 Advances in Materials Physics and Chemistry, 2013, 3, 314-319 Published Online December 2013 (http://www.scirp.org/journal/ampc) http://dx.doi.org/10.4236/ampc.2013.38043 Open Access AMPC A New Heptamethine Cyanine-Based Near-Infrared Fluorescent Probe for Divalent Copper Ions with High Selectivity Zhixiang Han1,3*, Qingqing Yang1, Lihui Liang2*, Xiaobing Zhang3 1School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang, China 2Hunan Provincial People’s Hospital, Changsha, China 3State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry & Chemical Engineering, Hunan University, Changsha, China Email: *zhixianghan69@126.com, *416284649@qq.com Received November 4, 2013; revised December 5, 2013; accepted December 16, 2013 Copyright © 2013 Zhixiang Han et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Zhixiang Han et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian. ABSTRACT A new near-infrared fluorophore 2-(2-Aminoethyl) pyridine-tricarbocyanine (1) was rationally designed and synthe- sized as a fluorescent probe for detection of Cu2+ with high selectivity. The response of Probe 1 is based on the fluores- cence quenching upon binding to Cu2+. The sensing performance of the proposed Cu2+-sensitive Probe 1 was then in- vestigated. The probe can be applied to the quantification detection of Cu2+ with a linear concentration range covering from 4.8 × 10−7 to 1.6 × 10−4 mol/L and a detection limit of 9.3 × 10−8 mol/L. The experimental results showed that the response of 1 to Cu2+ was independent of pH in medium condition (pH 6.0 - 8.0), and exhibited excellent selectivity towards Cu2+ over other common metal cations. Keywords: Near-Infrared Dye; Fluorescent Probe; Cu2+ Ions 1. Introduction The design and synthesis of fluorescent probes for se- lective and sensitive detection of metal ions have at- tracted wide-spread interests of chemists, biologists, clinical biochemists and environmentalists in recent years [1]. Copper is an essential trace element in both plants and animals, including humans. Among the essen- tial heavy metals, the abundance of copper ranks the third in human body. It participates in many biological processes, such as haemoglobin synthesis (in utilization of Fe and regeneration of Hb), development of connec- tive tissue, normal functions of the central nervous sys- tem, and oxidative phosphorylation [2-4]. Nevertheless, copper of high concentration is highly toxic to some or- ganisms such as many bacteria and viruses [5]. Owing to its toxicity for bacteria, elevated concentrations of copper would ham- per the self-purification capability of the sea or rivers, and destroy the biological reprocessing systems in water. Copper is also found to be harmful to human at high concentration and has been suspected to cause the damage of infant liver in recent years. Accordingly, searching for efficient and reproducible analytical meth- ods for the copper assay is of great importance for envi- ronment and human health. Many analytical methods for detection of copper, in- cluding atomic absorption spectrometry (AAS) [6], in- ductively coupled plasma-mass spectroscopy (ICP-MS) [7], inductively coupled plasma-atomic emission spec- trometry (ICP-OES) [8], spectrophotometry [9], voltam- metry [10] and fluorescence spectroscopy [11], have been developed so far. Among these methods, fluores- cence spectroscopy offers significant advantages due to its nondestructive character, high sensitivity and speci- ficity, and the availability of a wide range of indicative dyes. Several fluorophores have been used to design flu- orescent probes for divalent copper ions including cal- cein [12], rhodamine [13], naphthalimide [14,15], pyrene [16,17], tris(2,2’-bipyridine)-ruthenium(II) [18], ben- *Corresponding author. 