 Chinese Medicine, 2013, 4, 137-147 Published Online December 2013 (http://www.scirp.org/journal/cm) http://dx.doi.org/10.4236/cm.2013.44017 Open Access CM PIP, Not FiO2 Regulates Expression of MMP-9 in the Newborn Rabbit VILI with Different Mechanical Ventilation Strategies Shaodong Hua1, Xiaoying Z hang 1, Shengli An2, Xiuxiang Liu3, Zhichun Feng4* 1Department of Pediatrics, BaYi Children’s Hospital of the General Military Hospital of Beijing PLA, Beijing, China 2Department of Biostatistics, South Medical University, Guangzhou, China 3The Hospital Affilicated Binzhou Medicall University, Binzhou, China 4Department of Pediatrics, BaYi Children’s Hospital of the General Military Hospital of Beijing PLA, Beijing, China Email: *fengzhichun81@163.com Received September 13, 2013; revised October 30, 2013; accepted November 16, 2013 Copyright © 2013 Shaodong Hua et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Results from experimental and clinical studies have shown that mechanical ventilation or/and hyperoxia may aggravate a pre-existing lung injury or even cause lung injury in healthy lungs by affecting the expression of MMP-9, but the MMP-9 effects are controversial. How are MMP-9 regulated when multicausative factors of injury such as different FiO2, PIP, and respiratory time (RT) impose simultaneously on lungs? Methods: Newborn New Zea- land white rabbits were randomly allocated to an unventilated air control group or to one of the 2 × 3 × 3 ventilation strategies by using a factorial design, with different FiO2, PIP, and RT. Then, lung wet-to-dry ratio (W/D), lung histo- pathology scores, transmission electron microscope, and cells in BALF were analyzed in these different groups. MMP-9 levels were studied by immunohistochemistry and ELISA. Results: MMP-9 levels were significantly different among 3 PIP ventilation regimes (F = 7.215) and MPIP group was the highest among 3 PIP groups. The lung histopathology score in 100% oxygen was significantly higher than in 45% oxygen group (F = 9.037) and MPIP group was the lowest among 3 PIP groups (F = 57.515) and RT 6 h was more serious than RT 1 h. MMP-9 positively correlated with mono- cytes, but negatively correlated with neutrophils and lung injury histopathology scores. Conclusions: Different PIP and FiO2 exert simultaneously on newborn lung in newborn rabbits ventilation, only mechanical stretch stimulation affects MMP-9 synthesis. Advisable mechanical stretch can promote MMP-9 expression and has protective role in lung in VILI. HPIP causes barotraumas and LPIP induces atelectrauma. Keywords: Mechanical Ventilation; Lung Injury; Matrix Metalloproteinase; Newborn Rabbit; Fraction of Inspired Oxygen; Peak Inspiratory Pressure 1. Introduction Mechanical ventilation (MV) is a life-saving therapy that can also damage the lungs. Matrix Metalloproteinase-9 (MMP-9) can degrade the complex components structure of the lungs and airway, such as extracellular matrix (ECM) and the basement membrane to participate in the lungs and airway reconstruction [1]. On the relationship between MMP-9 and lung injury, there were plenty of studies showing that expression of MMP-9 was regulated by factors of MV [2,3] and high concentrations of oxygen [4] as well as the expression of MMP-9 increases led to lung injury [5,6], but there were also some studies on protective role in MMP-9 [7,8], absence of MMP-9 wor- sens mechanical ventilation-induced lung injury (VILI) [9]. These conclusions are based on single-factor condi- tion model and different condition animal models have different experimental results [10]. However, clinically, VILI was multi-factorial, not only including oxygen con- centrations, peak inspiratory pressure (PIP), but also including duration of ventilation and so on. To support gas exchange, the parameters about oxygen concentra- tions and PIP are usually regulated. Importantly, PIP, hyperoxia and duration of ventilation (respiratory time, RT) can induce lung injury, but it has not yet to be de- termined whether these 3 factors regulated individually *Corresponding author.  S. D. HUA ET AL. 138 or simultaneously the expression of MMP-9 causing lung injury when multi-factors impose simultaneously on lungs. Did these factors interact, and/or was one more dominant than the other? Assessment of lung injury was only carried out in animal experiment.We hypothesized that these causative factors of injury could not simul- taneously promote MMP-9 producing, otherwise, VILI was impossible to be cured. 2. Methods 2.1. Ethics The use of animals was approved by hospital of Beijing Institutional Animal Care and Use Committee (IACUC) and conformed to the guidelines of the National Institutes of Health for the care and use of laboratory animals. 2.2. Animals and Experimental Protocol We employed 114 newborn New Zealand white rabbits (postnatal days, 1 - 5; 44.84 g). The rabbits were ran- domly allocated to either an unventilated air control group (n = 6) or to one of the 2 × 3 × 3 ventilation strate- gies by using a factorial design FiO2: FiO2 = 100% and FiO2 = 45%; PIP: high PIP (HPIP) = 25 cmH2O, mid PIP (MPIP) = 18 cmH2O and low PIP (LPIP) = 10 cmH2O; respiratory time (RT): 1 h, 3 h and 6 h; Each group had 6 rabbits, and there were108 rabbits in the ventilated groups. 2.3. Mechanical Ventilation The rabbits were anesthetized with intraperitoneal so- dium pentobarbital, 25 mg/kg. Their body-temperatures were maintained at 39˚C by a heating pad. A tracheo- stomy was performed near the thyroid eminence, and an endotracheal tube (1.3 × 25 mm2 intravenous catheter needles) was inserted via tracheostomy (the depth was 1.5 - 2.0 cm), and the endotracheal tube was regulated on the basis of the symmetry of thorax fluctuation after ven- tilation to avoid atelectasis. Then the rabbits were venti- lated (Siemens-900C, Germany) with a fixed positive end-expiratory pressure (PEEP) at 2 cmH2O with a res- piratory rate of (RR) 50 min−1, and an inspiratory time of 0.33 sec at differing levels of FiO2 and PIP depending on the RT according to a factorial design. No additional fluid support was given in any of the conducted experi- ments. At the end of the experiment, the rabbits from each experiment group were euthanized at 1, 3, and 6 h with a lethal dose of pentobarbital (100 mg/kg, i.p.). Im- mediately after sacrifice, lungs were isolated and meas- urements were performed as described below. 