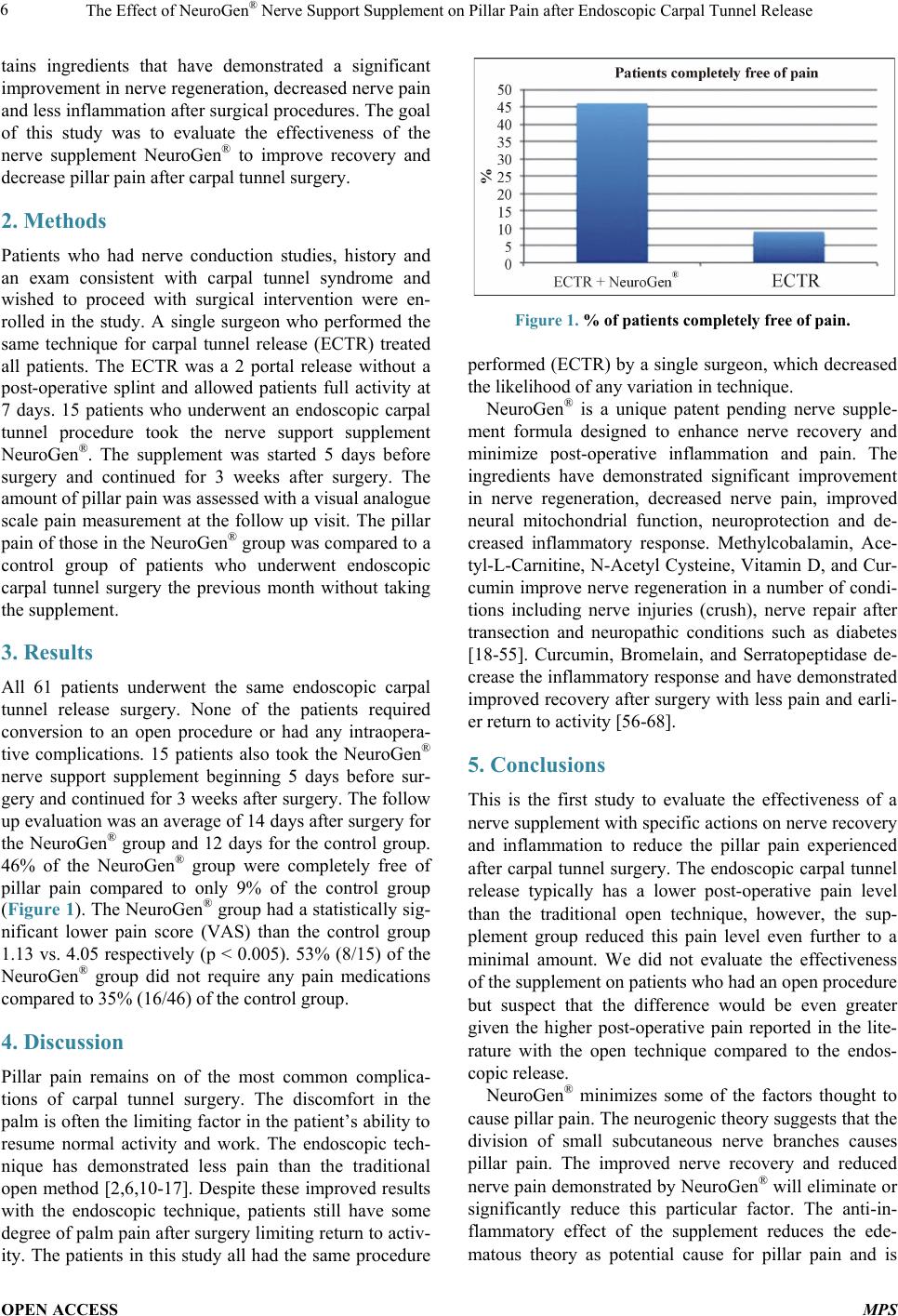 The Effect of NeuroGen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release OPEN ACCESS MPS tains ingredients that have demonstrated a significant improvement in nerve regeneration, decreased nerve pain and less inflammation after surgical procedures. The goal of this study was to evaluate the effectiveness of the nerve supplement NeuroGen® to improve recovery and decrease pillar pain after carpal tunnel surgery. 2. Methods Patients who had nerve conduction studies, history and an exam consistent with carpal tunnel syndrome and wished to proceed with surgical intervention were en- rolled in the study. A single surgeon who performed the same technique for carpal tunnel release (ECTR) treated all patients. The ECTR was a 2 portal release without a post-operative splint and allowed patients full activity at 7 days. 15 patients who underwent an endoscopic carpal tunnel procedure took the nerve support supplement NeuroGen®. The supplement was started 5 days before surgery and continued for 3 weeks after surgery. The amount of pillar pain was assessed with a visual analogue scale pain measurement at the follow up visit. The pillar pain of those in the NeuroGen® group was compared to a control group of patients who underwent endoscopic carpal tunnel surgery the previous month without taking the supplement. 3. Results All 61 patients underwent the same endoscopic carpal tunnel release surgery. None of the patients required conversion to an open procedure or had any intraopera- tive complications. 15 patients also took the NeuroGen® nerve support supplement beginning 5 days before sur- gery and continued for 3 weeks after surgery. The follow up evaluation was an averag e of 14 days after surgery for the NeuroGen® group and 12 days for the control group. 46% of the NeuroGen® group were completely free of pillar pain compared to only 9% of the control group (Figu re 1). The NeuroGen® group had a statistical ly sig- nificant lower pain score (VAS) than the control group 1.13 vs. 4.05 respectively (p < 0.0 05). 53% (8/15) of the NeuroGen® group did not require any pain medications compare d to 35% (16/46) of t he c ontrol gr o up. 4. Discussion Pillar pain remains on of the most common complica- tions of carpal tunnel surgery. The discomfort in the palm is often the limiting factor in the patient’s ability to res ume normal activity and work. The endoscopic tech- nique has demonstrated less pain than the traditional open method [2,6,10-17]. Despite these improved results with the endoscopic technique, patients still have some degree of palm pain after surgery limiting return to activ- ity. The patients in this study all had the same procedure Figure 1. % of patients c ompletely free of pain. performed (ECTR) by a single surgeon , which decreased the likelihood of any variation in technique. NeuroGen® is a unique patent pending nerve supple- ment formula designed to enhance nerve recovery and minimize post-operative inflammation and pain. The ingredients have demonstrated significant improvement