 Advances in Infectious Diseases, 2013, 3, 281-289 Published Online December 2013 (http://www.scirp.org/journal/aid) http://dx.doi.org/10.4236/aid.2013.34043 Open Access AID 281 Mortality of HIV-I nfec ted Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors Armel Poda1,2*#, Arsène Hema2#, Jacques Zoungrana2, Nongodo Firmin Kaboré2, Bebar Euloges Kamboulé2, Ibrahim Soré2, Guillaume Bado2, Abdoul-Salam Ouédraogo1,3, Nicolas Meda4,5, Adrien Bruno Sawadogo2,3 1School of Medicine, University of Bobo Dioulasso, Bobo Dioulasso, Burkina Faso; 2Day Care Hospital for HIV-Patients of Bobo Dioulasso, Department of Infectious Diseases, Souro Sanou Teaching Hospital, Bobo Dioulasso, Burkina Faso; 3Department of Virology and Bacteriology, Souro Sanou Teaching Hospital, Bobo Dioulasso, Burkina Faso; 4School of Medicine, University of Ouagadougou, Ouagadougou, Burkina Faso; 5Muraz Center, Bobo Dioulasso, Burkina Faso. Email: *armelpoda@yahoo.fr Received September 8th, 2013; revised October 8th, 2013; accepted October 15th, 2013 Copyright © 2013 Armel Poda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: In sub Saharan Africa, small size surveys have demonstrated early high mortality among infected patients on antiretroviral therapies (ART). Few studies have been conducted in large cohorts of HIV-patients in public health care system in West Africa. Objectives: Our study aims to determine mortality rate and its predictors in a cohort of patients on ART in a public daycare hospital in Burkina Faso. Methods: We have carried out a retrospective cohort study. All HIV-infected patients on ART between January 1st 2008 and December 31st 2011 were included in the study. Survival probability was estimated by the Kaplan-Meier method. Cox regression analysis was used to identify associated factors to mortality. Results: A total of 2243 HIV-infected patients were included in the study. During the follow-up, 218 pa- tients representing 9.7% were lost. About 104 patients representing 4.6% were transferred and 1691 representing 75.4% were still in the therapeutic cohort. There were 230 death cases for a total of 4282 persons-years, (5.4 deaths for 100 persons-years; 95% CI: 4.8 - 6.3). The survival probabilities after 6 months, 1 year and 2 years were 92.6%, 91% and 88.9% respectively. For the multivariate analysis, the following factors were independently associated to death: male gender, BMI < 18.5 kg/m2, WHO stage 3 and 4, HIV-2, T-CD4 lymphocytes < 200/µl, haemoglobin rate < 8 g/dl and creatinine clearance < 60 ml/m2. Conclusions: Our study provides for the first time mortality rates and its predictors among HIV-patients on antiretroviral treatment in a large cohort in public health sector in Burkina Faso. It highlights the importance of early HIV screening to limit ART initiation at advanced HIV infection stages. Keywords: Antiretroviral Therapy; Burkina Faso; HIV; Mortality; West Africa 1. Introduction Nowadays, antiretroviral treatments (ART) do not only aim to reduce morbidity and mortality caused by HIV in- fection and improving infected patients’ life. They also aim to prevent HIV infection [1], therefore, they have the interest of reaching universal access to ART goals (ART co- verage superior or equal to 80% to those in need among in- fected patients). Few African countries were able to make it [2]. However, in sub Saharan Africa, the number of patients on ART is increasing [2]. Landlocked West Afri- can country, Burkina Faso is also part of this trend with 36,248 patients on ART in December 2011 [2]. Obviously, ART have drastically reduced HIV-related morbidity and mortality [2-6]. However, one can note residual mortality among HIV-patients both in Southern and Northern coun- tries [7]. The Souro Sanou Teaching Hospital is a public reference health centre in Burkina Faso and in West Af- rica for the ambulatory care to HIV-infected patients [8]. Few studies have been carried out among large cohorts in West Africa on mortality during ART in public health care system. In such conditions where patients on ART *Corresponding author. #These authors have contributed equally to the work.  Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 282 are increasing, we found it necessary to make investiga- tions among the Bobo-Dioulasso cohort which includes 3089 patients on ART at the day of December 31st 2011. The objective of this study was therefore to determine mortality rates and related factors among a cohort of ART patients in Burkina Faso, West Africa. 