2.4. Measurements The left lung was weighed and subsequently dried for 2 days in an oven at 70˚C for estimating the wet-to-dry ratios (W/D). Bronchoalveolar lavage fluid (BALF) was obtained by instilling 1.0 ml saline 3 times by using a T catheter (Abbott, Sligo, Ireland) into the left trachea to lavage the left lung, and approximately 2.7 ml of BALF was retrieved per rabbit. Subsequently, BALF was cen- trifuged at 2000 g for 10 min, supernatants were snap- frozen in liquid nitrogen for later analysis, and wright- stained smears of cytospin slides of tracheal aspirates were examined for cell density (cells/ × 400 high power field) with white blood cells (WBC) and differential WBC counts (percentage) being done by an observer masked to the group identities. 2.5. Lung Histopathology To analyze the histopathology of the lungs, the right lung lower lobe was fixed in 4% formalin and embedded in paraffin. Sections of 4 μm in thickness were stained with hematoxylin and eosin (HE) and analyzed by a patho- logist who was blinded to the group identities. To score lung injury, we used a modified VILI histopathology scoring system as previously described [11,12]. An over- all score of VILI was obtained on the basis of the sum- mation of all the scores from air control or ventilated lungs (n = 6 per group). 2.6. Electron Microscopy Electron microscopy was performed to investigate the morphological changes in different ventilation groups. Lung tissues were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.4 for 18 h. Lung tissues were post-fixed for 1.5 h in 1% osmium tetroxide (OsO4), dis- solved in 0.1 M phosphate buffer at pH 7.4, dehydrated in an ascending acetone series, embedded in epon, sec- tioned at 70 nm, stained with uranyl-acetate and lead nitrate, and examined under an H-7500 transmission electron microscope (HITACHI, Japan). 2.7. Matrix Metalloproteinase-9 Assay At each RT point, the right lung middle lobe was har- vested and weighted 0.12 g pulmonary tissue samples and frozen at −70˚C until use. The concentrations of MMP-9 in lung tissue homogenate were assayed using a commerically available kit according to the manufac- ture's protocol (Rabbit MMP-9 ELISA Kit, Catalog No: E0553Rb, Wuhan EIAab Science.co., Ltd., China; http://www.eiaab.com). The concentrations of MMP-9 in lung tissue homogenate were expressed ng· mL−1. 2.8. Immunohistochemistry Immunohistochemical analysis of protein expression was performed on paraffin slides with the use of SABC kits Open Access CM  S. D. HUA ET AL. 139 (Boster Biological Technology, Ltd., Wuhan, China), Dako peroxidase kit (Dako, CA) and DAB reagent (DAKO, Denmark) as previously described [13], Tissue sections were incubated with primary antibodies (bioti- nylated anti rabbit MMP-9) and appropriate secondary antibodies (biotinylated goat anti rabbit). The sections were lightly counterstained with hematoxylin and bound antibody was visualized according to the standard avidin- biotinperoxidase complex protocol with a microscope (Nikon, Japan). The primary antibody was replaced by PBS for negative control slides and the known-positive slice was used as the positive control slides. The immu- noreactivities of the lung tissue specimens were scored independently by two pathologists who were blinded to the protocol and experimental groups using the following scheme: the yellow intensity of positive immunoreactiv- ity stained: 0 = no stain; 1 = stramineous; 2 = buffy; 3 = brown and the area of positive yellow stained (0 = 0; 1 = 0 - 1/3; 2 = 1/3 - 2/3; 3 = 2/3 - 1). MMP-9 expression was examined randomly in five HPFs (magnification ×400), and the total scores of the two parts represented the expression of MMP-9. 2.9. Statistical Analysis All data in the results section are expressed as mean ± standard deviation. 2 × 3 × 3 factorial design analysis of variance (ANOVA) was performed. Interaction signifi- cant needed to analyze simple effect with one-way ANOVA after fixed certain factor. Post Hoc Test for multiple comparisons, if equal variances assumed, LSD was perform; equal variances not assumed, Tamhane’s T2 test was performed. The χ2 test was used to compare the distribution of atelectasis. Bivariate correlation was used to determine the correlation of variable. The statis- tical significance level was set at p < 0.05. 3. Results 3.1. FiO2, PIP and RT Contribute to W/D. There Are Interactions to W/D between FiO2 and PIP Factorial design analysis of variance results show: there were significances in different FiO2 (F = 7.164, p = 0.009) and 100% oxygen group was higher than 45% oxygen groups, or in different PIP (F = 27.563, p = 0.000) groups and 18 cmH2O was the lowest among 3 PIP groups, or in different RT groups (F = 3.233, p = 0.044) and RT6 group was higher than RT1 group (p = 0.016). Further- more, there were interaction effect between FiO2 and PIP (F = 3.674, p = 0.029) (R squared = 0.479, Adjusted R squared = 0.381). The simple effect was analyzed. When FiO2 was 100% or 45%, One way ANOVA showed that 18 cmH2O group was the lowest in 3 PIP groups , re- spectively, (p = 0.000, 0.010) or (p = 0.000, 0.000). As for PIP, there was significance in different FiO2 groups and 100% oxygen groups was higher than 45% oxygen when PIP was fixed at 25 cmH2O (F = 4.209, p = 0.048) or 18 cmH2O (F = 10.241, p = 0.003), whilst, there was no significance in different FiO2 groups when PIP was fixed at 10 cmH2O (F = 0.270, p = 0.607) (Table 1). 