in nerve regeneration, decreased nerve pain, improved neural mitochondrial function, neuroprotection and de- creased inflammatory response. Methylcobalamin, Ace- tyl-L-Carnitine, N-Acetyl Cysteine, V itamin D, and Cur- cumin improve nerve regeneration in a number of condi- tions including nerve injuries (crush), nerve repair after transection and neuropathic conditions such as diabetes [18-55]. Curcumin, Bromelain, and Serratopeptidase de- crease the inflammatory response and have demonstrated improved recov ery after surgery with less pain and ear li- er return to activity [56-68]. 5. Conclusions This is the first study to evaluate the effectiveness of a nerve supplement with specific actions on nerve recovery and inflammation to reduce the pillar pain experienced after carpal tunnel surgery. The endoscopic carpal tunnel release typically has a lower post-operative pain level than the traditional open technique, however, the sup- plement group reduced this pain level even further to a minimal amount. We did not evaluate the effectiveness of the supplement on patients who had an open procedure but suspect that the difference would be even greater given the higher post-operative pain reported in the lite- rature with the open technique compared to the endos- copic release. NeuroGen® minimizes some of the factors thought to cause pillar pain. The neurogenic theory suggests that the division of small subcutaneous nerve branches causes pillar pain. The improved nerve recovery and reduced nerve pain demonstrated by NeuroGen® will eliminate or significantly reduce this particular factor. The anti-in- flammatory effect of the supplement reduces the ede- matous theory as potential cause for pillar pain and is  The Effect of NeuroGen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release OPEN ACCESS MPS well documented to enhance the recovery from trauma and various surgeries. The ligamentous or muscular theory suggests some aspect of muscular pull on forming scar as a possible role in pillar pain. Several of the ingre- dients minimize the scar tissue formation and have been used in plastic surgery cases for this specific effect. The NeuroGen® nerve supplement significantly im- proves the recovery following carpal tunnel surgery by decreasing the amount of pillar pain. This decrease in pain results in less post-operative medication required and an earlier return to activity. Further studies will eva- luate the effectiveness of the supplement on nerve regen- eration after surgical decompression. REFERENCES [1] R. A. Brown, R. H. Gelberman, J. G. Seiler, S. O. Abra- hamsson, A. J. Weiland, J. R. Urbaniak, et al., “Carpal Tunnel Release. A Prospective, Randomized Assessment of Open and Endoscopic Methods,” Journal of Bone & Joint Surgery, Vol. 75, No. 9, 1993, pp. 1265-1275. [2] C. Dumontier, C. Sokolow, C. Leclercq and P. Chauvin, “Early Results of Conventional versus Two-Portal En- doscopic Carpal Tunnel Release. A Prospective Study,” Journal of Hand Surgery, Vol. 20, No. 5, 1995, pp. 658- 662. http://dx.doi.org/10.1016/S0266-7681(05)80130-5 [3] G. Foucher and J. Braga Da Silva, “Endoscopic Opening of the Carpal Canal,” Chirurgie, Vol. 120, No. 12, 1994, pp. 100-104. [4] S. S. Bande, L. L. De Sme t and G. G. Fabr y, “The Results of Carpal Tunnel Release: Open versus Endoscopic Tech- nique,” Journal of Hand Surgery, Vol. 19, No. 1, 1994, pp. 14-17. http://dx.doi.org/10.1016/0266-7681(94)90039-6 [5] J. Menon, “Endoscopic Carpal Tunnel Release: Prelimi- nary Report,” Arthroscopy, Vol. 10, No. 1, 1994, pp. 31- 38. http://dx.doi.org/10.1016/S0749-8063(05)80290-7 [6] I. Atroshi, G.-U. Larsson, E. Ornstei n, M. Hofer, R. Johnsson and J. Ranstam, “Outcomes of Endoscopic Sur- gery Compared with Open Surgery for Carpal Tunnel Syndrome among Employed Patients: Randomised Con- trolled Trial,” BMJ, Vol. 33 2, No. 7556, 2006, p. 1473. http://dx.doi.org/10.1136/bmj.38863.632789.1F [7] J. Oertel, H. W. S. Schroeder and M. R. Gaab, “Dual - Portal Endoscopic Release of the Transverse Ligament in Carpal Tunnel Syndrome: Results of 411 Procedures with Special Reference to Technique, Efficacy, and Complica- tions,” Neurosurgery, Vol. 59, No. 2, 2006, pp. 333-340. http://dx.doi.org/10.1227/01.NEU.0000223500.25131.99 [8] A. Biy a ni and E. Downes, “A Open Twin Incision Tech- nique of Carpal Tunnel De compression with Reduced In- cidence of Scar Tenderness,” The Journal of Hand Sur- gery: Journal of the British Society for Surgery of the Hand, Vol. 18, No. 3, 1993, pp. 331-334. http://dx.doi.org/10.1016/0266-7681(93)90055-K [9] K. S. Ludlow, J. L. Merla, J. A. Cox and L. N. Hurst, “Pillar Pain as a Postoperative Complication of Carpal Tunnel Release: A Review of the Literature,” Journal of Hand Therapy, Vol. 10, No. 4, 2012, pp. 277-282. [10] J. M. Agee, H. R. J. McCarroll, R. D. Tortosa, D. A. Berry, R. M. Szabo and C. A. Peimer, “Endoscopic Re- lease of the Carpal Tunnel: A Randomized Prospective Multicenter Study,” Journal of Hand Surgery (American Volume), Vol. 17, No. 6, 1992, pp. 987-995. http://dx.doi.org/10.1016/S0363-5023(09)91044-9 [11] M. W. Erdmann, “Endoscopic Carpal Tunnel Decompres- sion,” Journal of Hand Surgery, Vol. 19, No. 1, 1994, pp. 5-13. http://dx.doi.org/10.1016/0266-7681(94)90038-8 [12] G. G. G. Hallock and D. A. D. Lutz, “Prospective Com- parison of Minimal Incision ‘Ope n ’ and Two-Portal En- doscopic Carpal Tunnel Release,” Plastic and Recon- structive Surgery, Vol. 96, No. 4, 1995, pp. 941-947. http://dx.doi.org/10.1097/00006534-199509001-00027 [13] A. P. Worseg, R. Kuzbari, K. Kor ak , K. Höcker, C. Wie- derer, M. Tschabitscher, et al., “Endoscopic Carpal Tun- nel Release Using a Single-Portal System,” British Jour- nal of Plastic Surgery, Vol. 49, No. 1, 1996, pp. 1-10. http://dx.doi.org/10.1016/S0007-1226(96)90179-4 [14] T. Futami, “Surgery for Bilateral Carpal Tunnel Syn- drome. Endoscopic and Open Release Compared in 10 Patients,” Acta Orthopaedica Scandinavica, Vol. 66, No. 2, 1995, pp. 153-155. http://dx.doi.org/10.3109/17453679508995510 [15] J. C. Payne, R. S. Bergman and D. J. Ettinger, “Endos- copic Carpal Tunnel Release,” Journal of the