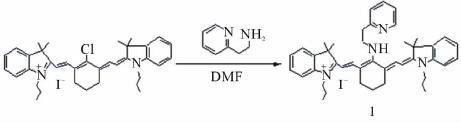 Z. X. HAN ET AL. 315 zoxazole [19], porphyrin [20,21], spiropyran [22,23], BODIPY [24], and so on. Unfortunately, limitations of the currently available probes for Cu2+ include low sensi- tivity, and/or excitation profiles in the ultraviolet or visi- ble region, which can damage living samples and cause interfering autofluorescence from native cells. The light in the near-infrared region (NIR) around 650 - 900 nm can penetrate more deeply into tissues, which is of importance to study on living organism im- aging. Moreover, it has a further advantage that auto- fluorescence is not observed upon NIR excitation. Hep- tamethine cyanine dyes [25], one of the important kinds of NIR dyes, has been widely used in various fields, and been employed as fluorescent labels in the studies of fluorescence imaging with biological mechanisms. And a few probes based on heptamethine cyanine dyes have been employed to detect metal ions or small molecules [26]. However, to the best of our knowledge, only few NIR fluorescent probes based on cyanine dyes have been reported for divalent copper ion assay detection [27]. Searching for new NIR probe for copper detection with high selectivity is still an active field as well as a chal- lenge for the analytical chemistry research. Herein, we report the synthesis and properties of a novel NIR fluorescent probe 2-(2-Aminoethyl) pyridine- tricarbocyanine (1) for the detection of Cu2+ with good selectivity and high sensitivity. Tricarbocyanine and 2-(2-aminoethyl) pyridine were selected as the reporter and cheleator, respectively. The probe exhibited stable response towards Cu2+ over the concentration range from 4.8 × 10−7 to 1.6 × 10−4 mol/L with a working pH range from pH 6.0 to 8.0. 2. Experimental 2.1. Reagents Before being used, N, N’-dimethylformamide (DMF) was subjected to simple distillation from K2CO3. IR-780 iodide was purchased from Sigma-Aldrich. 2-(2-Amino- ethyl) pyridine was purchased from Alfa Aesar. All other chemicals were of analytical reagent grade, purchased from Shanghai chemical Reagent Corporation (Shanghai, China), and used without further purification. Twice dis- tilled water was used throughout all experiments. Thin layer chromatography (TLC) was carried out using silica gel 60 F254, and column chromatography was conducted over silica gel (100 - 200 mesh), both of which were ob- tained from the Qingdao Ocean Chemicals (Qingdao, China). 2.2. Synthesis of Compound 1 Synthetic route for Compound 1 was depicted in Scheme 1. Briefly, IR-780 iodide (11.3 mg, 0.0167 mmol) and 2-(2-Amino-ethyl) pyridine (20.4 mg, 0.167 mmol) were Scheme 1. Synthetic pathway of Compound 1. dissolved in anhydrous DMF (3 mL) in a 25 mL round bottom flask. The mixture was stirred at 80˚C for 4 h under an argon atmosphere. The solvent was removed under reduced pressure, then purified on silica gel chro- matography eluted with CH2Cl2/ethanol (100:1, V/V) to afford the desired product as a blue solid (7.1 mg, yield 56%). 