3.2. PIP, RT 2 Factors Contribute to WBCs in BALF. There Are Interactions to Cells between FiO2 and RT as well as PIP and RT Factorial design ANOVA (Table 2) showed that the number of WBCs in BALF was similar in the 2 FiO2 groups (F = 0.122, p = 0.728), whereas PIP (F = 78.437, p < 0.001) and RT (F = 9.114, p < 0.001) had a signifi- cant effect on the number of WBCs. Moreover, there were interaction effects between FiO2 and RT (F = 6.206, p = 0.003) or between PIP and RT (F = 3.468, p = 0.011) (R Squared = 0.693, Adjusted R Squared = 0.636). The simple effect was analyzed. when FiO2 was 100%, ONE- WAY ANOVA showed that there were no significance in cells among RT 1 h, 3 h and 6 h groups (F = 2.386, p = 0.102), but fixed FiO2 was 45%, there were significance in cells among RT 1 h, 3 h and 6 h groups (F = 3.481, p = 0.038), multiple comparisons with Tamhane were no significance. when fixed RT was 3 h, 100% oxygen group was lower than 45% oxygen group in cells (F = 5.393, p = 0.026). But there were no significance when fixed RT was 1 h (F = 0.051, p = 0.822) and 6 h (F = 1.027, p = 0.318). When fixed PIP was 25 cmH2O, ONE-WAY ANOVA showed that there were signifi- cance in CELLS among RT 1 h, 3 h, 6 h groups (F = 3.923, p = 0.030) and RT 6 h group was the highest compared with RT 1 h and 3 h (p = 0.024, 0.019). When fixed PIP was 18 cmH2O, ONE-WAY ANOVA showed that there were significance in cells among 3 RT groups (F = 8.862, p = 0.001) and RT 3 h group was the highest compared with RT 1 h and 6 h (p = 0.000, 0.013). When fixed PIP was 10 cmH2O, ONE-WAY ANOVA showed that there were significance in cells among 3 RT groups (F = 6.611, p = 0.004) and RT 1 h group was the lowest compared with RT3 h and 6 h (p = 0.001, 0.040). As for fixed RT 1 h,3 h and 6 h, ONE-WAY ANOVA showed that there were significance in cells among 3 PIP groups, (F = 39.001, 11.188, 33.732, respectively, p = 0.000) and 25 cmH2O group was the highest compared with 18 and 10 cmH2O group in fixed RT 1 h, 3 h and 6 h. 3.3. FiO2, PIP, RT 3 Factors Contribute to Neutrophil in BALF. There Are Interactions to Neutrophil between FiO2 and PIP as well as PIP and RT Factorial design ANOVA showed that the neutrophil levels were significantly different in these ventilation regimes (Table 2). These results show that neutrophil le- Open Access CM 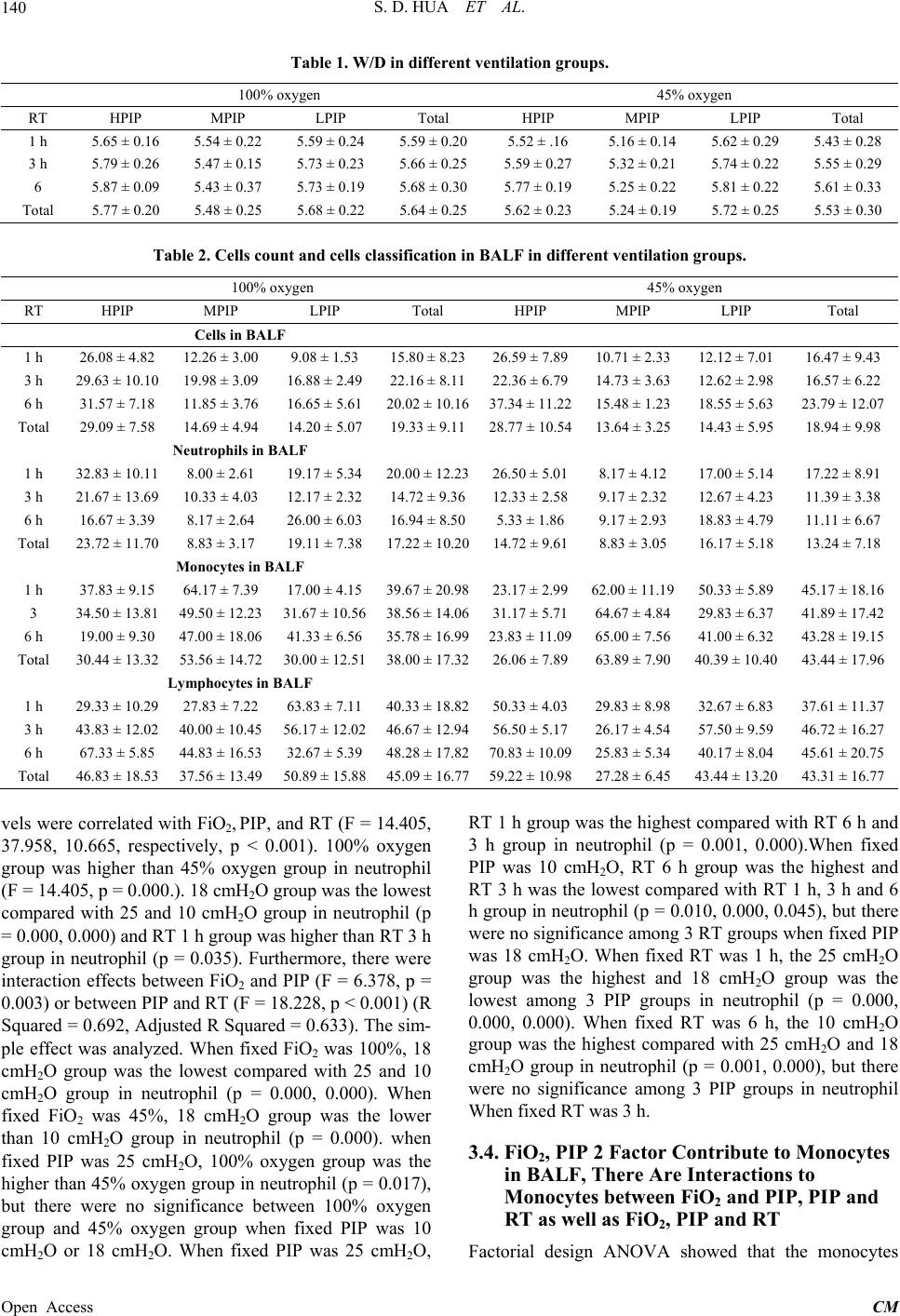 S. D. HUA ET AL. Open Access CM 140 Table 1. W/D in different ventilation groups. 100% oxygen 45% oxygen RT HPIP MPIP LPIP Total HPIP MPIP LPIP Total 1 h 5.65 ± 0.16 5.54 ± 0.22 5.59 ± 0.24 5.59 ± 0.20 5.52 ± .16 5.16 ± 0.14 5.62 ± 0.29 5.43 ± 0.28 3 h 5.79 ± 0.26 5.47 ± 0.15 5.73 ± 0.23 5.66 ± 0.25 5.59 ± 0.27 5.32 ± 0.21 5.74 ± 0.22 5.55 ± 0.29 6 5.87 ± 0.09 5.43 ± 0.37 5.73 ± 0.19 5.68 ± 0.30 5.77 ± 0.19 5.25 ± 0.22 5.81 ± 0.22 5.61 ± 0.33 Total 5.77 ± 0.20 5.48 ± 0.25 5.68 ± 0.22 5.64 ± 0.25 5.62 ± 0.23 5.24 ± 0.19 5.72 ± 0.25 5.53 ± 0.30 Table 2. Cells count and cells classification in BALF in different ventilation groups. 100% oxygen 45% oxygen RT HPIP MPIP LPIP Total HPIP MPIP LPIP Total Cells in BALF 1 h 26.08 ± 4.82 12.26 ± 3.00 9.08 ± 1.53 15.80 ± 8.2326.59 ± 7.8910.71 ± 2.3312.12 ± 7.01 16.47 ± 9.43 3 h 29.63 ± 10.10 19.98 ± 3.09 16.88 ± 2.4922.16 ± 8.1122.36 ± 6.7914.73 ± 3.6312.62 ± 2.98 16.57 ± 6.22 6 h 31.57 ± 7.18 11.85 ± 3.76 16.65 ± 5.6120.02 ± 10.1637.34 ± 11.2215.48 ± 1.2318.55 ± 5.63 23.79 ± 12.07 Total 29.09 ± 7.58 14.69 ± 4.94 14.20 ± 5.0719.33 ± 9.1128.77 ± 10.5413.64 ± 3.2514.43 ± 5.95 18.94 ± 9.98 Neutrophils in BALF 1 h 32.83 ± 10.11 8.00 ± 2.61 19.17 ± 5.3420.00 ± 12.2326.50 ± 5.018.17 ± 4.12 17.00 ± 5.14 17.22 ± 8.91 3 h 21.67 ± 13.69 10.33 ± 4.03 12.17 ± 2.3214.72 ± 9.3612.33 ± 2.589.17 ± 2.32 12.67 ± 4.23 11.39 ± 3.38 6 h 16.67 ± 3.39 8.17 ± 2.64 26.00 ± 6.0316.94 ± 8.505.33 ± 1.86 9.17 ± 2.93 18.83 ± 4.79 11.11 ± 6.67 Total 23.72 ± 11.70 8.83 ± 3.17 19.11 ± 7.3817.22 ± 10.2014.72 ± 9.618.83 ± 3.05 16.17 ± 5.18 13.24 ± 7.18 Monocytes in BALF 1 h 37.83 ± 9.15 64.17 ± 7.39 17.00 ± 4.1539.67 ± 20.9823.17 ± 2.9962.00 ± 11.1950.33 ± 5.89 45.17 ± 18.16 3 34.50 ± 13.81 49.50 ± 12.23 31.67 ± 10.5638.56 ± 14.0631.17 ± 5.7164.67 ± 4.8429.83 ± 6.37 41.89 ± 17.42 6 h 19.00 ± 9.30 47.00 ± 18.06 41.33 ± 6.5635.78 ± 16.9923.83 ± 11.0965.00 ± 7.5641.00 ± 6.32 43.28 ± 19.15 Total 30.44 ± 13.32 53.56 ± 14.72 30.00 ± 12.5138.00 ± 17.3226.06 ± 7.8963.89 ± 7.9040.39 ± 10.40 43.44 ± 17.96 Lymphocytes in BALF 1 h 29.33 ± 10.29 27.83 ± 7.22 63.83 ± 7.1140.33 ± 18.8250.33 ± 4.0329.83 ± 8.9832.67 ± 6.83 37.61 ± 11.37 3 h 43.83 ± 12.02 40.00 ± 10.45 56.17 ± 12.0246.67 ± 12.9456.50 ± 5.1726.17 ± 4.5457.50 ± 9.59 46.72 ± 16.27 6 h 67.33 ± 5.85 44.83 ± 16.53 32.67 ± 5.3948.28 ± 17.8270.83 ± 10.0925.83 ± 5.3440.17 ± 8.04 45.61 ± 20.75 Total 46.83 ± 18.53 37.56 ± 13.49 50.89 ± 15.8845.09 ± 16.7759.22 ± 10.9827.28 ± 6.4543.44 ± 13.20 43.31 ± 16.77 vels were correlated with FiO2, PIP, and RT (F = 14.405, 37.958, 10.665, respectively, p < 0.001). 100% oxygen group was higher than 45% oxygen group in neutrophil (F = 14.405, p = 0.000.). 