American Osteopathic Association, Vol. 94 , No. 4, 1994, pp. 295- 298. [16] A. Thoma, K. Veltri, T. Haines and E. Duku, “A Syste- matic Re view of Reviews Comparing the Effectiveness of Endoscopic and Open Carpal Tunnel Decompression,” Plastic and Reconstructive Surgery, Vol. 113, No. 4, 2004, pp. 1184-1191. http://dx.doi.org/10.1097/01.PRS.0000110202.08818.C1 [17] R. Bhattacharya, P. D. Birdsall, P. Finn and J. Stothard, “A Randomized Controlled Trial of Knifelight and Open Carpal Tunnel Release,” Journal of Hand Surgery, Vol. 29, No. 2, 2004, pp. 113-115. http://dx.doi.org/10.1016/j.jhsb.2003.09.001 [18] A. S. Morani a nd S. L. Bodhankar, “Early Co-Adminis- tration of Vitamin E Acetate and Methylcobalamin Im- proves Thermal Hyperalgesia and Motor Nerve Conduc- tion Velocity Following Sciatic Nerve Crush Injury in Rats,” Pharmacological Reports, Vol. 62, No. 2, 2010, pp. 405-409. [19] X. Kong, X. Sun and J. Zhang, “The Protective Role of Mecobalamin Following Optic Nerve Crush in Adult Rats,” Eye Science, Vol. 20, No. 3, 2004, pp. 171-177. [20] V. V. Mikhailov and V. M. Avakumov, “Mechanism of the Effect of Methylcobalamin on the Recovery of Neu- romuscular Functions in Mechanical and Toxin Denerva- tion,” Farmakologiia i Toksikologiia, Vol. 46, No. 6, 1983, pp. 9-12. [21] K. Oka da, H. Tanaka, K. Temporin, M. Okamoto, Y. Ku- roda, H. Moritomo, et al., “Methylcobalamin Increases Erk1/2 and Akt Activities through the Methylation Cycle and Promotes Nerve Regeneration in a Rat Sciatic Nerve Injury Model,” Ex perimental Neurology, Vol. 222 , No. 2,  The Effect of NeuroGen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release OPEN ACCESS MPS 2010, pp. 191-203. http://dx.doi.org/10.1016/j.expneurol.2009.12.017 [22] K. Yamatsu, T. Kaneko, A. Kitahara and I. Ohkawa, “Pharmacological Studies on Degeneration and Regene- ration of Peripheral Nerves. (1) Effects of Methylcobala- min and Cobamide on EMG Patterns and Loss of Muscle Weight in Rats with Crushed Sciatic Nerve,” Nippon Ya- kurigaku Zasshi, Vol. 72, No. 2, 1976, pp. 259-268. http://dx.doi.org/10.1254/fpj.72.259 [23] A. Yamatsu, R. Matsumi, H. Atomi and T. Imanaka, “Iso- lation and Characterization of a Novel Poly(Vinyl Alco- hol)-Degrading Bacterium, Sphingopyxis sp. PVA3,” Ap- plied Microbiology and Biotechnology, Vol. 72, No. 4, 2006, pp. 804-811. http://dx.doi.org/10.1007/s00253-006-0351-4 [24] T. Watanabe, R. Kaji, N. Oka, W. Bara and J. Kimura, “Ultra-High Dose Methylcobalamin Promotes Nerve Re- generation in Experimental Acrylamide Neuropathy,” Journal of the Neurological Sciences, Vol. 122 , No. 2, 1994, pp. 140-14 3. http://dx.doi.org/10.1016/0022-510X(94)90290-9 [25] K. Yamazaki, K. Oda, C. Endo, T. Kikuchi and T. Wa- kabayashi, “Methylcobalamin (Met hyl-B12) Promotes Re- generation of Motor Nerve Terminals Degenerating in Anterior Gracile Muscle of Gracile Axonal Dystrophy (GAD) Mutant Mouse,” Neuroscience Letters, Vol. 170, No. 1, 1994, pp. 195-197. http://dx.doi.org/10.1016/0304-3940(94)90272-0 [26] W. C. Liao, J. R. C hen , Y. J. Wang and G. F. Tseng, “Methylcobalamin, but