1H NMR (400 MHz, CDCl3): δ 0.93 (t, 6H, J = 7.4 Hz), 1.55(s, 12H), 1.63 - 1.73(m, 6H), 2.45 (t, 4H, J = 6.0 Hz), 3.21(t, 2H, J = 6.4 Hz), 3.92 (t, 4H, J = 6.8 Hz), 4.14(d, 2H, J = 6.4 Hz), 5.76 (m, 2H), 7.05(t, 2H, J = 6.8 Hz), 7.15(d, 2H, J = 8.0 Hz), 7.27 - 7.33(m, 4H), 7.43(m, 2H), 7.60(d, 2H, J = 12.8 Hz), 7.75(m, 1H), 8.50(d, 1H, J = 5.2 Hz), 8.68(br, 1H). ESI-MS: [M-I−] = 625.3, calculated: [M]+ = 625.9. 2.3. Apparatus 1H NMR spectra were recorded on a INOVE-400 (Varian) spectrometer operating at 400 MHz. All chemical shifts are reported in the standard δ notation of parts per mil- lion. LC-MS analyses were performed using an Agilent 1100 HPLC/MSD spectrometer; UV-Vis absorption spectra were recorded with a Shimadzu MultiSpec-1501 spectrophotometer. All fluorescence measurements were carried out on a HITACHI F4500 (Japan) with excitation slit set at 10.0 nm and emission at 20.0 nm. The pH measurements were carried out on Mettler-Toledo Delta 320 pH meter (Shanghai, China). 2.4. Measurement Procedures 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) buffer {0.1 mol/L, pH = 7.4, I = 0.1 (NaNO3)} were prepared by dissolving appropriate HEPES and NaNO3 in water, adjusting to pH = 7.4 by 1.0 mol/L NaOH with the volume to 1000 ml in a volumetric flask. A 2.0 × 10−5 mol/L stock solution of 1 was prepared by dissolving 1 in CH3CN. A stock standard solution of Cu2+ (0.01 mol/L) was prepared by dissolving an appro- priate amount copper in water and adjusting the volume to 100 ml, then further diluted to 1 × 10−3 - 1 × 10−7 mol/L stepwise. The buffered solutions of wide pH range were obtained by adjustment of 0.1 mol/L HEPES solu- tion with HCl or NaOH solution. The complex solution of Cu2+/1 was prepared by adding 5.0 mL the stock solu- tion of 1 and 1.0 mL mentioned above solution of Cu2+ in a 10 mL volumetric flask. Then the mixture was diluted to 10mL with pH 7.4 HEPES buffer solution. In the ob- Open Access AMPC 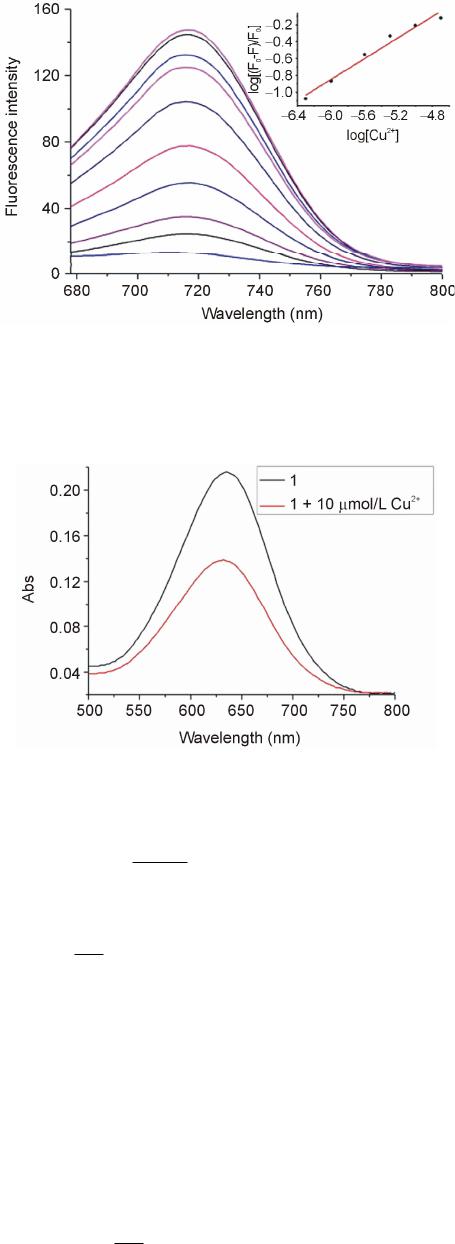 Z. X. HAN ET AL. 316 tained solution, the concentrations were 1 × 10−5 mol/L of 1 and 1 × 10−3 - 1 × 10−8 mol/L of Cu2+. Blank solu- tion of 1 was prepared under the same conditions without Cu2+. All the solution above were protected from light and kept at 4˚C for further use. 3. Results and Discussion 3.1. Spectral Characteristics Figure 1 showed the fluorescence spectra of 1 in HEPES buffer solutions with different concentrations of Cu2+, which recorded at excitation wavelength of 640 nm and emission wavelength of 670 - 800 nm. The spectrum of free 1 exhibited very strong fluorescence emission in a buffer solution. Addition of Cu2+ to a solution of 1, fluo- rescence signal exhibited a remarkable quenching. The fluorescence intensity of 1 was gradually decreased with increasing Cu2+ concentration. These results provided a proof for the formation of an inclusion complex of 1 with Cu2+, which constituted the basis for the determination of Cu2+ concentration with 1. It is worthy to note that the fluorescence intensity of Probe 1 can be recovered upon addition of the coordinating reagents