18 cmH2O group was the lowest compared with 25 and 10 cmH2O group in neutrophil (p = 0.000, 0.000) and RT 1 h group was higher than RT 3 h group in neutrophil (p = 0.035). Furthermore, there were interaction effects between FiO2 and PIP (F = 6.378, p = 0.003) or between PIP and RT (F = 18.228, p < 0.001) (R Squared = 0.692, Adjusted R Squared = 0.633). The sim- ple effect was analyzed. When fixed FiO2 was 100%, 18 cmH2O group was the lowest compared with 25 and 10 cmH2O group in neutrophil (p = 0.000, 0.000). When fixed FiO2 was 45%, 18 cmH2O group was the lower than 10 cmH2O group in neutrophil (p = 0.000). when fixed PIP was 25 cmH2O, 100% oxygen group was the higher than 45% oxygen group in neutrophil (p = 0.017), but there were no significance between 100% oxygen group and 45% oxygen group when fixed PIP was 10 cmH2O or 18 cmH2O. When fixed PIP was 25 cmH2O, RT 1 h group was the highest compared with RT 6 h and 3 h group in neutrophil (p = 0.001, 0.000).When fixed PIP was 10 cmH2O, RT 6 h group was the highest and RT 3 h was the lowest compared with RT 1 h, 3 h and 6 h group in neutrophil (p = 0.010, 0.000, 0.045), but there were no significance among 3 RT groups when fixed PIP was 18 cmH2O. When fixed RT was 1 h, the 25 cmH2O group was the highest and 18 cmH2O group was the lowest among 3 PIP groups in neutrophil (p = 0.000, 0.000, 0.000). When fixed RT was 6 h, the 10 cmH2O group was the highest compared with 25 cmH2O and 18 cmH2O group in neutrophil (p = 0.001, 0.000), but there were no significance among 3 PIP groups in neutrophil When fixed RT was 3 h. 3.4. FiO2, PIP 2 Factor Contribute to Monocytes in BALF, There Are Interactions to Monocytes between FiO2 and PIP, PIP and RT as well as FiO2, PIP and RT Factorial design ANOVA showed that the monocytes  S. D. HUA ET AL. 141 counts in 100% oxygen group were lower than that of 45% oxygen group (F = 9.305, p = 0.003). There were significance in 3 PIP groups (F = 106.749, p = 0.000) and 18 cmH2O group was the highest than 25 and 10 cmH2O (p = 0.000, 0.000), but RT were no difference (F = 0.952, p = 0.390). Furthermore, there were interaction effects between FiO2 and PIP (F = 7.588, p = 0.001) or between PIP and RT (F = 5.092, p = 0.001) or among PIP, RT and FiO2 (R Squared = 0.771, Adjusted R Squared = 0.728). The simple effect was analyzed. When fixed FiO2 was 100%, 18 cmH2O group was the highest compared with 25 and 10 cmH2O groups in monocytes (p = 0.000, 0.000). When fixed FiO2 was 45%, 18 cmH2O group was the highest and 25 cmH2O group was the lowest among 3 PIP groups in monocytes (p = 0.000, 0.000, 0.000). When fixed PIP was 18 cmH2O or 10 cmH2O, 45% oxy- gen group was higher 100% oxygen group in monocytes (F = 6.887 or 7.339. p = 0.013 or 0.010), but 25 cmH2O group was no difference. As for interaction effects be- tween PIP and RT, when fixed PIP was 25 cmH2O,RT 3 h was higher than RT 6 h in monocytes (p = 0.009); When fixed PIP was 10 cmH2O, RT 6 h was higher than RT 3 h in monocytes (p = 0.007); however, there was no difference among 3 RT group in monocytes. When fixed RT 1 h or 3 h, 18 cmH2O group was the highest among 3 PIP groups(all of p were 0.000); When fixed RT 6 h, 18 cmH2O group was the highest and 25 cmH2O was the lowest among 3 PIP groups (p = 0.000, 0.000, 0.030) (Table 2). 3.5. PIP and RT Contributes to Lymphocytes. There Are Interactions to Lymphocytes between FiO2 and PIP, PIP and RT as well as FiO2, PIP and RT Factorial design ANOVA showed that the lymphocytes counts in 100% and 45% oxygen group were no differ- ence (F = 1.081, p = 0.301). There were significance in 3 PIP groups (F = 51.462, p = 0.000) and 18 cmH2O group was the lowest among 3 PIP groups (p = 0.000, 0.000). RT were difference (F = 9.366, p = 0.000.), but Post Hoc Test for 3 RT groups were no difference with Tamhane (p = 0.092, 0.157, 1.000). Furthermore, there were inter- action effects between FiO2 and PIP (F = 17.386,p = 0.001) or between PIP and RT (F = 20.852, p = 0.000) or among FiO2, RT and PIP (F = 11.785, p = 0.000). (R Squared = 0.762, Adjusted R Squared = 0.717) (Table 2). 3.6. FiO2, RT, PIP 3 Factors Contribute to Lung Injury Histopathology Scores Factorial design ANOVA showed that the lung histopa- thology scores in 100% oxygen was significantly higher than in 45% oxygen group (F = 9.037, p = 0.003) (Table 3) and 18 cmH2O group lung histopathology scores was the lowest among 3 PIP groups (F = 57.515, p < 0.000). RT were significant differences (F = 3.586, p = 0.032) and RT 6 h groups was higher than RT 1 h group (p = 0.010). However, there were no interaction effects among FiO2, RT and PIP. 3.7. PIP Contributes to MMP-9 Levels. There Was Interaction to MMP-9 between FiO2 and PIP in Lung Tissue Bomogenate Factorial design ANOVA showed that the MMP-9 levels were significantly different among 3 PIP ventilation re- gimes (F = 7.215, p = 0.932) and 18 cmH2O groups was the highest than the other 2 PIP groups (p = 0.000, 0.008), but there were no significance between the 25 and 10 cmH2O. FiO2 and RT did not contribute to MMP-9 (F = 0.007, 0.401; p = 0.932, 0.671, respectively). There were interaction to MMP-9 between FiO2 and PIP. When fixed PIP was 25 cmH2O, there were no significance between 100% and 45% oxygen group in MMP-9 (F = 0.583, p = 0.450). When fixed PIP was 18 cmH2O, 100% oxygen group was higher than 45% oxygen group in MMP-9 (F = 4.403, p = 0.043). When fixed PIP was 10 cmH2O, 100% oxygen group was lower than 45% oxygen group in MMP-9 (F = 4.392, p = 0.044). when fixed FiO2 was 100%, there were significances among 3 PIP groups (F = 10.622, p = 0.000) and the 18 cmH2O group was the highest than the others PIP groups (p = 0.002, 0.003) in MMP-9, ut there was no significance between 25 and 10 cmH2O.There were no significance among 3 PIP groups in MMP-9 (F = 1.248, p = 0.296) when fixed FiO2 was 45% (Table 4). 3.8. Pathology Ten of 108 rabbits were induced with pulmonary atelec- tasis (Figure 1(c), Table 5), and PIP (χ2 = 6.834, p < 0.05) or RT (χ2 = 8.154, p < 0.05) induced atelectasis significantly, but FiO2 did not (χ2 = 0.441, p > 0.05). Al- though the histopathological changes in the ventilation groups were greatly different from the control groups (Figure 2(a)), they shared the common structural chan- ges among these ventilation group. Change in lung struc- ture with patchy areas of parenchymal thickening and small airspaces interspersed with areas of enlarged air- spaces, with inflammatory cell infiltrated. Pathological features from the exudative phase to the early prolifera- tive phase of diffuse alveolar damage such as: epithelial destruction, capillary congestion, interstitial oedema, in- tra-alveolar oedema, haemorrhage, mononuclear infiltration, polymorphonuclear infiltration, interlobular septal thicken- ing, hyaline membrane formation, uneven alveolar ventila- tion and microatelectasis were observed in the present ex- perimental groups. The hemorrhage in the 100% oxygen Open Access CM 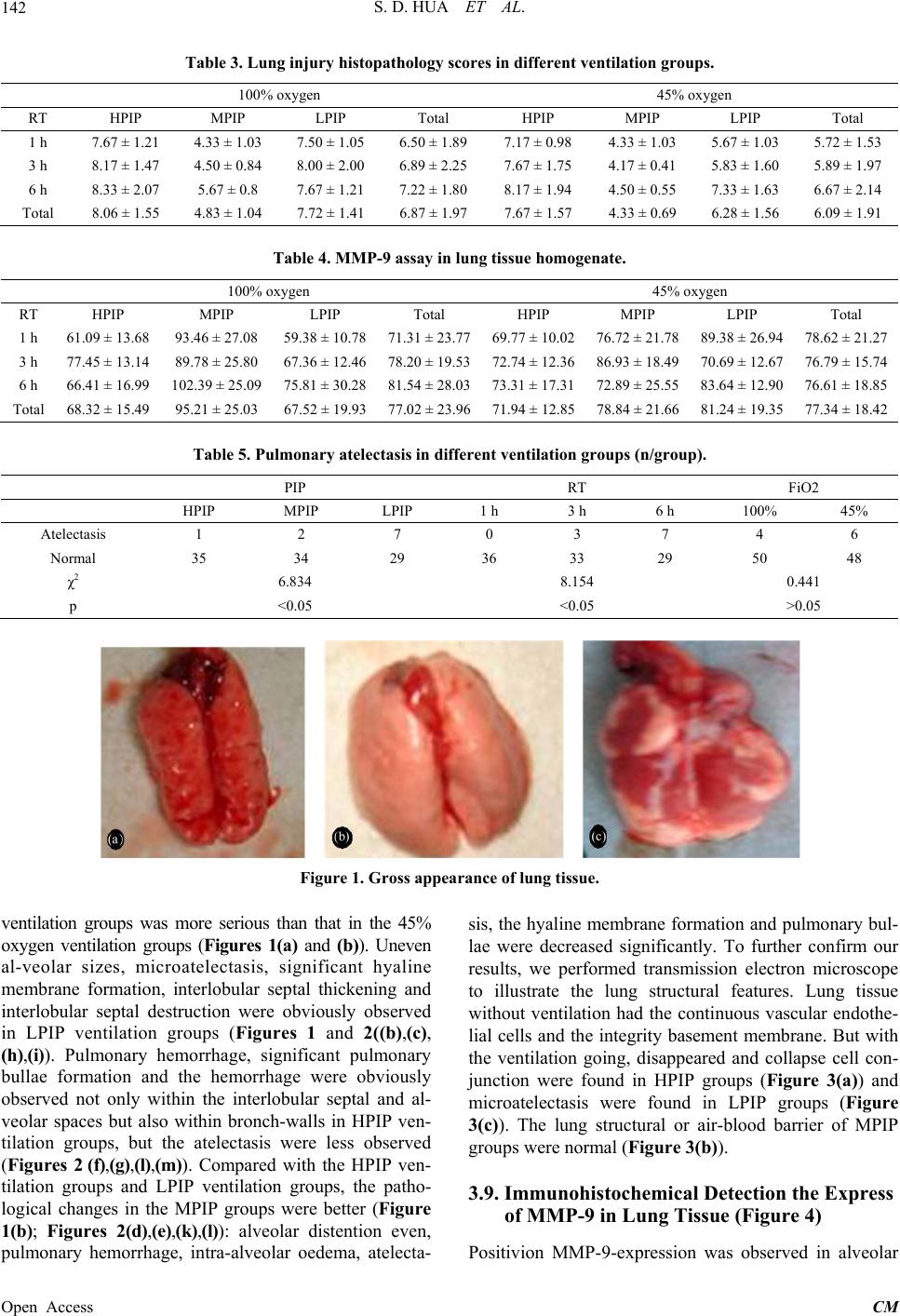 S. D. HUA ET AL. Open Access CM 142 Table 3. Lung injury histopathology scores in different ventilation groups. 