Not Methylprednisolone or Pleio- trophin, Accelerates the Recovery of Rat Bice ps after Ul- nar to Musculocutaneous Nerve Transfer,” Neuroscience, Vol. 171, No. 3, 2010, pp. 934-949. http://dx.doi.org/10.1016/j.neuroscience.2010.09.036 [27] A. A. Sima, M. Calvani, M. Mehra and A. Amato, “Acetyl-L-Carnitine Improves Pain, Nerve Regeneration, and Vibratory Perception in Patients with Chronic Di- abetic Neuropathy: An Analysis of Two Randomized Placebo-Controlled Trials,” Diabetes Care, Vol. 28, No. 1, 2004, pp. 89-94. http://dx.doi.org/10.2337/diacare.28.1.89 [28] C. De Angel is, C. Scarfò, M. Falcinelli, E. Perna, E. Re da, M. T. Ramacci, et al., “Acetyl-L-Carnitine Prevents Age- Dependent Structural Alterations in Rat Peripheral Nerves and Promotes Regeneration Following Sciatic Nerve In- jury in Young and Senescent Rats,” Experimental Neu- rology, Vol. 128, No. 1, 1994, pp. 103-114. http://dx.doi.org/10.1006/exnr.1994.1117 [29] Z. T. Kokkalis, P. N. Soucacos and J. K. Terzis, “Effect of Acetyl-L-Carnitine on Axonal Sprouting Following Donor Nerve Injury Distal to an End-to-Side Neurorrha- phy Model,” Journal of Reconstructive Microsurgery, Vol. 25, No. 8, 2009, pp. 483-495. http://dx.doi.org/10.1055/s-0029-1234027 [30] C. De Angelis, C. Scarfò, M. Falcinelli, E. Reda , M. T. Ramacci and L. Angelucci, “Levocarnitine Acetyl Stimu- lates Peripheral Nerve Regeneration and Neuromuscular Junction Remodelling Following Sciatic Nerve Injury,” International Journal of Clinical Pharmacology Research, Vol. 12, No. 5-6, 1992, pp. 269-279. [31] E. Fernandez, R. Pallini, L. Lauretti, E. Marchese, V. Bozzini, A. Sbriccoli, et al., “Levocarnitine Acetyl Pro- motes The Regeneration of Peripheral Sensory Axons and Reduces the Hypertrophy of Axotomized Spinal Cord Motoneurons in Rats,” Drugs under Experimental and Clinical Research , Vol. 17, No. 12, 1991, 563-570. [32] A. McKay Hart, M. Wiberg and G. Terenghi, “Pharma- cological Enhancement of Peripheral Nerve Regeneration in the Rat by Systemic Acetyl-L-Carnitine Treatment,” Neuroscience Letters, Vol. 334, No. 3, 2002, pp. 181-185. http://www.sciencedirect.com/science/article/pii/s030439 4002009825 http://dx.doi.org/10.1016/S0304-3940(02)00982-5 [33] A. D. H. Wilson, A. Hart, M. Wiberg and G. Terenghi, “Acetyl-l-Carnitine Increases Nerve Regeneration and Target Organ Reinnervation—A Morphological Study,” Journal of Plastic, Reconstructive & Aesthetic Surgery, Vol. 63, No. 7, 2009, pp. 1186-1195. http://dx.doi.org/10.1016/j.bjps.2009.05.039 [34] S. Karsidag, A. Akcal, S. Sahin, S. Karsi dag, F. Kabuk- cuoglu and K. Ugurlu, “Neurophysiological and Morpho- logical Responses to Treatment with Acetyl-L-Carnitine in a Sciatic Nerve Injury Model: Preliminary Data,” Journal of Hand Surgery (European Volume), Vol. 37, No. 6, 2012, pp. 529-536. http://dx.doi.org/10.1177/1753193411426969 [35] A. D. H. Wilson, A. Har t, T. Brännström, M. Wiberg and G. Terenghi, “Primary Sensory Neuronal Rescue with Systemic Acetyl-L-Carnitine Following Peripheral Axo- tomy. A Dose-Response Analysis,” British