ethylenediamine- tetraacetic acid (EDTA). In order to better understand the variation of fluores- cence intensity with the concentrations of Cu2+, the ab- sorption spectra of 1 in the absence and presence of Cu2+ were recorded (Figure 2). In the absorption spectrum of 1, the results showed a strong absorption band at 643nm in the absence of Cu2+, while the addition of Cu2+ ions decreased with no obvious shift in absorbance at 643 nm. From the fluorescence spectra and UV-is spectra, it is indicated that the fluorescence changes of 1 were more likely to be caused by the change of quantum yield rather than spectral shifts. Similar results were reported by Tang et al. [28]. In addition, Probe 1 works well and no detectable change in the linear range, detection limit or other ana- lytical performance is found after it has been stored for several weeks in the dark at 4˚C, which implies that the NIR fluorescent probe used is stable. 3.2. Principle of Operation The complexation equilibrium of 1 (A) with Cu2+ (B) with an association constant K can be expressed by the following equation: K nm mBnAA B (1) where Cu2+ (B) and 1 (A) is established by formation of a complex with a complexing ratio of m:n. According to the modified Stern–Volmer equation [29], the relation- ships for the changes of fluorescence intensities, the concentration of Cu2+ [B] in solution and the concentra- tion of 1 [A] in solution can be expressed as follows: Figure 1. Fluorescence emission spectrum of 1 (10 µmol/L) with different concentration of Cu2+ (From top to bottom: 0, 0.5, 1.0, 2.5, 5.0, 10, 20, 50, 100, 200 µmol/L) in CH3CN/H2O (1:1, v/v) solution. Inset shows the linear responses with divalent copper ions concentrations. Figure 2. Chages in the UV-vis spectra of 1 (10 µmol/L) (black line) upon the addition of Cu2+ (10 µmol/L) (red line). 1 0n q FF m AB (2) Assuming 0 FF , one can obtain: loglog1 loglog q F nAm F B (3) Here F0 and F denote the fluorescence intensities of 1 in the absence and presence of Cu2+, respectively. Kq is fluorescence quenching constant. The calibration curve was constructed by recording the fluorescence intensity values of 1 in the presence of different Cu2+ concentra- tion. In the range between 1.0 × 10−7 and 5.0 × 10−5 mol/L Cu2+, the fluorescence intensity is linearly de- pendent on the Cu2+ concentration. The dependency can be described by the following equation: 2 log0.9343log Cu4.80 F F (4) It is obvious from Equation (4) that m is the slope of Open Access AMPC 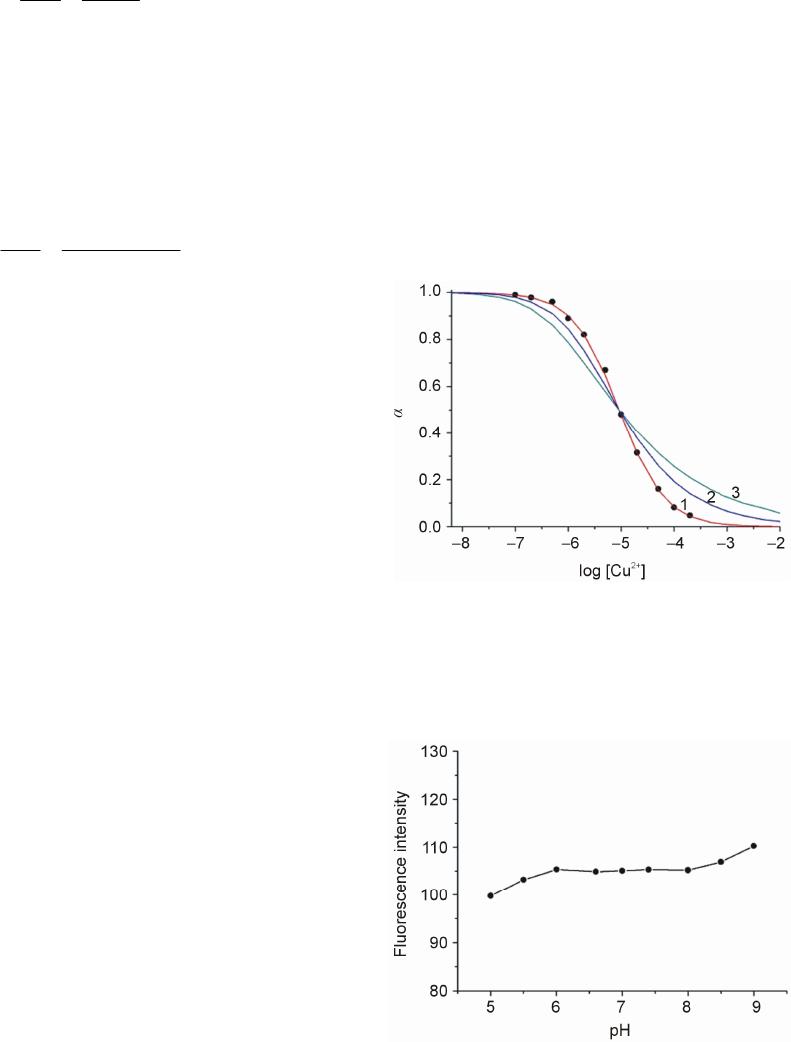 Z. X. HAN ET AL. 317 log(ΔF/F) versus log[Cu2+], which was calculated to be 1 approximately. Quenching constant (Kq) is 6.3 × 104. The relative fluorescence intensity α is defined as the ra- tio of free A [A]f, to the total amount of A [A]t, in the so- lution. It can be experimentally determined by measur- ing the fluorescence intensity of 1 in the solution: ft bt t A F FF (5) Here Fb is the fluorescence intensity of 1 in the blank buffer solution and Ft represents the fluorescence inten- sity of 1 in the solution when 1 is completely complexed with Cu2+. F is the fluorescence intensity of 1 actually measured when in contact with Cu2+ solutions of a given concentration. The relationship between the α and Cu2+ concentration [B] can be represented