2. Methods 2.1. Study Area The study was conducted at the Teaching Hospital in Bobo-Dioulasso and specifically at the Day Care Hospi- tal for HIV-patients. Bobo-Dioulasso is the second major city after Ouagadougou the capital city, in Burkina Faso 2.2. Study Design We conducted a retrospective cohort study. We included all HIV patients on ART between January 1st 2008 and December 31st 2011. These patients were followed up from the initiation of the ART to June 30th 2012 (cut off point). 2.3. Data Collection Data have been collected during medical examinations with ESOPE software (Epi concept). ESOPE is software for monitoring patients with HIV infection in limited re- sources countries. Data collected at the first visit includ- ed: socio-demographic data, medical history, WHO clas- sification, biological and clinical examinations, data such as weight, height, etc. Samplings for biological examina- tions were carried out on patients with empty stomach. Examinations carried out at the first visit included hae- mogram, TCD4 lymphocytes counting, and creatinine checking. Data collected on ESOPE software were trans- ferred to STATA-12 Corporation, Texas for analysis. 2.4. Statistical Analyses Patients’ socio-demographic, clinical and biological char- acteristics were described in number and percentage for qualitative variables. Quantitative variables were de- scribed through their medians and inter-quartile intervals (IQR). The Pearson chi2 test was used to compare pro- portions. The Student t test was used to test equality be- tween the two averages (signification threshold 5%). To calculate the mortality rate we used at the numerator the total number of deaths that occurred during the follow up and at the denominator how long patients were followed up in the cohort from the first day of the treatment. Survival probability was evaluated by the Kaplan-Me- ier method. The association between socio-demographic, clinical and immunologic factors of mortality was assess- ed with the Iogrank test (signification threshold = 5%). The effect of predictors significantly associated to the prognosis was then studied through a multivariate analy- sis with Cox proportional risks model. 2.5. Ethical Issues This study was done in the process of routine clinical care. Collected information was kept confidential and names of patients were not included in the data collection process. 3. Results 3.1. Characteristics of the Study Population A total of 2243 HIV infected patients were on ART be- tween January 2008 and December 2011. These included 636 males (28.4%) and 1607 females (71.6%) aged be- tween 16 to 78 years with an average age of 37 (IQR 32 - 45) (Table 1). Married patients represented 54.3%, un- educated ones were 46.4% and 58.1% were unem- ployed. HIV-1 was the most common infection (91.2%) and was discovered in 59.1% of cases during an opportunistic infection. At their first medical examination 54.6% of patients were at 3 and 4 clinical stages, according to the WHO classification. The average Body Mass Index (BMI) was 20.5 kg/m2 (IQR 18 - 23) and 29.3% had a BMI < 18.5. The pre-therapeutic biological assessment showed 7.1% of severe anaemia (hb < 8 g/dl); 10% of kidney insufficiency (creatinine clearance < 60 ml/m2). The me- dian number of T-CD4 lymphocytes at the initiation of the ART was 186 cells/µl (IQR 95 - 268) and 6.4% of the patients had more than 350 cells/µl. The median time limit between the first medical visit and the initiation of the ART was estimated to 44.0 days (IQR 21 - 206) and the median duration of the treatment was 20.0 months (IQR 9 - 35). The current medication consisted in the association of two nucleoside reverse transcriptase inhibitors (NRTIs) with a non-nucleoside reverse transcriptase inhibitor (NNRTI). The most com- mon Anti Retroviral regimens prescribed included AZT + 3TC + EFV (40.7%), AZT + 3TC + NVP (31.4%). 3.2. Cohort Follow-Up and Survival Progression During the follow-up, we lost 218 patients (9.7%). Among males we lost of follow-up 4.3% of cases and among females we noted 4.8% of lost of follow-up. Around 104 (4.6%) were transferred to different health centres. Among males, 8.9% were transferred and among females 10% were transferred (p = 0.44). 