Journal of Plastic Surgery, Vol. 56, No. 8, 2003, pp. 732-739. http://dx.doi.org/10.1016/j.bjps.2003.08.005 [36] A. M. Hart, M. Wiberg, M. You le and G. Terenghi, “Sys - temic Acetyl-L-Carnitine Eliminates Sensory Neuronal Loss after Peripheral Axotomy: A New Clinical Ap- proach in the Management of Peripheral Nerve Trauma,” Experimental Brain Research, Vol. 145, No. 2, 2002, pp. 182-189. [37] E. Fernandez, R. Pallini, C. Gangitano, A. Del Fá, C. O. Sangiacomo, A. Sbriccoli, J. R. Ricoy and G. F. Rossi, “Effects of L-Carnitine, L-Acetylcarnitine and Ganglio- sides on the Regeneration of the Transected Sciatic Nerve in Rats,” Neurological Research, Vol. 11, No. 1, 1989, pp. 57-62. [38] V. K. Kostopoulos, C. L. Davis and J. K. Terzis, “Effects of Acetylo-L-Carnitine in End-to-Side Neurorrhaphy: A Pilot Study,” Microsurgery, Vol. 29, No. 6, 2009, pp. 456-463. http://www.ncbi.nlm.nih.gov/pubmed/19308954 [39] G. Terenghi, A. Ha r t and M. Wiberg, “The Nerve Injury and the Dying Neurons: Diagnosis and Prevention,” Jour- nal of Hand Surgery, European Volume, Vol. 36, No. 9, 2011, pp. 730-734. http://scholar.google.com/scholar?q=related:Me0jiiPdpc0 J:scholar.google.com/&hl=en&num=30&as_sdt=0,5&as_ ylo=2011&as_yhi=2011 [40] E. Fernandez, R. Pal lini, C. Gangitano, A. D el Fá, C. Oli- vieri-Sangiacomo, A. Sbriccoli, J. Ricoy and G. F. Rossi, “Studies on the Degenerative and Regenerative Pheno- mena Occurring after Transection and Repair of the Scia- tic Nerve in Rats: Effects of Acetyl-L-Carnitine,” Inter-  The Effect of NeuroGen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release OPEN ACCESS MPS national Journal of Clinical Pharmacology Research, Vol. 10, No. 1-2, 1990, pp. 85-99. [41] E. Fernandez, R. Pallini, L. Lauretti, F. La Marca, A. Scogna and G. F. Rossi, “Motonuclear Changes after Cranial Nerve Injury and Regeneration,” Archives Ita- liennes Biologie, Vol. 135, No. 4, 1997, pp. 343-351. [42] R. De Simone, L. Aloe, L. Ferraris and M. T. Ramacci, “Levocarnitine Acetyl Treatment Promotes Iris Reinner- vation Following 6-Hydroxydopamine-Induced Noradre- nergic Denervation in Rats,” Int ernational Journal of Clinical Pharmacology Research, Vol. 12, No. 5-6, 1992, pp. 281-287. [43] K. Kotil, M. Kirali, M. Eras, T. Bilge and H. Uzun, “Neuroprotective Effects of Acetyl-L-Carnithine in Ex- perimental Chronic Compression Neuropathy. A Prospec- tive, Randomized and Placebo-Control Trials,” Turkish Neurosurgery, Vol. 17, No. 2, 2007, pp. 67-77. [44] A. D. H. Wilson, A. Hart, T. Brännström, M. Wiberg and G. Terenghi, “Delayed Acety l -L-Carnitine Administration and Its Effect on Sensory Neuronal Rescue after Peri- pheral Nerve Injury,” Journal of Plastic Reconstructive & Aesthetic Surgery, Vol. 60, No. 2, 2007, pp. 114-118. [45] E. Fernandez, R. Pallini, L. Lauretti, E. Marchese, C. Gangitano, A. Del Fá, C. Olivieri-Sangiacomo, A. Sbric- coli and G. F. Rossi, “Levoc arnitine Acety l Prevents Cell Death Following Long-Term Section of the Vagus Nerve in Rats,” International Journal of Clinical Pharmacology Research, Vol. 12, No. 5-6, 1992, pp. 289-297. [46] A. Love, M. A. Cotter and N. E. Cameron, “Effects of the Sulphydryl Donor N-Acetyl-L-Cysteine on Nerve Con- duction, Perfusion, Maturation and Regeneration Follow- ing Freeze Damage In Diabetic Rats,” European Journal of Clinical Investigation, Vol. 26 , No. 8, 1996, pp. 698- 706. [47] D. Weli n, L. N. Novikova, M. Wiberg, J.