as: 1 1 1 n n t nK AB m (6) The response of 1 for different concentrations of Cu2+ was shown in Figure 3. Three curves are calculated us- ing Equation (6) with different K and ratios of Cu2+ and 1. It can be seen that the best curve was 1:1 complex ratio and an appropriate K of 1.09 × 105 fits form the experi- mental data. The curve can serve as the calibration curve for the detection of Cu2+ concentration. A practically usable range for quantitative determination covered from 4.8 × 10−7 to 1.6 × 10−4 mol/L (0.05 ≤ α ≤ 0.95) [29]. The detection limit was 9.3 × 10−8 mol/L (defined as three times standard deviation of blank solution). 3.3. Effect or pH The effects of pH on the fluorescence intensity of 1 in the presence of Cu2+ were carried out at a pH range from 5.0 to 9.0 with fixed the Cu2+ concentration at 5 μmol/L (Figure 4). In lower pH value, the fluorescence intensity of 1 decreased with decreasing pH value, which might be caused by the protonation of Compound 1 without bind- ing with the metal ion. On the other hand, too high pH would lead to form the precipitation of Cu(OH)2, and reduce its complexation with 1. In a wide range of pH from 6.0 to 8.0, acidity did not affect the determination of Cu2+ with Compound 1. In other words, the response behavior of Compound 1 is independent of pH in me- dium condition, which is convenient for practical appli- cations of the proposed probe in determination of Cu2+. 3.4. Selectivity Under the same conditions, the ability of 1 to recognize Cu2+ was further investigated by mixture 100 μmol/L Cu2+ with the other background anions and metal ions. The experiments were carried out by recording the changes of the fluorescence intensity before and after adding the interferants into the pH 7.4 HEPES buffer solution. As shown from Figure 5, one can see that the proposed probe exhibited a relatively high selectivity for Cu2+ ions over a large number of mono-, bi-, and triva- lent cations. Fortunately, normal interferents like Hg2+ do not interfere, which is better than that of the probe re- ported in literatures. 3.5. Preliminary Analytical Application The proposed probe was applied to the determination of copper ions in water samples of Xiang River. The river water samples were simply filtrated and showed that no Cu2+ was present in them. All the water samples were spiked with standard Cu2+ solutions at different concen- tration levels, and then analyzed their concentrations with proposed Probe 1. Results are shown in Table 1. One can see that the recovery study of spiked Cu2+ Figure 3. Relative fluorescence intensity α of 1 as a function of log[Cu2+] in CH3CN/H2O (1:1, v/v) solution. The curves fitting the experimental data were calculated from Equation (6). (1) m:n = 1:1, K= 1.09 × 105; (2) m:n = 1:2, K= 1.09 × 1010; (3) m:n = 1:3, K= 1.46 × 1015. (●) data points experi- mentally obtained. Figure 4. Effect of pH on the emission of 1 (10 μmol/L) with 5 μmol/L Cu2+ at 715 nm in CH3CN/H2O (1:1, v/v) solution. Excitation was provided at 640 nm. Open Access AMPC 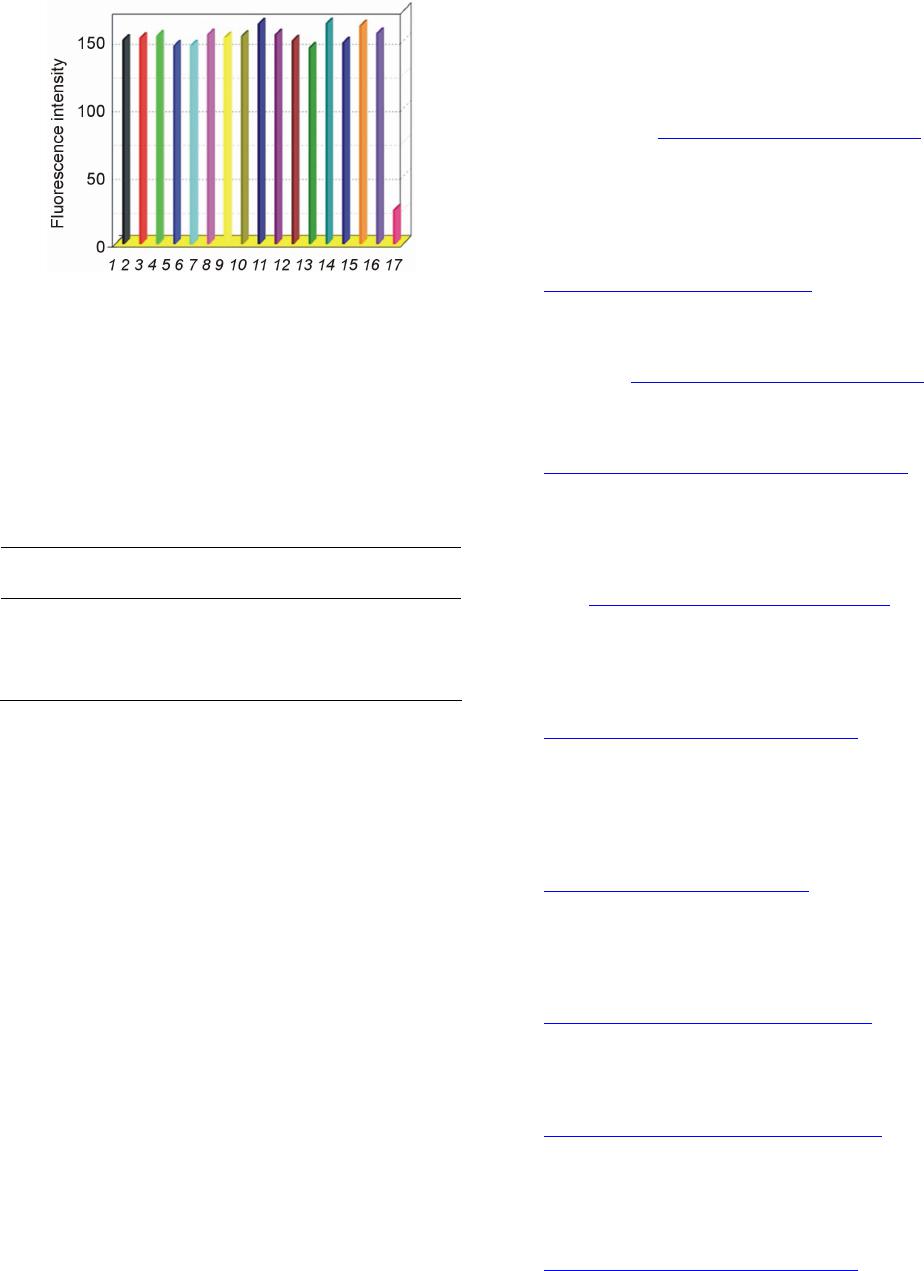 Z. X. HAN ET AL. 318 Figure 5. Emission change of 1 at 715 nm upon addition of each cation in CH3CN/H2O (1:1, v/v) solution at pH 7.40 {0.1 mol/L HEPES, I = 0.1 (NaNO3)}: 1, none; 2, Zn2+; 3, Cd2+; 4, Mn2+; 