230 deaths rep- resenting 10.3% were registered. Among males we Regis- tered 15.4% of deaths and among females, 8.2% of Open Access AID 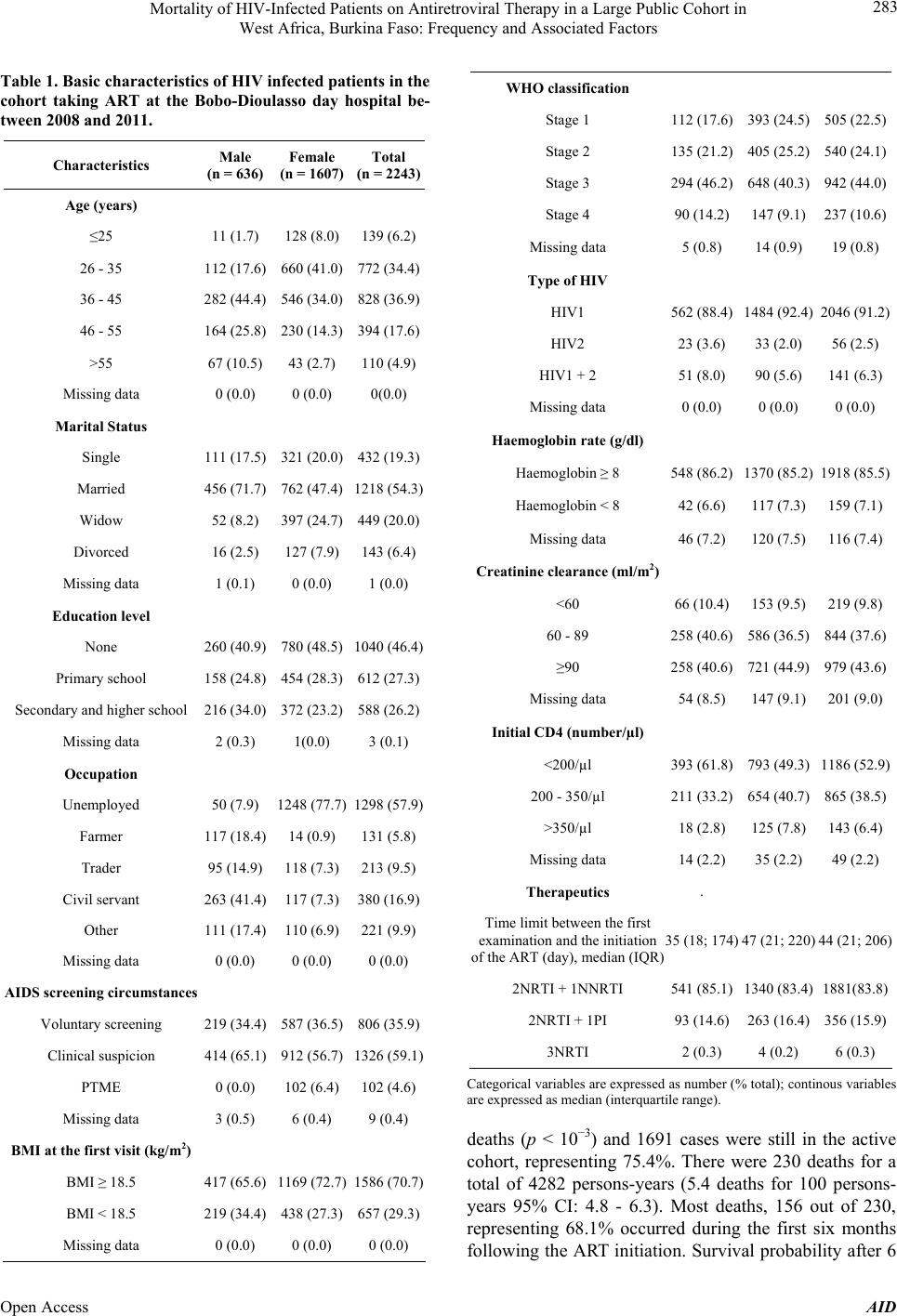 Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 283 Table 1. Basic characteristics of HIV infected patients in the cohort taking ART at the Bobo-Dioulasso day hospital be- tween 2008 and 2011. Characteristics Male (n = 636) Female (n = 1607) Total (n = 2243) Age (years) ≤25 11 (1.7) 128 (8.0) 139 (6.2) 26 - 35 112 (17.6) 660 (41.0) 772 (34.4) 36 - 45 282 (44.4) 546 (34.0) 828 (36.9) 46 - 55 164 (25.8) 230 (14.3) 394 (17.6) >55 67 (10.5) 43 (2.7) 110 (4.9) Missing data 0 (0.0) 0 (0.0) 0(0.0) Marital Status Single 111 (17.5) 321 (20.0) 432 (19.3) Married 456 (71.7) 762 (47.4) 1218 (54.3) Widow 52 (8.2) 397 (24.7) 449 (20.0) Divorced 16 (2.5) 127 (7.9) 143 (6.4) Missing data 1 (0.1) 0 (0.0) 1 (0.0) Education level None 260 (40.9) 780 (48.5) 1040 (46.4) Primary school 158 (24.8) 454 (28.3) 612 (27.3) Secondary and higher school 216 (34.0) 372 (23.2) 588 (26.2) Missing data 2 (0.3) 1(0.0) 3 (0.1) Occupation Unemployed 50 (7.9) 1248 (77.7) 1298 (57.9) Farmer 117 (18.4) 14 (0.9) 131 (5.8) Trader 95 (14.9) 118 (7.3) 213 (9.5) Civil servant 263 (41.4) 117 (7.3) 380 (16.9) Other 111 (17.4) 110 (6.9) 221 (9.9) Missing data 0 (0.0) 0 (0.0) 0 (0.0) AIDS screening circumstances Voluntary screening 219 (34.4) 587 (36.5) 806 (35.9) Clinical suspicion 414 (65.1) 912 (56.7) 1326 (59.1) PTME 0 (0.0) 102 (6.4) 102 (4.6) Missing data 3 (0.5) 6 (0.4) 9 (0.4) BMI at the first visit (kg/m2) BMI ≥ 18.5 417 (65.6) 1169 (72.7) 1586 (70.7) BMI < 18.5 219 (34.4) 438 (27.3) 657 (29.3) Missing data 0 (0.0) 0 (0.0) 0 (0.0) WHO classification Stage 1 112 (17.6) 393 (24.5)505 (22.5) Stage 2 135 (21.2) 405 (25.2)540 (24.1) Stage 3 294 (46.2) 648 (40.3)942 (44.0) Stage 4 90 (14.2) 147 (9.1) 237 (10.6) Missing data 5 (0.8) 14 (0.9) 19 (0.8) Type of HIV HIV1 562 (88.4) 1484 (92.4)2046 (91.2) HIV2 23 (3.6) 33 (2.0) 56 (2.5) HIV1 + 2 51 (8.0) 90 (5.6) 141 (6.3) Missing data 0 (0.0) 0 (0.0) 0 (0.0) Haemoglobin rate (g/dl) Haemoglobin ≥ 8 548 (86.2) 1370 (85.2)1918 (85.5) Haemoglobin < 8 42 (6.6) 117 (7.3) 159 (7.1) Missing data 46 (7.2) 120 (7.5) 116 (7.4) Creatinine clearance (ml/m2) <60 66 (10.4) 153 (9.5) 219 (9.8) 60 - 89 258 (40.6) 586 (36.5)844 (37.6) ≥90 258 (40.6) 721 (44.9)979 (43.6) Missing data 54 (8.5) 147 (9.1) 201 (9.0) Initial CD4 (number/µl) <200/µl 393 (61.8) 793 (49.3)1186 (52.9) 200 - 350/µl 211 (33.2) 654 (40.7)865 (38.5) >350/µl 18 (2.8) 125 (7.8) 143 (6.4) Missing data 14 (2.2) 35 (2.2) 49 (2.2) Therapeutics . Time limit between the first examination and the initiation of the ART (day), median (IQR) 35 (18; 174) 47 (21; 220)44 (21; 206) 2NRTI + 1NNRTI 541 (85.1) 1340 (83.4)1881(83.8) 2NRTI + 1PI 93 (14.6) 263 (16.4)356 (15.9) 3NRTI 2 (0.3) 4 (0.2) 6 (0.3) Categorical variables are expressed as number (% total); continous variables are expressed as median (interquartile range). deaths (p < 10−3) and 1691 cases were still in the active cohort, representing 75.4%. There were 230 deaths for a total of 4282 persons-years (5.4 deaths for 100 persons- years 95% CI: 4.8 - 6.3). Most deaths, 156 out of 230, representing 68.1% occurred during the first six months following the ART initiation. Survival probability after 6 Open Access AID 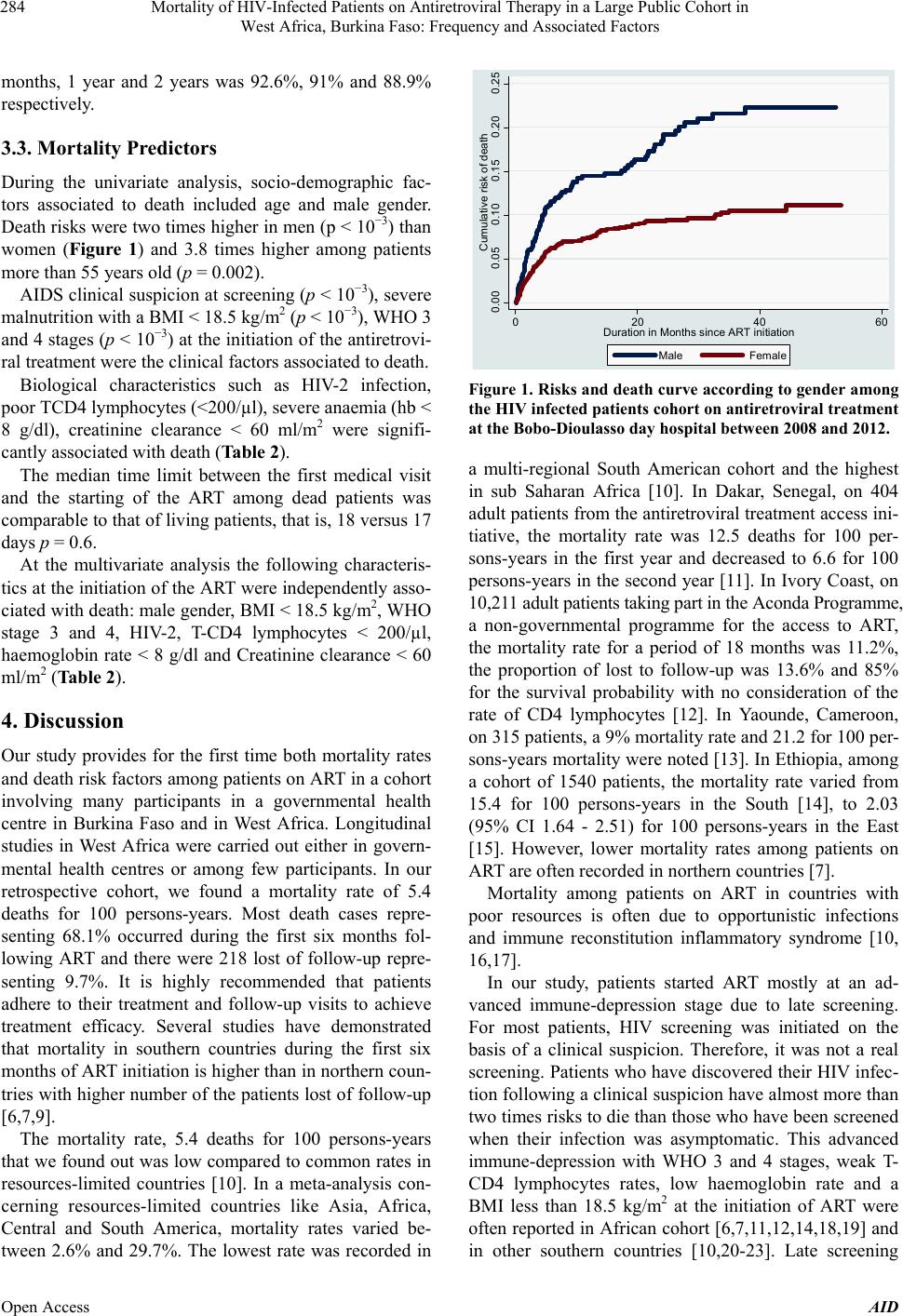 Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 284 months, 1 year and 2 years was 92.6%, 91% and 88.9% respectively. 3.3. Mortality Predictors During the univariate analysis, socio-demographic fac- tors associated to death included age and male gender. Death risks were two times higher in men (p < 10−3) than women (Figure 1) and 3.8 times higher among patients more than 55 years old (p = 0.002). AIDS clinical suspicion at screening (p < 10−3), severe malnutrition with a BMI < 18.5 kg/m2 (p < 10−3), WHO 3 and 4 stages (p < 10−3) at the initiation of the antiretrovi- ral treatment were the clinical factors associated to death. Biological characteristics such as HIV-2 infection, poor TCD4 lymphocytes (<200/µl), severe anaemia (hb < 8 g/dl), creatinine clearance < 60 ml/m2 were signifi- cantly associated with death (Table 2). The median time limit between the first medical visit and the starting of the ART among dead patients was comparable to that of living patients, that is, 18 versus 17 days p = 0.6. At the multivariate analysis the following characteris- tics at the initiation of the ART were independently asso- ciated with death: male gender, BMI < 18.5 kg/m2, WHO stage 3 and 4, HIV-2, T-CD4 lymphocytes < 200/µl, haemoglobin rate < 8 g/dl and Creatinine clearance < 60 ml/m2 (Table 2). 