100% oxygen 45% oxygen RT HPIP MPIP LPIP Total HPIP MPIP LPIP Total 1 h 7.67 ± 1.21 4.33 ± 1.03 7.50 ± 1.05 6.50 ± 1.89 7.17 ± 0.98 4.33 ± 1.03 5.67 ± 1.03 5.72 ± 1.53 3 h 8.17 ± 1.47 4.50 ± 0.84 8.00 ± 2.00 6.89 ± 2.25 7.67 ± 1.75 4.17 ± 0.41 5.83 ± 1.60 5.89 ± 1.97 6 h 8.33 ± 2.07 5.67 ± 0.8 7.67 ± 1.21 7.22 ± 1.80 8.17 ± 1.94 4.50 ± 0.55 7.33 ± 1.63 6.67 ± 2.14 Total 8.06 ± 1.55 4.83 ± 1.04 7.72 ± 1.41 6.87 ± 1.97 7.67 ± 1.57 4.33 ± 0.69 6.28 ± 1.56 6.09 ± 1.91 Table 4. MMP-9 assay in lung tissue homogenate. 100% oxygen 45% oxygen RT HPIP MPIP LPIP Total HPIP MPIP LPIP Total 1 h 61.09 ± 13.68 93.46 ± 27.08 59.38 ± 10.7871.31 ± 23.7769.77 ± 10.0276.72 ± 21.78 89.38 ± 26.94 78.62 ± 21.27 3 h 77.45 ± 13.14 89.78 ± 25.80 67.36 ± 12.4678.20 ± 19.5372.74 ± 12.3686.93 ± 18.49 70.69 ± 12.67 76.79 ± 15.74 6 h 66.41 ± 16.99 102.39 ± 25.09 75.81 ± 30.2881.54 ± 28.0373.31 ± 17.3172.89 ± 25.55 83.64 ± 12.90 76.61 ± 18.85 Total 68.32 ± 15.49 95.21 ± 25.03 67.52 ± 19.9377.02 ± 23.9671.94 ± 12.8578.84 ± 21.66 81.24 ± 19.35 77.34 ± 18.42 Table 5. Pulmonary atelectasis in different ventilation groups (n/group). PIP RT FiO2 HPIP MPIP LPIP 1 h 3 h 6 h 100% 45% Atelectasis 1 2 7 0 3 7 4 6 Normal 35 34 29 36 33 29 50 48 χ2 6.834 8.154 0.441 p <0.05 <0.05 >0.05 Figure 1. Gross appearance of lung tissue. ventilation groups was more serious than that in the 45% oxygen ventilation groups (Figures 1(a) and (b)). Uneven al-veolar sizes, microatelectasis, significant hyaline membrane formation, interlobular septal thickening and interlobular septal destruction were obviously observed in LPIP ventilation groups (Figures 1 and 2((b),(c), (h),(i)). Pulmonary hemorrhage, significant pulmonary bullae formation and the hemorrhage were obviously observed not only within the interlobular septal and al- veolar spaces but also within bronch-walls in HPIP ven- tilation groups, but the atelectasis were less observed (Figures 2 (f),(g),(l),(m)). Compared with the HPIP ven- tilation groups and LPIP ventilation groups, the patho- logical changes in the MPIP groups were better (Figure 1(b); Figures 2(d),(e),(k),(l)): alveolar distention even, pulmonary hemorrhage, intra-alveolar oedema, atelecta- sis, the hyaline membrane formation and pulmonary bul- lae were decreased significantly. To further confirm our results, we performed transmission electron microscope to illustrate the lung structural features. Lung tissue without ventilation had the continuous vascular endothe- lial cells and the integrity basement membrane. But with the ventilation going, disappeared and collapse cell con- junction were found in HPIP groups (Figure 3(a)) and microatelectasis were found in LPIP groups (Figure 3(c)). The lung structural or air-blood barrier of MPIP groups were normal (Figure 3(b)). 3.9. Immunohistochemical Detection the Express of MMP-9 in Lung Tissue (Figure 4) Positivion MMP-9-expression was observed in alveolar 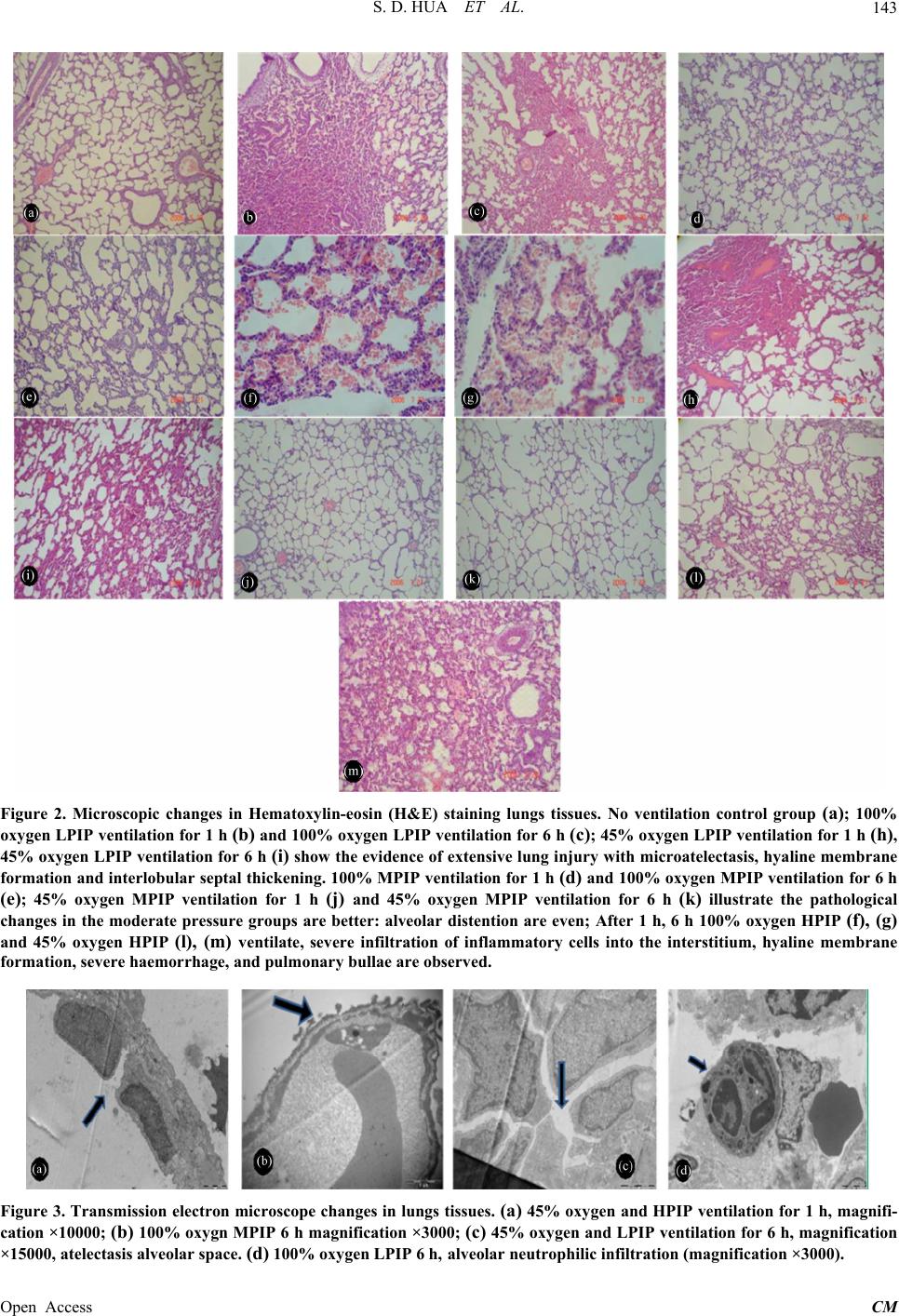 S. D. HUA ET AL. 143 Figure 2. Microscopic changes in Hematoxylin-eosin (H&E) staining lungs tissues. No ventilation control group (a); 100% oxygen LPIP ventilation for 1 h (b) and 100% oxygen LPIP ventilation for 6 h (c); 45% oxygen LPIP ventilation for 1 h (h), 45% oxygen LPIP ventilation for 6 h (i) show the evidence of extensive lung injury with microatelectasis, hyaline membrane formation and interlobular septal thickening. 100% MPIP ventilation for 1 h (d) and 100% oxygen MPIP ventilation for 6 h (e); 45% oxygen MPIP ventilation for 1 h (j) and 45% oxygen MPIP ventilation for 6 h (k) illustrate the pathological changes in the moderate pressure groups are better: alveolar distention are even; After 1 h, 6 h 100% oxygen HPIP (f), (g) and 45% oxygen HPIP (l), (m) ventilate, severe infiltration of inflammatory cells into the interstitium, hyaline membrane formation, severe haemorrhage, and pulmonary bullae are observed. Figure 3. Transmission electron microscope changes in lungs tissues. (a ) 45% oxygen and HPIP ventilation for 1 h, magnifi- cation ×10000; (b) 100% oxygn MPIP 6 h magnification ×3000; (c) 45% oxygen and LPIP ventilation for 6 h, magnification ×15000, atelectasis alveolar space. (d) 100% oxygen LPIP 6 h, alveolar neutrophilic infiltration (magnification ×3000). Open Access CM 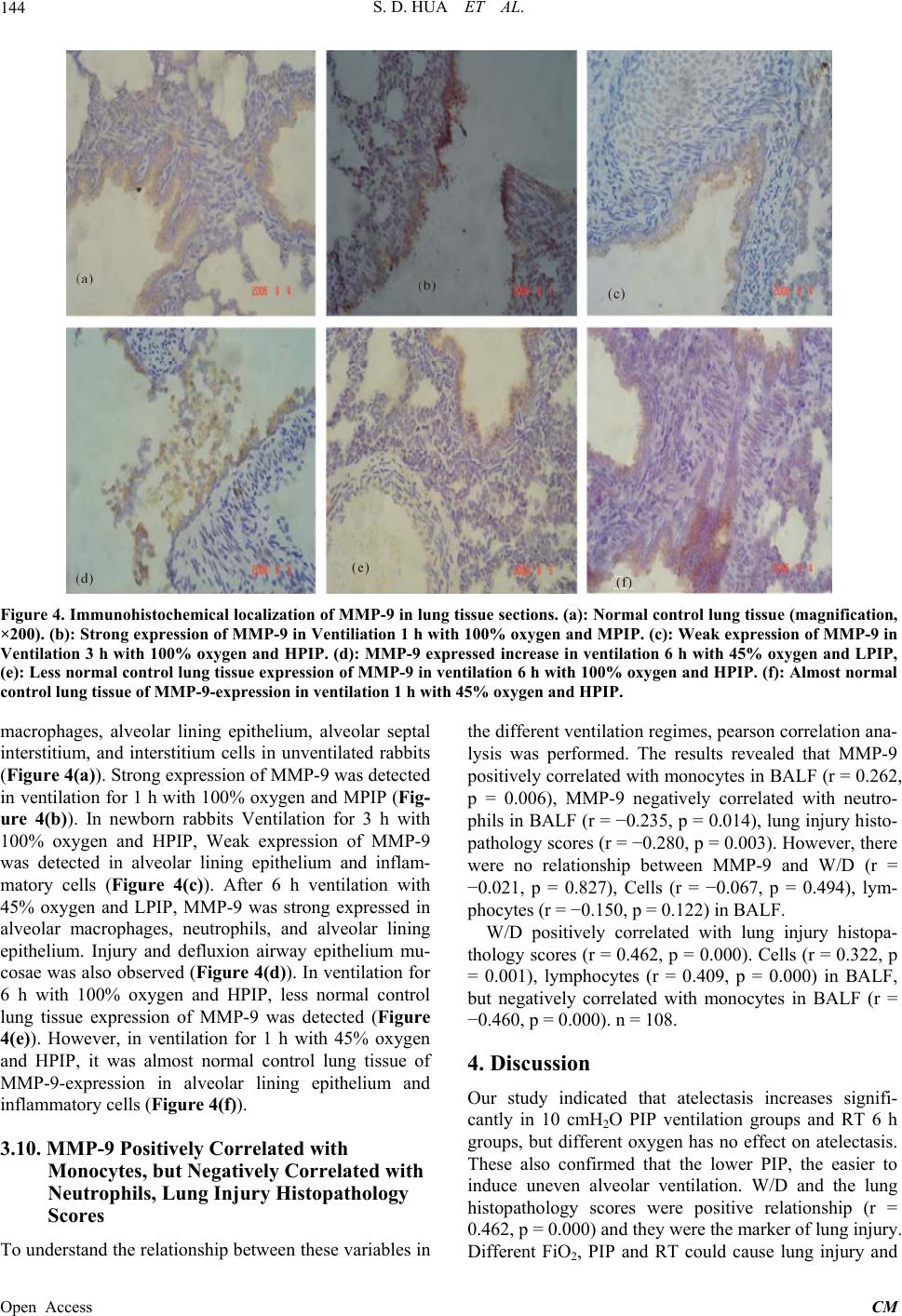 S. D. HUA ET AL. 144 Figure 4. Immunohistochemical localization of MMP-9 in lung tissue sections. (a): Normal control lung tissue (magnification, ×200). (b): Strong expression of MMP-9 in Ventiliation 1 h with 100% oxygen and MPIP. (c): Weak expression of MMP-9 in Ventilation 3 h with 100% oxygen and HPIP. (d): MMP-9 expressed increase in ventilation 6 h with 45% oxygen and LPIP, (e): Less normal control lung tissue expression of MMP-9 in ventilation 6 h with 100% oxygen and HPIP. (f): Almost normal control lung tissue of MMP-9-expression in ventilation 1 h with 45% oxygen and HPIP. macrophages, alveolar lining epithelium, alveolar septal interstitium, and interstitium cells in unventilated rabbits (Figure 4(a)). Strong expression of MMP-9 was detected in ventilation for 1 h with 100% oxygen and MPIP (Fig- ure 4(b)). In newborn rabbits Ventilation for 3 h with 100% oxygen and HPIP, Weak expression of MMP-9 was detected in alveolar lining epithelium and inflam- matory cells (Figure 4(c)). After 6 h ventilation with 45% oxygen and LPIP, MMP-9 was strong expressed in alveolar macrophages, neutrophils, and alveolar lining epithelium. Injury and defluxion airway epithelium mu- cosae was also observed (Figure 4(d)). In ventilation for 6 h with 100% oxygen and HPIP, less normal control lung tissue expression of MMP-9 was detected (Figure 4(e)). However, in ventilation for 1 h with 45% oxygen and HPIP, it was almost normal control lung tissue of MMP-9-expression in alveolar lining epithelium and inflammatory cells (Figure 4(f)). 