5, Ca2+; 6, Mg2+; 7, K+; 8, Na+; 9, Li+; 10, Al3+; 11, Co2+; 12, Ni2+; 13, Pb2+; 14, Hg2+; 15, Fe3+; 16, Ag+ 17, Cu2+. With the exception of Cu2+, Hg2+ and Al3+ where 10−4 mol/L of cation were added, each solution contained 10−3 mol/L of interest. The concentration of 1 was 10 μmol/L and excitation was provided at 640 nm. Table 1. Recovery study of spiked determination of copper in Xiang River water with proposed Probe 1. Sample Cu2+ spiked (mol/L) Cu2+ recovered (mol/L) Recovery (%) 1 0 0 – 2 1.0 × 10−5 (1.02a ± 0.03b) × 10−5 102.0 3 5.0 × 10−6 (4.93a ± 0.04b) × 10−6 98.6 aAverage were calculated with n = 3, bStandard deviations. determined by the 1-based probe showed satisfactory re- sults. The present probe is useful for the determination of Cu2+ in real samples. 4. Conclusion In summary, a new near-infrared fluorescent probe was designed and synthesized for the detection of Cu2+ based on quenching the fluorescence of tricarbocyanine chro- mophore with 1-Cu2+ complexation. Compared to re- ported fluorescent probes, 1-based probe showed high selectivity and large stokes shift over existing reagents and methods for the fluorescence determination of Cu2+ in neutral medium. And the proposed method can be used for the determination of Cu2+ in real samples. 5. Acknowledgements This work was supported by the National Natural Sci- ence Foundation of China (Grant 20505008, 21105038), “973” National Key Basic Research Program of China (2007CB310500), Ministry of Education of China (NCET-07-0272), and Hunan Natural Science Founda- tion (06JJ4010, 07JJ3025). REFERENCES [1] A. P. de Silva, H. Q. Gunaratne, T. Gunnlaugsson, A. J. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, “Signaling Recognition Events with Fluorescent Sensors and Switches,” Chemical Reviews, Vol. 97, No. 5, 1997, pp. 1515-1566. http://dx.doi.org/10.1021/cr960386p [2] E. Vuori, A. Huunan-Seppälä and J. O. Kilpiö, “Biologi- cally Active Metals in Human Tissues. I. The Effect of Age and Sex on the Concentration of Copper in Aorta, Heart, Kidney, Liver, Lung, Pancreas and Skeletal Mus- cle,” Scandinavian Journal of Work, Environment & Health, Vol. 4, No. 2, 1978, pp. 167-175. http://dx.doi.org/10.5271/sjweh.2712 [3] D. Radisky and J. Kaplan, “Regulation of Transition Metal Transport across the Yeast Plasma Membrane,” The Journal of Biological Chemistry, Vol. 274, 1999, pp. 4481-4484. http://dx.doi.org/10.1074/jbc.274.8.4481 [4] B. M. Rode and Y. Suwannachot, “The Possible Role of Cu(II) for the Origin of Life,” Coordination Chemistry Reviews, Vol. 190-192, 1999, pp. 1085-1099. http://dx.doi.org/10.1016/S0010-8545(99)00159-9 [5] C. Barranguet, F. P. van den Ende, M. Rutgers, A. M. Breure, M. Greijdanus, J. J. Sinke and W. Admiraal, “Copper-Induced Modifications of the Trophic Relations in Riverine Algal-Bacterial Biofilms,” Environmental To- xicology and Chemistry, Vol. 22, No. 6, 2003, pp. 1340- 1349. http://dx.doi.org/10.1002/etc.5620220622 [6] N. Pourreza and R. Hoveizavi, “Simultaneous Preconcen- tration of Cu, Fe and Pb as Methylthymol Blue Com- plexes on Naphthalene Adsorbent and Flame Atomic Absorption Determination,” Analytica Chimica Acta, Vol. 549, No. 1-2, 2005, pp. 124-128. http://dx.doi.org/10.1016/j.aca.2005.06.037 [7] J. S. Becker, M. V. Zoriy, C. Pickhardt, N. Palomero-Gal- lagher