4. Discussion Our study provides for the first time both mortality rates and death risk factors among patients on ART in a cohort involving many participants in a governmental health centre in Burkina Faso and in West Africa. Longitudinal studies in West Africa were carried out either in govern- mental health centres or among few participants. In our retrospective cohort, we found a mortality rate of 5.4 deaths for 100 persons-years. Most death cases repre- senting 68.1% occurred during the first six months fol- lowing ART and there were 218 lost of follow-up repre- senting 9.7%. It is highly recommended that patients adhere to their treatment and follow-up visits to achieve treatment efficacy. Several studies have demonstrated that mortality in southern countries during the first six months of ART initiation is higher than in northern coun- tries with higher number of the patients lost of follow-up [6,7,9]. The mortality rate, 5.4 deaths for 100 persons-years that we found out was low compared to common rates in resources-limited countries [10]. In a meta-analysis con- cerning resources-limited countries like Asia, Africa, Central and South America, mortality rates varied be- tween 2.6% and 29.7%. The lowest rate was recorded in 0.00 0.05 0.10 0.15 0.20 0.25 Cumulative risk of death 020 40 60 Dur a tio n in M onths since AR T in itiati o n Male Female Figure 1. Risks and death curve according to gender among the HIV infected patients cohort on antiretroviral treatment at the Bobo-Dioulasso day hospital between 2008 and 2012. a multi-regional South American cohort and the highest in sub Saharan Africa [10]. In Dakar, Senegal, on 404 adult patients from the antiretroviral treatment access ini- tiative, the mortality rate was 12.5 deaths for 100 per- sons-years in the first year and decreased to 6.6 for 100 persons-years in the second year [11]. In Ivory Coast, on 10,211 adult patients taking part in the Aconda Programme, a non-governmental programme for the access to ART, the mortality rate for a period of 18 months was 11.2%, the proportion of lost to follow-up was 13.6% and 85% for the survival probability with no consideration of the rate of CD4 lymphocytes [12]. In Yaounde, Cameroon, on 315 patients, a 9% mortality rate and 21.2 for 100 per- sons-years mortality were noted [13]. In Ethiopia, among a cohort of 1540 patients, the mortality rate varied from 15.4 for 100 persons-years in the South [14], to 2.03 (95% CI 1.64 - 2.51) for 100 persons-years in the East [15]. However, lower mortality rates among patients on ART are often recorded in northern countries [7]. Mortality among patients on ART in countries with poor resources is often due to opportunistic infections and immune reconstitution inflammatory syndrome [10, 16,17]. In our study, patients started ART mostly at an ad- vanced immune-depression stage due to late screening. For most patients, HIV screening was initiated on the basis of a clinical suspicion. Therefore, it was not a real screening. Patients who have discovered their HIV infec- tion following a clinical suspicion have almost more than two times risks to die than those who have been screened when their infection was asymptomatic. This advanced immune-depression with WHO 3 and 4 stages, weak T- CD4 lymphocytes rates, low haemoglobin rate and a BMI less than 18.5 kg/m2 at the initiation of ART were often reported in African cohort [6,7,11,12,14,18,19] and n other southern countries [10,20-23]. Late screening i Open Access AID 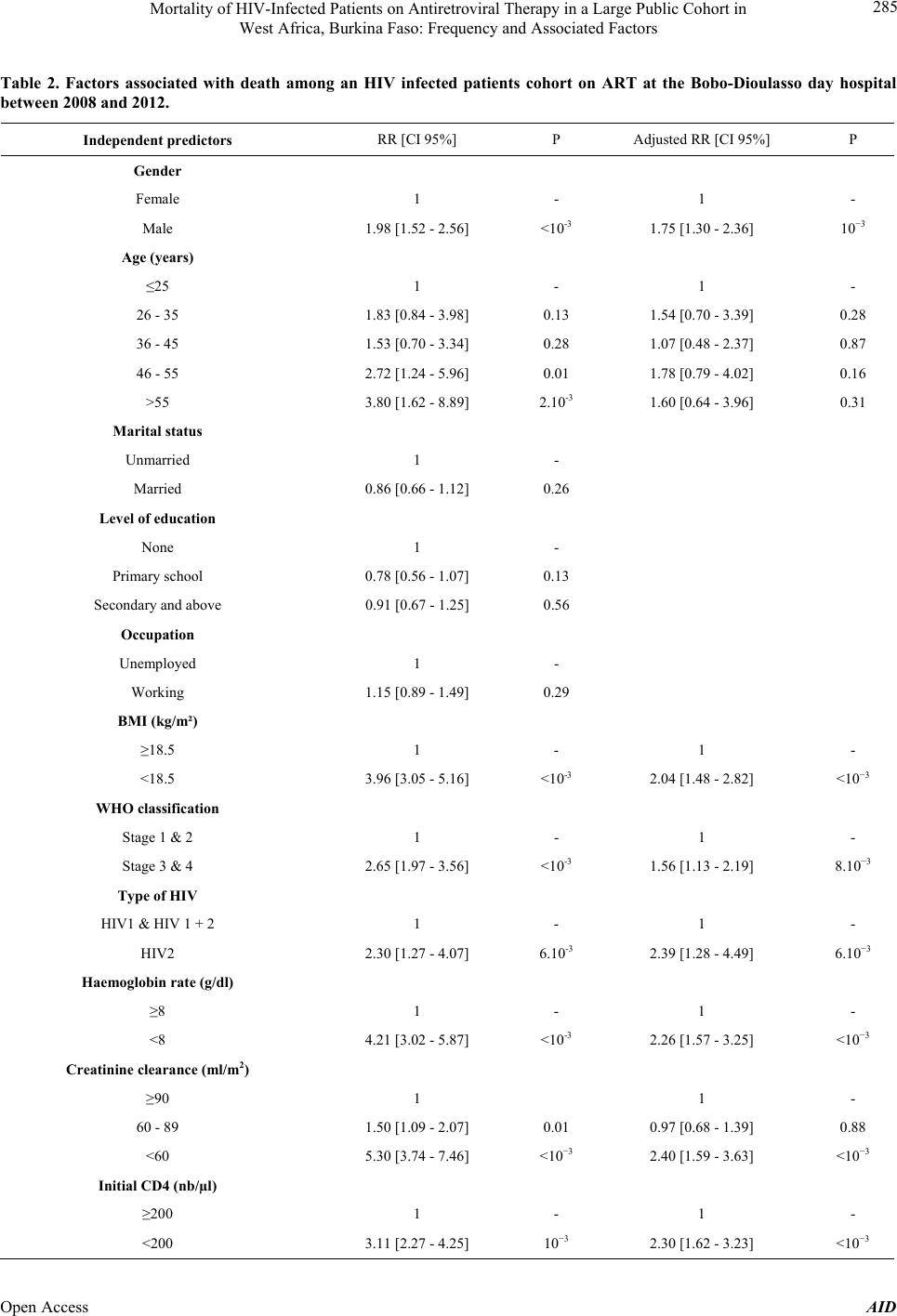 Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors Open Access AID 285 Table 2. Factors associated with death among an HIV infected patients cohort on ART at the Bobo-Dioulasso day hospital between 2008 and 2012. Independent predictors RR [CI 95%] P Adjusted RR [CI 95%] P Gender Female 1 - 1 - Male 1.98 [1.52 - 2.56] <10-3 1.75 [1.30 - 2.36] 10−3 Age (years) ≤25 1 - 1 - 26 - 35 1.83 [0.84 - 3.98] 0.13 1.54 [0.70 - 3.39] 0.28 36 - 45 1.53 [0.70 - 3.34] 0.28 1.07 [0.48 - 2.37] 0.87 46 - 55 2.72 [1.24 - 5.96] 0.01 1.78 [0.79 - 4.02] 0.16 >55 3.80 [1.62 - 8.89] 2.10-3 1.60 [0.64 - 3.96] 0.31 Marital status Unmarried 1 - Married 0.86 [0.66 - 1.12] 0.26 Level of education None 1 - Primary school 0.78 [0.56 - 1.07] 0.13 Secondary and above 0.91 [0.67 - 1.25] 0.56 Occupation Unemployed 1 - Working 1.15 [0.89 - 1.49] 0.29 BMI (kg/m²) ≥18.5 1 - 1 - <18.5 3.96 [3.05 - 5.16] <10-3 2.04 [1.48 - 2.82] <10−3 WHO classification Stage 1 & 2 1 - 1 - Stage 3 & 4 2.65 [1.97 - 3.56] <10-3 1.56 [1.13 - 2.19] 8.10−3 Type of HIV HIV1 & HIV 1 + 2 1 - 1 - HIV2 2.30 [1.27 - 4.07] 6.10-3 2.39 [1.28 - 4.49] 6.10−3 Haemoglobin rate (g/dl) ≥8 1 - 1 - <8 4.21 [3.02 - 5.87] <10-3 2.26 [1.57 - 3.25] <10−3 Creatinine clearance (ml/m2) ≥90 1 1 - 60 - 89 1.50 [1.09 - 2.07] 0.01 0.97 [0.68 - 1.39] 0.88 <60 5.30 [3.74 - 7.46] <10−3 2.40 [1.59 - 3.63] <10−3 Initial CD4 (nb/µl) ≥200 1 - 1 - <200 3.11 [2.27 - 4.25] 10−3 2.30 [1.62 - 3.23] <10−3  Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 286 and advanced immune-depression on ART initiation are the major causes for early mortality of patients taking ART among cohorts in countries with poor resources. Throughout the survey we found out that men were nearly two times more exposed to death risks than wo- men. A strong relationship between male gender and mortality when taking ART was reported in other African cohorts [11,24,25]. In our centre, there was no significant difference between men and women lost to follow up. In Kenya, a survey demonstrated that the rate of men in follow up programmes is lower than that of women [26]. Besides, men have a bad observance of the ART [27]. However, a survey in China showed that death risk on ART is higher when the patient does not regularly have medical visits and that women do not regularly attend checkups as men [28]. Through our results, death risk was higher among HIV-2 infected patients compared to HIV-1 and HIV-1 + 2 infected patients in a multivariate analysis. A study conducted in Ouagadougou, Burkina Faso shows that si- milar results are reported in non-governmental centres treating HIV infected patients [29]. In this study among patients with advanced immune-depression, there is a hi- gher mortality rate and a lower immune restoration among HIV-2 patients compared to HIV-1 patients. In one hand, results on immunity restoration were dis- covered in Gambia [30] and Ivory Coast [12]. On the other hand, in the Gambian survey mortality was higher among HIV-1 patients compared to HIV-2 and HIV-1 + 2 patients [30]. In the study carried out in Ivory Coast, there is no statistical difference in the mortality rate re- garding the type of HIV [12]. In a prospective cohort survey conducted in Guinea Bissau between 1990 and 2007, 223 HIV-1 infected patients participated in the study. Those with a HIV-2 infection in addition to HIV-1 presented a slow progression towards AIDS and had a higher TCD4 lymphocytes rate [31]. We also noticed that 9.8% of patients in our cohort had a creatinine clearance < 60 ml/m2 at the ART initiation. In Zambia, a survey came out to the same proportions. Similarly, the authors of the study found a very close relation between the high rate of creatinine at ART initia- tion and death occurrence [32]. However, it is demon- strated that in countries with poor resources, where there are not always etiologic investigations in case of high creatinine, the initiation of ART improved patients’ kid- ney function [33]. Factors associated to kidney impair- ment among HIV infected patients initiating ART com- bine diabetes, high blood pressure, heart diseases, tradi- tional treatment and HIV-related factors such as low TCD4 lymphocytes, high viral load, WHO stage III and IV, and low BMI [34,35]. Nowadays according to WHO recommendations [36] tenofovir is widely used in Africa, so there are many problems with patients suffering from a kidney insufficiency when starting an ART. Though it is very efficient and well tolerated [36-38], tenofovir worsens existing kidney lesions among African cohorts [39-41]. Finally, in our study, age and level of education were not considered as risk factors. In Ethiopia, patients with a primary school education level have higher death risks than uneducated ones [14]. Our study has weaknesses: the Bobo-Dioulasso day hospital is a national reference teaching hospital with an experienced team. It provides free services including bio- logical assessment and most opportunistic infection treat- ments. Our results are not representing the situation in the overall country, especially the HIV care in remote health districts where there are few practitioners and peo- ple have limited resources. However, a survey done by DWB-Holland tries to show that mortality in both verti- cal and integrated programmes is similar [42]. Because of the retrospective aspect of our study, data collection and the quality of some information have limitations. Some data are also missing and some factors such as viral load, opportunistic infections, death causes, and ob- servance assessment were not taken into account. Finally, the number of those lost to follow up and the fact that we have no information on them could be a factor which does not make for the true assessment of mortality rates. 