3.10. MMP-9 Positively Correlated with Monocytes, but Negatively Correlated with Neutrophils, Lung Injury Histopathology Scores To understand the relationship between these variables in the different ventilation regimes, pearson correlation ana- lysis was performed. The results revealed that MMP-9 positively correlated with monocytes in BALF (r = 0.262, p = 0.006), MMP-9 negatively correlated with neutro- phils in BALF (r = −0.235, p = 0.014), lung injury histo- pathology scores (r = −0.280, p = 0.003). However, there were no relationship between MMP-9 and W/D (r = −0.021, p = 0.827), Cells (r = −0.067, p = 0.494), lym- phocytes (r = −0.150, p = 0.122) in BALF. W/D positively correlated with lung injury histopa- thology scores (r = 0.462, p = 0.000). Cells (r = 0.322, p = 0.001), lymphocytes (r = 0.409, p = 0.000) in BALF, but negatively correlated with monocytes in BALF (r = −0.460, p = 0.000). n = 108. 4. Discussion Our study indicated that atelectasis increases signifi- cantly in 10 cmH2O PIP ventilation groups and RT 6 h groups, but different oxygen has no effect on atelectasis. These also confirmed that the lower PIP, the easier to induce uneven alveolar ventilation. W/D and the lung histopathology scores were positive relationship (r = 0.462, p = 0.000) and they were the marker of lung injury. Different FiO2, PIP and RT could cause lung injury and Open Access CM  S. D. HUA ET AL. 145 the degree of lung injury in 100% oxygen groups was more severe than in 45% oxygen groups. Lung injury in RT 6 h groups was more severe than in RT 1 h groups. The fact that lung injury in MPIP group was the lightest among 3 PIP groups was confirmed by W/D, the lung histopathology scores and lung histopathology. There was significant pulmonary hemorrhage in pulmonary alveoli and bronch-walls in HPIP groups, so only MPIP caused uniform alveolar distention (Figure 1(b)), HPIP was easy to cause barotraumas (Figure 1(a)) [14,15] and LPIP was easy to atelectasis (Figure 1(c)). MMP-9 is a metalloproteinase secreted by a wide va- riety of cell types. In the lung, MMP-9 is synthesized by normal resident structural and inflammatory cells such as bronchial epithelial cells [16], alveolar epithelial cells [17], and alveolar macrophages [18]. All of these cell types can greatly increase their MMP-9 secretion after stimulation [16-18]. In our experiment, the cell count and cell classification were researched in BALF and the re- sults found that MMP-9 was not correlated with the cell count, lymphocytes and W/D. It is agreed with Gushima report that MMP-9 expression was not correlated with the number of total cells or lymphocytes [19], MMP-9 was negatively correlated with lung histopathology scores and neutrophils, but MM-9 was positively corre- lated with alveolar macrophages. On the basis of these findings, it has been suggested that MMP-9 may derive from alveolar macrophages. Macrophages are a type of inflammatory cell that synthesizes hundreds of bioactive substances and enzymes. Macrophages are sensitive to cyclic pressure stretching and pressure-stretching stimu- lus. Macrophages respond to pressure-stretching strain by secreting MMP-9 and the chemokine IL-8 [20]. All of these results (fractorial design ANOVA, lung histopa- thology and immunohistochemisty) confirmed that the higher alveolar macrophages, the higher the level of MMP-9, the lower alveolar neutrophilic granulocyte, the lighter lung injury. MMP-9 is the production of macro- phages. Normally, protected lung cell is alveolar macro- phages, but not neutrophilic granulocyte [21]. Absence of MMP-9 led to a more severe injury with neutrophil in- crease in the alveolar spaces in 100% oxygen LPIP 6 h (Figure 3(d)). It appeared that MMP-9 has advantage over VILI. Mice lacking MMP-9 developed more severe lung damage after high-pressure ventilation than their wildtype counterparts [9], and MMP-9 deficiency wors- ened lung injury in a model of bronchopulmonary dys- plasia [8]. MMP-9 had protective role in O3-induced lung neutrophilic inflammation and hyperpermeability. MMP-9 deficiency was associated with enhanced airway epithelial injury and neutrophil recruitment [7]. MMP-9 deficiency impairs host defense against abdominal sepsis [22]. Other authors have shown a similar protective role in MMP-9 in different models of lung injury [7-9,22-24]. It is known that proteolytic function of MMP-9 affects cytokine and chemokine levels as well as their activities. MMP-9 could cleave different cytokine and chemokines, like IL-1β [25]. MMP-9 protected against ventilator- induced lung injury by decreasing alveolar neutrophilic infiltration, probably by modulation of the cytokine re- sponse in the air spaces [9]. MMP-9 was first identified in neutrophils and could also be expressed by neutrophils [26], but neutrophils-derived MMP-9 differs from MMP- 9 expressed by other cell types in two major ways. First, mature neutrophils do not synthesize MMP-9 de novo. Rather, MMP-9 is produced during the late stages of maturation of neutrophils precursors in the bone marrow [27]. These may explain why the numbers of neutrophils were negatively correlated with total MMP-9 level in our study. There was no significant difference in MMP-9 be- tween 100% oxygen ventilation groups and 45% oxygen ventilation groups. It indicated that FiO2 was not the regulation factor for the express of MMP-9 