and K. Zilles, “Imaging of Copper, Zinc, and Other Elements in Thin Section of Human Brain Samples (Hip- pocampus) by Laser Ablation Inductively Coupled Plas- ma Mass Spectrometry,” Analytical Chemistry, Vol. 77, No. 10, 2005, pp. 3208-3216. http://dx.doi.org/10.1021/ac040184q [8] Z. H. Li, L. Zhang, Z. P. Zang, X. J. Chang and X. J. Zou, “Attapulgite Modified with 2-Hydroxy-1-Naphthaldehyde as Selective Solid-Phase Extractant for Determination of Copper(II) in Environmental Samples by ICP-OES,” Mi- crochim Acta, Vol. 171, No. 1-2, 2010, pp. 161-168. http://dx.doi.org/10.1007/s00604-010-0407-0 [9] J. J. Pinto, C. Moreno and M. Garcı́a-Vargas, “A Very Sensitive Flow System for the Direct Determination of Copper in Natural Waters Based on Spectrophotometric Detection,” Talanta, Vol. 64, No. 2, 2004, pp. 562-565. http://dx.doi.org/10.1016/j.talanta.2004.03.009 [10] V. Beni, V. Ogurtsov, N. Bakunin, D. W. Arrigan and M. Hill, “Development of A Portable Electroanalytical Syst- em for the Stripping Voltammetry of Metals: Determina- tion of Copper in Acetic Acid Soil Extracts,” Analytica Chimica Acta, Vol. 552, No. 1-2, 2005, pp. 190-200. http://dx.doi.org/10.1016/j.aca.2005.07.058 [11] Y. Zheng, X. Cao, J. Orbulescu, V. Konka, F. M. Andre- Open Access AMPC  Z. X. HAN ET AL. Open Access AMPC 319 opoulos, A. M. Pham and R. M. Leblanc, “Peptidyl Fluo- rescent Chemosensors for the Detection of Divalent Cop- per,” Analytical Chemistry, Vol. 75, No.7, 2003, pp. 1706-1712. http://dx.doi.org/10.1021/ac026285a [12] L. A. Saari and W. R. Seitz, “Immobilized Calcein for Metal Ion Preconcentration,” Analytical Chemistry, Vol. 56, No. 4, 1984, pp. 810-813. http://dx.doi.org/10.1021/ac00268a051 [13] X. Q. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, “Fluorescent Chemosensors Based on Spiroring-Opening of Xanthenes and Related Derivatives,” Chemical Re- views, Vol. 112, No. 3, 2012, pp. 1910-1956. http://dx.doi.org/10.1021/cr200201z [14] X. H. Qian, Y. Xiao, Y. F. Xu, X. F. Guo, J. H. Qian and W. P. Zhu, “ ‘Alive’ Dyes as Fluorescent Sensors: Fluoro- phore, Mechanism, Receptor and Imagesin Living Cells,” Chemical Communications, Vol. 46, 2010, pp. 6418-6436. http://dx.doi.org/10.1039/c0cc00686f [15] X. H. Zhao, Q. J. Ma, X. B. Zhang, B. Huang, Q. Jiang, J. Zhang, G. L. Shen and R. Q. Yu, “A Highly Selective Fluorescent Sensor for Cu2+ Based on a Covalently Im- mobilized Naphthalimide Derivative,” Analytical Science, Vol. 26, No. 5, 2010, pp. 585-590. http://dx.doi.org/10.2116/analsci.26.585 [16] R. Martínez, F. Zapata, A. Caballero, A. Espinosa , A. Tarraga and P. Molina, “2-Aza-1,3-butadiene Derivatives Featuring an Anthracene or Pyrene Unit: Highly Selec- tive Colorimetric and Fluorescent Signaling of Cu2+ Ca- tion,” Organic Letters, Vol. 8, No. 15, 2006, pp. 3235- 3238. http://dx.doi.org/10.1021/ol0610791 [17] H. J. Kim, J. Hong, A. Hong, S. Ham, J. H. Lee and J. S. Kim, “Cu2+-Induced Intermolecular Static Excimer For- mation of Pyrenealkylamine,” Organic. Letters, Vol. 10, No. 10, 2008, pp. 1963-1966. http://dx.doi.org/10.1021/ol800475d [18] C. L. He, F. L. Ren, X. B. Zhang, Y. Y. Dong and Y. Zhao, “A Fluorescent Chemosensor for Copper(II) Based on a Carboxylic Acid-functionalized Tris(2,2’-bipyri- dine)-ruthenium(II) Complex,” Analytical