-O. Kellerth and L. N. Novikov, “Effects of N-Acetyl-Cysteine on the Survival and Regeneration of Sural Sensory Neurons in Adult Rats,” Brain Research, Vol. 1287, 2009, pp. 58-66. [48] T. Karlidag, M. Yildiz, S. Yalcin, N. Colakoglu, I. Kay- gusuz and E. Sapmaz, “Evaluation of the Effect of Me- thylprednisolone and N-Acetylcystein on Anastomotic Degeneration and Regeneraton of the Facial Nerve,” Au- ris Nasus Larynx , Vol. 39, No. 2, 2012, pp. 145-150. [49] J. Brown, J. I. Bianco, J. J. McGrath and D. W. Eyles, “1,25-Dihydroxyvitamin D3 Induces Nerve Growth Fac- tor, Promotes Neurite Outgrowth and Inhibits Mitosis in Embryonic Rat Hippocampal Neurons,” Neuroscience Letters, Vol. 343, No. 2, 2003, pp. 139-143 . http://dx.doi.org/10.1016/S0304-3940(03)00303-3 [50] M. Fukuoka, K. Sakurai, T. Ohta, M. Kiyoki and I. Ka- tayama, “Tacalcitol, an Active Vitamin D3, Induces Nerve Growth Factor Production in Human Epidermal Keratinocytes,” Skin Pharmacology and Skin Physiology, Vol. 14, No. 4, 2001, pp. 226-233. http://dx.doi.org/10.1159/000056351 [51] J.-F. Chabas, O. Alluin, G. Ra o, S. Garcia, M.-N. Lavaut, J. J. Risso, et al ., “Vitamin D 2Potentiates Axon Regene- ration,” Journal of Neurotrauma, Vol. 25, No. 10, 2008, pp. 1247-1256. http://dx.doi.org/10.1089/neu.2008.0593 [52] M. Goudarzvand, M Javan, J. Mirnajafi-Zadeh, S. Moza- fari and T. Tiraihi, “Vitamins E and D3 Attenuate De- myelination and Potentiate Remyelination Processes of Hippocampal Formation of Rats Following Local Injec- tion of Ethidium Bromide,” cellular and Molecular Neu- robiology, Vol. 30, No. 2, 2010, pp. 289-299. [53] Y. Xu, B. Ku, L. Cui, X. Li, P. A. Barish, T. C. Foster and W. O. Ogle, “Curcumin Reverses Impaired Hippo- campal Neurogenesis and Increases Serotonin Receptor 1A mRNA and Brain-Derived Neurotrophic Factor Ex- pression in Chronically Stressed Rats,” Brain Research, Vol. 1162, 2007, pp. 9-18. [54] K.-K. Liao, M.-J. Wu, P.-Y. Chen, S.-W. Huang, S.-J. Chiu, C.-T. Ho and J. H. Yen, “Curcuminoids Promote Neurite Outgrowth in PC12 Cells through MAPK/ERK- and PKC -Dependent Pathways,” Journal of Agricultural Food Chemistry, Vol. 60, No. 1, 2012, pp. 433-443. [55] M. A. Jalaludin, “Methylcobalamin Treatment of Bell’s Pal sy ,” Methods and Findings in Experimental and Clini- cal Pharmacology, Vol. 17, No. 8, 1995, pp. 539-544. [56] A. D. Kandhare, K. S. Raygude, P. Ghosh, A. E. Ghule and S. L. Bodhankar, “Therapeutic Role of Curcumin in Prevention of Biochemical and Behavioral Aberration Induced by Alcoholic Neuropathy in La boratory Animals,” Neuroscience Letters, Vol. 511, No. 1, 2012, pp. 18-22. [57] D. J. Fitzhugh, S. Shan, M. W. Dewhirst and L. P. Hale, “Bromelain Treatment Decreases Neutrophil Migration to