in this venti- lation animal model. However, the expression of MMP-9 has significant difference in different PIP, in other words, the different mechanical stretch regulated the expression of MMP-9. The application of high pressures to lungs during mechanical ventilation can induce a severe injury type, known as ventilator-induced lung injury [28,29]. Physical stimulus can lead to an inflammatory response within the respiratory system and in distal organs [30]. Several of these pathways result in the synthesis, release, and activation of MMPs [2,31]. Mechanical stretch dif- ferentially affects MMP-2/9 and their inhibitors in fetal lung cells [32]. Furthermore, advisable mechanical stretch could promote MMP-9 secretion and decrease the lung injury by our study. The mechanism may be that advisable mechanical stretch activated and enlarged the signal password of MMP-9, leading to MMP-9 synthesis and discharge increase, so the expression of MMP-9 was up-regulated. However, HPIP ventilation formed obvious pulmonary bullae and destroyed the normal pulmonary alveoli structures as well as interrupted the signal con- nection between cell and cell (Figure 3(a)). Finally, the signal password of MMP-9 was broken and the MMP-9 could not be synthesized. Over-mechanical stretch of epithelial cells decreased MMP-9 activity and the MMP- 9/TIMP-1 ratio by 60% - 70% [32]. Accordingly, lung injury was inevitable. In LPIP ventilation, there were obvious uneven alveolar ventilation and microatelectasis, suggesting that the stimulation signal transmission of mechanical stretch was uneven in pulmonary alveoli and could not active the signal password of MMP-9, because MMP-9 is not produced constitutively, but needs a trig- ger to be expressed [33]. MMP-9 is synthesized and stored in the granules of neutrophils and eosinophils in the bone marrow, but is secreted from the cells outside of Open Access CM  S. D. HUA ET AL. 146 the bone marrow in an inducible manner [34,35]. There- fore, atelectasis cannot pass the signal to alveolar epithe- lial cell, macrophages and fibroblasts and cannot induce MMP-9 synthesis. MMP-9 expression decreased in LPIP and caused lung injury. In conclusion, different PIP and different oxygen con- centrations exert simultaneously on newborn lung in new- born rabbits ventilation; only mechanical stretch stimula- tion affects MMP-9 synthesis. Advisable mechanical stretch can promote MMP-9 expression and has protec- tive role in lung in VILI. HPIP causes barotraumas and LPIP induces atelectrauma. 5. Acknowledgements We thank Prof. Yanping Chen (Department of Biostatis- tics, Southern medical University) for his valuable advice in relation to this study. REFERENCES [1] P. L. Davies, O. B. Spiller, M. L. Beeton, et al., “Rela- tionship of Proteinases and Proteinase Inhibitors with Microbial Presence in Chronic Lung Disease of Prema- turity,” Thorax, Vol. 65, No. 3, 2010, pp. 246-251. http://dx.doi.org/10.1136/thx.2009.116061 [2] H. D. Foda, E. E. Rollo, M. Drews, et al., “Ventilator- Induced Lung Injury Upregulates and Activates Gelati- nases and EMMPRIN: Attenuation by the Synthetic Ma- trix Metalloproteinase Inhibitor, Prinomastat (AG3340),” American Journal of Respiratory Cell and Molecular Bi- ology, Vol. 25, No. 6, 2001, pp. 717-724. http://dx.doi.org/10.1165/ajrcmb.25.6.4558f [3] J. H. Kim, M. H. Suk, D. W. Yoon, et al., “Inhibition of Matrix Metalloproteinase-9 Prevents Neutrophilic Inflam- mation in Ventilator-Induced Lung Injury,” American Journal of Physiology Lung Cellular and Molecular, Vol. 291, No. 4, 2006, pp. L580-L587. http://dx.doi.org/10.1152/ajplung.00270.2005 [4] S. Buckley, B. Driscoll, W. Shi, et al., “Migration and Gelatinases in Cultured Fetal, Adult, and Hyperoxic Al- veolar Epithelial Cells,” American Journal of Physiology Lung Cellular and Molecular, Vol. 281, No. 2, 2001, pp. L427-L434. [5] C. Delclaux, M. P. d’Ortho, C. Delacourt, et al., “Gelati- nases in Epithelial Lining Fluid of Patients with Adult Respiratory Distress Syndrome,” American Journal of Physiology, Vol. 272, No. 3, 1997, pp. L442-L451. [6] B. Ricou, L. Nicod, S. Lacraz, et al., “Matrix Metallopro- teinases and TIMP in Acute Respiratory Distress Syn- drome,” American Journal of Respiratory and Critical Care Medicine, Vol. 154, No. 2, 1996, pp. 346-352. http://dx.doi.org/10.1164/ajrccm.154.2.8756805 [7] H. K. Yoon, H. Y. Cho and S. R. Kleeberger, “Protective Role of Matrix Metalloproteinase-9 in Ozone-Induced Airway Inflammation,” Environmental Health Perspec- tives, Vol. 115, No. 11, 2007, pp. 1557-1563. http://dx.doi.org/10.1289/ehp.10289 [8] H. Lukkarinen, A. Hogmalm, U. Lappalainen, et al., “Matrix Metalloproteinase-9 Deficiency Worsens Lung Injury in a Model of Bronchopulmonary Dysplasia,” American Journal of Respiratory Cell and Molecular Bi- ology, Vol. 41, No. 1, 2009, pp. 59-68. http://dx.doi.org/10.1165/rcmb.2008-0179OC [9] G. M. Albaiceta, A. Gutierrez-Fernandez, D. Parra, et al., “Lack of Matrix Metalloproteinasec9 Worsens Ventila- tor-Induced Lung Injury,” American Journal of Physiol- ogy Lung Cellular and Molecular, Vol. 294, No. 3, 2008, pp. L535-L543. http://dx.doi.org/10.1152/ajplung.00334.2007 [10] V. Lagente, B. Manoury, S. Nenan, et al., “Role of Ma- trix Metalloproteinase in the Development of Airway In- flammation and Remodeling,” Brazilian Journal of Medi- cal and Biological Research, Vol. 38, No. 10, 2005, pp. 1521-1530. http://dx.doi.org/10.1590/S0100-879X2005001000009 [11] E. K. Wolthuis, A. P. Vlaar, G. Choi, et al., “Mechanical Ventilation Using Non-Injurious Ventilation Settings Causes Lung Injury in the Absence of Pre-Existing Lung Injury in Healthy Mice,” Critical Care, Vol. 13, No. 1, 2009, p. R1. http://dx.doi.org/10.1186/cc7688 [12] E. K. Wolthuis, A. P. Vlaar, G. Choi, et al., “Recombi- nant Human Soluble Tumor Necrosis Factor-Alpha Re- ceptor Fusion Protein