Science, Vol. 22, No. 12, 2006, pp. 1547-1551. http://dx.doi.org/10.2116/analsci.22.1547 [19] X. B. Zhang, J. Peng, C. L. He, G. L. Shen and R. Q. Yu, “A Highly Selective Fluorescent Sensor for Cu2+ Based on 2-(2’-Hydroxyphenyl)benzoxazole in a Poly(vinyl chloride) Matrix,” Analytica Chimica Acta, Vol. 567, No. 2, 2006, pp. 189-195. http://dx.doi.org/10.1016/j.aca.2006.03.025 [20] H. Y. Luo, X. B. Zhang, J. H. Jiang, C. Y. Li, J. Peng, G. L. Shen and R. Q. Yu, “An Optode Sensor for Cu2+ with High Selectivity Based on Porphyrin Derivative Ap- pended with Bipyridine,” Analytical Science, Vol. 23, No. 5, 2007, pp. 551-555. http://dx.doi.org/10.2116/analsci.23.551 [21] Y. Q. Weng, F. Yue, Y. R. Zhong and B. H. Ye, “A Cop- per(II) Ion-Selective On-Off-Type Fluoroionophore Based on Zinc Porphyrin-Dipyridylamino,” Inorganic Chemistry, Vol. 46, No. 19, 2007, pp. 7749-7755. http://dx.doi.org/10.1021/ic061709v [22] N. Shao, Y. Zhang, S. M. Cheung, R. H. Yang, W. H. Chan, T. Mo, K. A. Li and F. Liu, “Copper Ion-Selective Fluorescent Sensor Based on the Inner Filter Effect Using a Spiropyran Derivative,” Analytical Chemistry, Vol. 77, No. 22, pp. 2005, pp. 7294-7303. http://dx.doi.org/10.1021/ac051010r [23] N. Shao, J. Y. Jin, H. Wang, Y. Zhang, R. H. Yang and W. H. Chan, “Tunable Photochromism of Spirobenzopy- ran via Selective Metal Ion Coordination: An Efficient Visual and Ratioing Fluorescent Probe for Divalent Cop- per Ion,” Analytical Chemistry, Vol. 80, No. 9, 2008, pp. 3466-3475. http://dx.doi.org/10.1021/ac800072y [24] X. Qi, E. J. Jun, L. Xu, S. J. Kim, J. S. Hong and Y. J. Yoon and J. Yoon, “New BODIPY Derivatives as OFF- ON Fluorescent Chemosensor and Fluorescent Chemodo- simeter for Cu2+: Cooperative Selectivity Enhancement toward Cu2+,” The Journal of Organic Chemistry, Vol. 71, No. 7, 2006, pp. 2881-2884. http://dx.doi.org/10.1021/jo052542a [25] N. Narayanan and G. Patonay, “A New Method for the Synthesis of Heptamethine Cyanine Dyes: Synthesis of New Near-Infrared Fluorescent Labels,” The Journal of Organic Chemistry, Vol. 60, No. 8, 1995, pp. 2391-2395. http://dx.doi.org/10.1021/jo00113a018 [26] L. Yuan, W. Y. Lin, K. B. Zheng, L. W. He and W. M. He, “Far-red to Near Infrared Analyte-Responsive Fluo- rescent Probes Based on Organic Fluorophore Platforms for Fluorescence Imaging,” Chemical Society Reviews, Vol. 42, 2013, pp. 622-661. http://dx.doi.org/10.1039/c2cs35313j [27] P. Li, X. Duan, Z. Z. Chen, Y. Liu, T. Xie, L. B. Fang, X. R. Li, M. Yin and B. Tang, “A Near-Infrared Fluorescent Probe for Detecting Copper(II) with High Selectivity and Sensitivity and Its Biological Imaging Applications,” Chemical Communications, Vol. 47, 2011, pp. 7755-7757. http://dx.doi.org/10.1039/c1cc11885d [28] B. Tang, H. Huang, K. H. Xu, L. H. Tong, G. W. Yang, X. Liu and L. G. An, “Highly Sensitive and Selective Near- Infrared Fluorescent Probe for Zinc and Its Application to Macrophage Cells,” Chemical Communications, Vol. 34, 2006, pp. 3609-3611. http://dx.doi.org/10.1039/b606809j [29] W. H. Liu, Y. Wang, J. H. Tang, G. L. Shen and R. Q. Yu, “An Optical Fiber Sensor for Berberine Based on Immo- bilized 1,4-Bis(Naphth[2,1-d]Oxazole-2-yl)Benzene in a New Copolymer,” Talanta, Vol. 46, No. 4, 1998, pp. 679- 688. http://dx.doi.org/10.1016/S0039-9140(97)00330-5
|