Sites of Inflammation,” Clinical Immunology, Vol. 128, No. 1, 2008, pp. 66-74. [58] L. Gaspani, E. Limiroli, P. Ferrario and M. Bianchi, “In Vivo and in Vitro Effects of Bromelain on PGE(2) and SP Concentrations in the Inflammatory Exudate in Rats,” Pharmacology, Vol. 65, No. 2, 2002, pp. 83-86. [59] L. P. Hale, M. Chichlowski, C. T. Trinh and P. K. Greer, “Dietary Supplementation with Fresh Pineapple Juice De- creases Inflammation and Colonic Neoplasia in IL-10- Deficient Mice with Colitis,” Inflammatory Bowel Dis- eases, Vol. 16, No. 12, 2010, pp. 2012-2021. [60] S. Muller, R. Marz, M. Schmolz, B. Drewelow, K. Esch- mann and P. Meiser, “Placebo-Controlled Randomized Clinical Trial on the Immunomodulating Activities of Low- and High-Dose Bromelain after Oral Administra- tion—New Evidence on the Antiinflammatory Mode of Action of Bromelain,” P hyt ot herapy Research, Vol. 27, No. 2, 2012, pp. 199-204. [61] F. Inchingolo, M. Ta tullo, M. Marrelli, A. M. Inchingolo, V. Picciariello, A. D. Inchingolo, G. Di palma, D. Verme- san and R. Cagiano, “Clinical Trial with Bromelain in Third Molar Exodontia,” European Review of Medi cal Pharmacological Sc iences, Vol. 14, No. 9, 2010, pp. 771- 774. [62] M. Ma sson , “Bromelain in Blunt Injuries of the Locomo- tor Syste m. A Study of Observe d Applications in General Practice,” Fortschritte der Medizin, Vol. 113, No. 19, 1995, pp. 303-306. [63] S. Jadav, N. Patel, T. Shah, M. Gajera, H. Trivedi and B. Shah, “Comparison of Anti-Inflammatory Activity of Serratiopeptidase and Diclofenac in Albino Rats,” Jour-  The Effect of NeuroGen® Nerve Support Supplement on Pillar Pain after Endoscopic Carpal Tunnel Release OPEN ACCESS MPS nal of Pharmacology & Pharmacotherapeutics, Vol. 1, No. 2, 2010, pp. 116-117. [64] A. H. M. Viswanatha Swamy and P. A. Patil, “Effect of Some Clinically Used Proteolytic Enzymes on Inflamma- tion in Rats,” Indian Journal of Pharmacetical Sciences, Vol. 70, No. 1, 2008, pp. 114-117. [65] A. Mazzone, M. Catalani, M. Costanzo, A. Drusian, A. Mandoli, S. Russo, E. Guarini and G. Vesperini , “Evalua- tion of Serratia peptidase in Acute or Chronic Inflamma- tion of Otorhinolaryngology Pathology: A Multicentre, Double-Blind, Randomized Trial Versus Placebo,” Jour- nal of International Medic al Research, Vol. 18, No. 5, 1990, pp. 379-388. [66] T. H. Al-Khateeb and Y. Nusair, “Effec t of the Proteolyt- ic Enzyme Serrapeptase on Swelling, Pain and Trismus after Surgical Extraction of Mandibular Third Molars,” International Journal of Oral and Maxillofacial Surgery, Vol. 37, No. 3, 2008, pp. 264-268. [67] P. M. Esch, H. Gerngross and A. Fabian, “Reduction of Postoperative Swelling. Objective Measurement of Swel- ling of the Upper Ankle Joint in Treatment with Serra- peptase—A Prospective Study,” Fortschr itte der Medizin, Vol. 107, No. 4, 1989, pp. 67-68,71-72. [68] M. Tachibana, O. Mizukoshi, Y. Harada, K. Kawamoto and Y. Nakai, “A Multi-Centre, Double-Blind Study of Serrapeptase versus Placebo in Post-Antrotomy Buccal Swelling,” Pharmatherapeutica, Vol. 3, No. 8, 1984, pp. 526-530.
|