5. Conclusion In our cohort, the mortality rate was 5.4 deaths per 100 person-years. Male gender, BMI <18.5 kg/m2, WHO stages 3 and 4, the number of T-CD4 lymphocytes < 200/μl, hemoglobin rate < 8 g/dl, creatinine ≥120 µmol/l were factors independently associated with death. This study provides for the first time, mortality rates and death risk factors for patients on ART in a cohort with a large number of participants in a government centre in Burkina Faso and West Africa. It emphasizes once again the need for screening and early initiation of ART. 6. Acknowledgements The authors thank all patients of the Day Hospital of Bo- bo Dioulasso, the male nurses, and all non-medical staff of our department. REFERENCES [1] M. S. Cohen, Y. Q. Chen, M. Mc Cauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, et al., “Prevention of HIV-1 Infection with Early Antiretroviral Therapy,” New England Journal of Medicine, Vol. 365, No. 6, 2011, pp. 493-505. http://dx.doi.org/10.1056/NEJMoa1105243 [2] UNAIDS, “UNAIDS World AIDS Day Report 2012,” Open Access AID  Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 287 2012. http://www.unaids.org/ [3] F. J. Palella, M. Deloria-Knoll, J. S. Chmiel, A. S. Moor- man, K. C. Wood, A. E. Greenberg, et al., “Survival Be- nefit of Initiating Antiretroviral Therapy in HIV-Infected Persons in Different CD4+ Cell Strata,” Annals of Inter- nal M edici ne, Vol. 138, No. 8, 2003, pp. 620-626. http://dx.doi.org/10.7326/0003-4819-138-8-200304150-0 0007 [4] M. Egger, M. May, G. Chêne, A. N. Phillips, B. Leder- gerber, F. Dabis, et al., “Prognosis of HIV-1-Infected Pa- tients Starting Highly Active Antiretroviral Therapy: A Collaborative Analysis of Prospective Studies,” Lancet, Vol. 360, No. 9327, 2002, pp. 119-129. http://dx.doi.org/10.1016/S0140-6736(02)09411-4 [5] D. Coetzee, K. Hildebrand, A. Boulle, G. Maartens, F. Louis, V. Labatala, et al., “Outcomes after Two Years of Providing Antiretroviral Treatment in Khayelitsha, South Africa,” AIDS, Vol. 18, No. 6, 2004, pp. 887-895. [6] S. D. Lawn, A. D. Harries, X. Anglaret, L. Myer and R. Wood, “Early Mortality among Adults Accessing Antire- troviral Treatment Programmes in Sub-Saharan Africa,” AIDS, Vol. 22, No. 15, 2008, pp. 1897-1908. http://dx.doi.org/10.1097/QAD.0b013e32830007cd [7] P. Braitstein, M. W. Brinkhof, F. Dabis, M. Schechter, A. Boulle, P. Miotti, et al., “Mortality of HIV-1-Infected Pa- tients in the First Year of Antiretroviral Therapy: Com- parison between Low-Income and High-Income Coun- tries,” Lancet, Vol. 367, No. 9513, 2006, pp. 817-824. http://dx.doi.org/10.1016/S0140-6736(06)68337-2 [8] C. Fontaine, A. Hema, E. Kamboule, J. B. Guiard-Schmid, F. X. Lescure, L. Slama, et al., “Bobo Dioulasso Teach- ing Hospital Day-Care Hospital: A Reference Structure for the Management of HIV Infected Patients in Burkina Faso,” Vol. 40, No. 7, 2010, pp. 393-397. [9] S. Jaffar, P. Munderi and H. Grosskurth, “Adherence to Antiretroviral Therapy in Africa: How High Is It Real- ly?” Tropical Medicine and International, Vol. 13, No. 9, 2008, pp. 1096-1097. http://dx.doi.org/10.1111/j.1365-3156.2008.02131.x [10] A. Gupta, G. Nadkarni, W. T. Yang, A. Chandrasekhar, N. Gupte, G. P. Bisson, et al., “Early Mortality in Adults Ini- tiating Antiretroviral Therapy (ART) in Low- and Mid- dle-Income Countries (LMIC): A Systematic Review and Meta-Analysis,” PLoS One, Vol. 6, No. 12, 2011, Article ID: e28691. http://dx.doi.org/10.1371/journal.pone.0028691 [11] J. F. Etard, I. Ndiaye, M. Thierry-Mieg, N. F.Guèye, P. M. Guèye, I. Lanièce, et al., “Mortality and Causes of Death in Adults Receiving Highly Active Antiretroviral Therapy in Senegal: A 7-Year Cohort Study,” AIDS, Vol. 20, No. 8, 2006, pp. 1181-1189. http://dx.doi.org/10.1097/01.aids.0000226959.87471.01 [12] S. Toure, B. Kouadio, C. Seyler, M. Traore, N. Dakoury- Dogbo, J. Duvignac, et al., “Rapid Scaling-Up of Anti- retroviral Therapy in 10,000 Adults in Côte d’Ivoire: 2- Year Outcomes and Determinants,” AIDS, Vol. 22, No. 7, 2008, pp. 873-882. http://dx.doi.org/10.1097/QAD.0b013e3282f768f8 [13] M. Rougemont, B. E. Stoll, N. Elia and P. Ngang, “Anti- retroviral Treatment Adherence and Its Determinants in Sub-Saharan Africa: A Prospective Study at Yaoundé Central Hospital, Cameroon,” AIDS Research Therapy, Vol. 6, 2009, p. 21. http://dx.doi.org/10.1186/1742-6405-6-21 [14] S. Biadgilign, A. A. Reda and T. Digaffe, “Predictors of Mortality among HIV Infected Patients Taking Antiretro- viral Treatment in Ethiopia: A Retrospective Cohort Stu- dy,” AIDS Research Therapy, Vol. 9, No. 1, 2012, p. 15. http://dx.doi.org/10.1186/1742-6405-9-15 [15] D Jerene, A Naess and B Lindtjørn, “Antiretroviral The- rapy at a District Hospital in Ethiopia Prevents Death and Tuberculosis in a Cohort of HIV Patients,” AIDS Re- search Therapy, Vol. 3, 2006, p. 10. http://dx.doi.org/10.1186/1742-6405-3-10 [16] G. E. Poda, M. Seydi, N. M. Manga, A. B. Dieng and P. S. “Sow, Immune Reconstitution Syndrome in the Course of Antiretroviral Treatment in Senegal,” Medicine et Mala- dies Infectieuses, Vol. 39, No. 5, 2009, pp. 350-351. http://dx.doi.org/10.1016/j.medmal.2009.01.001 [17] R. M. Novak, J. T. Richardson, K. Buchacz, J. S. Chmiel, M. D. Durham, F. J. Palella, et al., “Immune Reconstitu- tion Inflammatory Syndrome: Incidence and Implications for Mortality,” AIDS, Vol. 26, No. 6, 2012, pp. 721-731. http://dx.doi.org/10.1097/QAD.0b013e3283511e91 [18] D. M. Moore, C. T. Yiannoutsos, B. S. Musick, J. Tappe- ro, R. Degerman, J. Campbell, et al., “Determinants of Early and Late Mortality among HIV-Infected Individuals Receiving Home-Based Antiretroviral Therapy in Rural Uganda,” Journal of Acquired Immune Deficency Syn- dromes, Vol. 58, No. 3, 2011, pp. 289-296. http://dx.doi.org/10.1097/QAI.0b013e3182303716 [19] J. S. Stringer, I. O. Zulu, J. Levy, E. M. Stringer, A. Mwango, B. H. Chi, V. Mtonga, et al., “Rapid Scale-Up of Antiretroviral Therapy at Primary Care Sites in Zambia: Feasibility and Early Outcomes,” Journal of the Ameri- can Medicine Association, Vol. 296, No. 7, 2006, pp. 782-793. http://dx.doi.org/10.1001/jama.296.7.782 [20] J. Van Griensven and S. Thai, “Predictors of Immune Re- covery and the Association with Late Mortality While on Antiretroviral Treatment in Cambodia,” Transactions of the Royal Society of the Tropical Medicine Hygiene, Vol. 105, No. 12, 2011, pp. 694-703. http://dx.doi.org/10.1016/j.trstmh.2011.08.007 [21] A. López-Martínez , N. M. O’Brien, Y. Caro-Vega, B. Crabtree-Ramírez and J. Sierra-Madero, “Different Base- line Characteristics and Different Outcomes of HIV-In- fected Patients Receiving HAART through Clinical Trials Compared with Routine Care in Mexico,” Journal of Ac- quired Immune Deficiency Syndromes, Vol. 59, No. 2, 2012, pp. 155-160. http://dx.doi.org/10.1097/QAI.0b013e31823ff035 [22] D. Cuong do, A. Thorson , A. Sönnerborg, N. P. Hoa, N. T. Chuc, H. D. Phuc, et al., “Survival and Causes of Death among HIV-Infected Patients Starting Antiretrovi- ral Therapy in North-Eastern Vietnam,” Scandinavian Journal of Infectious Diseases, Vol. 44, No. 3, 2012, pp. Open Access AID  Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors 288 201-208. http://dx.doi.org/10.3109/00365548.2011.631937 [23] F. Fregonese, I. J. Collins, G. Jourdain, S. Lecoeur, T. R. Cressey, N. Ngo-Giang-Houng, et al., “Predictors of 5- Year Mortality in HIV-Infected Adults Starting Highly Active Antiretroviral Therapy in Thailand,” Journal of Acquired Immune Deficiency Syndromes, Vol. 60, No. 6, 2012, pp. 91-98. http://dx.doi.org/10.1097/QAI.0b013e31824bd33f [24] M. Bastard, M. B. Fall, I. Lanièce, B. Taverne, A. Des- claux, R. Ecochard, et al., “Revisiting Long-Term Adhe- rence to Highly Active Antiretroviral Therapy in Senegal Using Latent Class Analysis,” Journal of Acquired Im- mune Deficiency Syndromes, Vol. 57, No. 1, 2011, pp. 55-61. http://dx.doi.org/10.1097/QAI.0b013e318211b43b [25] E. J. Mills, C. Bakanda, J. Birungi, K. Chan, R. S. Hogg, N. Ford, et al., “Male Gender Predicts Mortality in a Large Cohort of Patients Receiving Antiretroviral Thera- py in Uganda,” Journal of International AIDS Society, Vol. 14, p. 52. http://dx.doi.org/10.1186/1758-2652-14-52 [26] K. Wools-Kaloustian, S. Kimaiyo, L. Diero, A. Siika, J. Si- dle, C. T. Yiannoutsos, et al., “Viability and Effective- ness of Large-Scale HIV Treatment Initiatives in Sub-Sa- haran Africa: Experience from Western Kenya,” AIDS, Vol. 20, No. 1, 2006, pp. 41-48. http://dx.doi.org/10.1097/01.aids.0000196177.65551.ea [27] J. B. Nachega, M. Hislop, D. W. Dowdy, M. Lo, S. B. Omer, L. Regensberg, et al., “Adherence to Highly Ac- tive Antiretroviral Therapy Assessed by Pharmacy Claims Predicts Survival in HIV-Infected South African Adults,” Journal Acquired Immune Deficiency Syndromes, Vol. 46, No. 1, 2006, pp. 78-84. http://dx.doi.org/10.1097/01.qai.0000225015.43266.46 [28] Y. Zhang, Z. Dou, K. Sun, Y. Ma, R. Y. Chen, M. Bul- terys, et al., “Association between Missed Early Visits and Mortality among Patients of China National Free