Partly Attenuates Ventilator-In- duced Lung Injury,” Shock, Vol. 31, No. 3, 2009, pp. 262-266. http://dx.doi.org/10.1097/SHK.0b013e31817d42dd [13] P. Svedin, H. Hagberg, K. Sävman, et al., “Matrix Met- alloproteinase-9 Gene Knock-out Protects the Immature Brain after Cerebral Hypoxia-Ischemia,” The Journal of Neuroscience, Vol. 27, No. 7, 2007, pp. 1511-1518. http://dx.doi.org/10.1523/JNEUROSCI.4391-06.2007 [14] D. Dreyfuss and G. Saumon, “Ventilator-Induced Lung Injury: Lessons from Experimental Studies,” American Journal of Respiratory and Critical Care Medicine, Vol. 157, No. 1, 1998, pp. 294-323. http://dx.doi.org/10.1164/ajrccm.157.1.9604014 [15] D. Dreyfuss, P. Soler, G. Basset, et al., “High Inflation Pressure Pulmonary Edema: Respective Effects of High Pressure, High Tidal Volume and Positive End-Expira- tory Pressure,” American Review of Respiratory Disease, Vol. 137, No. 5, 1988, pp. 1159-1164. [16] P. M. Yao, J. M. Buhler, M. P. d’Ortho, et al., “C. Ex- pression of Matrix Metalloproteinase Gelatinases A and B by Cultured Epithelial Cells from Human Bronchial Ex- plants,” The Journal of Biological Chemistry, Vol. 271, No. 26, 1996, pp. 15580-15589. http://dx.doi.org/10.1074/jbc.271.26.15580 [17] A. Pardo, K. Ridge, B. Uhal, et al., “Lung Alveolar Epithelial Cells Synthesize Interstitial Collagenase and Gelatinases A and B in Vitro,” The International Journal of Biochemistry & Cell Biology, Vol. 29, No. 6, 1997, pp. 901-910. http://dx.doi.org/10.1016/S1357-2725(97)00030-7 [18] H. G. Welgus, E. J. Campbell, J. D. Cury, et al., “Neutral Metalloproteinases Produced by Human Mononuclear Phagocytes. Enzyme Profile, Regulation, and Expression Open Access CM  S. D. HUA ET AL. Open Access CM 147 during Cellular Development,” Journal of Clinical Inves- tigation, Vol. 86, No. 5, 1990, pp. 1496-1502. http://dx.doi.org/10.1172/JCI114867 [19] Y. Gushima, K. Ichikado, M. Suga, et al., “Expression of Matrix Metalloproteinases in Pigs with Hyperoxia-In- duced Acute Lung Injury,” European Respiratory Journal, Vol. 18, No. 5, 2001, pp. 827-837. http://dx.doi.org/10.1183/09031936.01.00049201 [20] J. Pugin, I. Dunn, P. Jolliet, et al. “Activation of Human Macrophages by Mechanical Ventilation in Vitro,” Ame- rican Journal of Physiology, Vol. 275, No. 6, 1998, pp. L1040-L1050. [21] D. F. Gibbs, R. L. Warner, S. J. Weiss, et al., “Charac- terization of Matrixmetallop Roteinases Produced by Rat Alveolar Macrophages,” American Journal of Respiratory Cell and Molecular Biology, Vol. 20, No. 6, 1999, pp. 1136-1144. http://dx.doi.org/10.1165/ajrcmb.20.6.3483 [22] R. Renckens, J. J. Roelofs, S. Florquin, et al., “Matrix Metalloproteinase-9 Deficiency Impairs Host Defense against Abdominal Sepsis,” The Journal of Immunology, Vol. 176, No. 6, 2006, pp. 3735-3741. [23] K. Bry, A. Hogmalm and E. Bäckström, “Mechanisms of Inflammatory Lung Injury in the Neonate: Lessons from a Transgenic Mouse Model of Bronchopulmonary Dyspla- sia,” Seminars in Perinatology, Vol. 34, No. 3, 2010, pp. 211-221. http://dx.doi.org/10.1053/j.semperi.2010.02.006 [24] S. Cabrera, M. Gaxiola, J. L. Arreola, et al., “Overex- pression of MMP9 in Macrophages Attenuates Pulmo- nary Fibrosis Induced by Bleomycin,” The International Journal of Biochemistry & Cell Biology, Vol. 39, No. 12, 2007, pp. 2324-2338. http://dx.doi.org/10.1016/j.biocel.2007.06.022 [25] A. Ito, A. Mukaiyama, Y. Itoh, et al., “Degradation of Interleukin 1beta by Matrix Metalloproteinases,” The Journal of Biological Chemistry, Vol. 271, No. 25, 1996, pp. 14657-14660. http://dx.doi.org/10.1074/jbc.271.25.14657 [26] W. C. Parks and R. P. Mecham, “Gelatinase B: Structure, Regulation, and Function,” In: Matrix Metalloproteinases, Academic Press, San Diego, 1998, pp. 115-148. [27] C. A. Owen, Z. Hu, B. Barrick, et al., “Inducible Expres- sion of Tissue Inhibitor of Metalloproteinases-Resistant Matrix Metalloproteinase-9 on the Cell Surface of Neu- trophils,” American Journal of Respiratory Cell and Mo- lecular Biology, Vol. 29, No. 3, 2003, pp. 283-294. http://dx.doi.org/10.1165/rcmb.2003-0034OC [28] A. S. Slutsky, “Ventilator-Induced Lung Injury: From Barotrauma to Biotrauma,” Respiratory Care, Vol. 50, No. 5, 2005, pp. 646-659. [29] C. C. dos Santos and A. S. Slutsky, “The Contribution of Biophysical Lung Injury to the Development of Bio- trauma,” Annual Review of Physiology, Vol. 68, 2006, pp. 585-618. http://dx.doi.org/10.1146/annurev.physiol.68.072304.113 443 [30] L. Gattinoni, E. Carlesso, P. Cadringher, et al., “Physical and Biological Triggers of Ventilator-Induced Lung In- jury and Its Prevention,” European Respiratory Journal, Vol. 47, 2003, pp. 15s-25s. http://dx.doi.org/10.1183/09031936.03.00021303 [31] F. Kheradmand, E. Werner, P. Tremble, et al., “Role of Rac1 and Oxygen Radicals in Collagenase-1 Expression Induced by Cell Shape Change,” Science, Vol. 280, No. 5365, 1998, pp. 898-902. http://dx.doi.org/10.1126/science.280.5365.898 [32] R. L. Hawwa, M. A. Hokenson, Y. Wang, et al., “Differ- ential Expression of MMP-2 and -9 and Their Inhibitors in Fetal Lung Cells Exposed to Mechanical Stretch: Re- gulation by IL-10,” Lung, Vol. 189, No. 4, 2011, pp. 341- 349. http://dx.doi.org/10.1007/s00408-011-9310-7 [33] G. Opdenakker, P. E. Van den Steen, J. Van Damme, “Gelatinase B: A Tuner and Amplifier of Immune Func- tions,” Trends in Immunology, Vol. 22, No. 10, 2001, pp. 571-579. http://dx.doi.org/10.1016/S1471-4906(01)02023-3 [34] J. J. Atkinson and R. M. Senior, “Matrix Metalloprotei- nase-9 in Lung Remodeling,” American Journal of Res- piratory Cell and Molecular Biology, Vol. 28, No. 1, 2003, pp. 12-24. http://dx.doi.org/10.1165/rcmb.2002-0166TR [35] S. Chakrabarti and K. D. Patel, “Matrix Metalloprotei- nase-2 (MMP-2) and MMP-9 in Pulmonary Pathology,” Experimental Lung Research, Vol. 31, No. 6, 2005, pp. 599-621. http://dx.doi.org/10.1080/019021490944232
|