An- tiretroviral Treatment Cohort,” Journal of Acquired Im- mune Deficiency Syndromes, Vol. 60, No. 1, 2012, pp. 59-67. http://dx.doi.org/10.1097/QAI.0b013e31824c3d9f [29] K. Harries, R. Zachariah, M. Manzi, P. Firmenich, R. Ma- thela, J. Drabo, et al., “Baseline Characteristics, Response to and Outcome of Antiretroviral Therapy among Patients with HIV-1, HIV-2 and Dual Infection in Burkina Faso,” Transactions of the Royal Society of Tropical Medicine Hygiène, Vol. 104, No. 2, 2010, pp. 154-161. http://dx.doi.org/10.1016/j.trstmh.2009.08.012 [30] I. Peterson, O. Togun, T. De Silva, F. Oko, S. Rowland- Jones, A. Jaye, et al., “Mortality and Immunovirological Outcomes on Antiretroviral Therapy in HIV-1 and HIV- 2-Infected Individuals in the Gambia,” AIDS, Vol. 25, No. 17, 2011, pp. 2167-2175. http://dx.doi.org/10.1097/QAD.0b013e32834c4adb [31] J. Esbjörnsson, F. Månsson, A. Kvist, P. E. Isberg, S. No- wroozalizadeh, A. J. Biague, et al., “Inhibition of HIV-1 Disease Progression by Contemporaneous HIV-2 Infec- tion,” New England Journal of Medicine, Vol. 367, No. 3, 2012, pp. 224-232. http://dx.doi.org/10.1056/NEJMoa1113244 [32] L. B. Mulenga, G. Kruse, S Lakhi, R. A. Cantrell, S. E. Reid, I. Zulu, et al., “Baseline Renal Insufficiency and Risk of Death among HIV-Infected Adults on Antiretro- viral Therapy in Lusaka, Zambia,” AIDS, Vol. 22, No. 14, 2008, pp. 1821-1827. http://dx.doi.org/10.1097/QAD.0b013e328307a051 [33] P. J. Peters, D. M. Moore, J. Mermin, J. T. Brooks, R. Downing, W. Were, et al., “Antiretroviral Therapy Im- proves Renal Function among HIV-Infected Ugandans,” Kidney International, Vol. 74, No. 7, 2008, pp. 925-929. http://dx.doi.org/10.1038/ki.2008.305 [34] V. Jotwani, Y. Li, C. Grunfeld, A. I. Choi and M. G. Shli- pak, “Risk Factors for ESRD in HIV-Infected Individuals: Traditional and HIV-Related Factors,” American Journal of Kidney Diseases, Vol. 59, No. 5, 2012, pp. 628-635. http://dx.doi.org/10.1053/j.ajkd.2011.10.050 [35] L. Msango, J. A. Downs, S. E. Kalluvya, B. R. Kidenya, R. Kabangila, W. D. Johnson Jr., et al., “Renal Dysfunc- tion among HIV-Infected Patients Starting Antiretroviral Therapy,” AIDS, Vol. 25, No. 11, 2011, pp. 1421-1425. http://dx.doi.org/10.1097/QAD.0b013e328348a4b1 [36] M. W. Tang, P. J. Kanki and R. W. Shafer, “A Review of the Virological Efficacy of the 4 World Health Organiza- tion-Recommended Tenofovir-Containing Regimens for Initial HIV Therapy,” Clinical Infectious Diseases, Vol. 54, No. 6, 2012, pp. 862-875. http://dx.doi.org/10.1093/cid/cir1034 [37] R. Landman, M. Poupard, M. Diallo, N. F. Ngom Gueye, N. Diakhate, B. Ndiaye et al., “Tenofovir-Emtricitabine- Efavirenz in HIV-I-Infected Adults in Senegal: A 96- Week Pilot Trial in Treatment-Naive Patients,” Journal of International Association of Physicians in AIDS Care, Vol. 8, No. 6, 2009, pp. 379-384. http://dx.doi.org/10.1177/1545109709344352 [38] R. Scherzer, M. Estrella, Y. Li, A. I. Choi, S. G. Deeks, C. Grunfeld, et al., “Association of Tenofovir Exposure with Kidney Disease Risk in HIV Infection,” AIDS, Vol. 26, No. 7, 2012, pp. 867-875. http://dx.doi.org/10.1097/QAD.0b013e328351f68f [39] P. De Beaudrap, M. B. Diallo, R. Landman, N. F. Guèye, I. Ndiaye, A. Diouf, et al., “Changes in the Renal Func- tion after Tenofovir-Containing Antiretroviral Therapy Initiation in a Senegalese Cohort (ANRS 1215),” AIDS Research Human Retroviruses, Vol. 26, No. 11, 2010, pp. 1221-1227. http://dx.doi.org/10.1089/aid.2009.0261 [40] R. Scherzer, M. Estrella, Y. Li, A. I. Choi, S. G. Deeks, C. Grunfeld, et al., “Association of Tenofovir Exposure with Kidney Disease Risk in HIV Infection,” AIDS, Vol. 26, No. 7, 2012, pp. 867-875. http://dx.doi.org/10.1097/QAD.0b013e328351f68f [41] A. Brennan, D. Evans, M. Maskew, S. Naicker, P. Ive, I. Sanne, et al., “Relationship between Renal Dysfunction, Nephrotoxicity and Death among HIV Adults on Teno- fovir,” AIDS, Vol. 25, No. 13, 2011, pp. 1603-1609. http://dx.doi.org/10.1097/QAD.0b013e32834957da [42] J. Greig, D. P. O’Brien, N. Ford, T. Spelman, K. Sabapa- thy and L. Shanks, “Similar Mortality and Reduced Loss to Follow-Up in Integrated Compared with Vertical Pro- Open Access AID  Mortality of HIV-Infected Patients on Antiretroviral Therapy in a Large Public Cohort in West Africa, Burkina Faso: Frequency and Associated Factors Open Access AID 289 grams Providing Antiretroviral Treatment in Sub-Saharan Africa,” Journal of Acquired Immune Deficiebcy Syn- dromes, Vol. 59, No. 5, 2012, pp. e92-e98. http://dx.doi.org/10.1097/QAI.0b013e31824206c7
|