 Vol.3, No.2, 124-135 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.32018 Copyright © 2011 SciRes. OPEN ACCESS Comparison of methods for evaluating stability and maturity of co-composting of municipal solid wastes and sewage sludge in semi-arid pedo-climatic condition Olfa Fourti*, Naceur Jedidi, Abdennaceur Hassen Centre des Recherches et des Technologies des Eaux, Laboratoire Traitement et Recyclage, Cité Mahrajène, Tunisie; *Corresponding Author: olfa_fourti@yahoo.fr Received 28 October 2010; revised 29 November 2010; accepted 3 December 2010. ABSTRACT The step of maturation (around 60 days) ap- peared less active as compared to the first step of pre-fermentation (around 90 days) since the values of temperature, recorded during the second step of maturation, are found generally less important than those recorded during the first step. A similar tendency of C/N ratio values decrease is generally observed in the two piles of wastes during the two steps of composting and these C/N ratio values determined in the pile of wastes free of sewage sludge (W1) are generally slightly higher than those observed in the pile added with dry sewage sludge (W2). The amount of total heavy metals (order of content: Zn > Pb > Ni > Cu > Cr > Cd) appeared very het- erogeneous and showed a large variation in the two piles of wastes. The use of sewage sludge in the pile W2 showed generally no apparent impact on the whole amount of total heavy met- als recorded in the finished product and the values recorded are usually lower than the metal concentration limits imposed by several countries. Microbial inventory and total DNA extracted from composting materials followed during all the two steps of composting showed a net variation over time, and revealed specifi- cally a good parallel progress according to the ambient temperature recorded inside the waste materials. It appeared also from this study that the microbial diversity is much nuanced in the case of windrow W2 added with sewage sludge as compared to that observed in the case of windrow W1 free of sewage sludge. Keywords: Temperature; Compost; Heavy Metals; Microbial Diversity; Microbial DNA Concentration 1. INTRODUCTION Composting is defined as a process of aerobic ther- mophilus microbial degradation or an exothermic bio- logical oxidation of various organic wastes (sewage sludge, animal manures, yard waste, crop residues, mu- nicipal solid waste, fish scraps and mortality, and food waste and food process wastes) by many populations of the indigenous microorganisms which lead to a stabi- lized, mature, deodorized, hygienic product, rich in hu- mic substances, easy to store and marketable as organic amendment or manure [1]. Composting aimed to reduce the volume of wastes going to the landfills and to facilitate recycling of nutri- ents in the organic wastes as a useful method of produc- ing a stabilized product that can be used as a source of nutrients and soil conditioner in the field. The composted product has the advantage of improving soil structure, increasing soil organic matter, suppressing soil-borne plant pathogens and enhancing plant growth. Immature composts may contain more growth inhibiting sub- stances than mature composts. Some of these growth inhibiting compounds include salts, free ammonia, phe- nolic substances, heavy metals, and organic acids. For optimum aerobic composting, moisture is neces- sary to support the metabolic processes of microorgan- isms. Composting materials should be maintained within a range of 40 to 65% moisture. A higher percentage will increase anaerobic decomposition, while lower moisture content will slow down the composting process as mi- croorganisms die or become dormant. Microbial diver- sity and activity during the composting process, mediat- ing stabilization of organic wastes, is an important factor and key to consider in composting. A large variety of mesophilic, thermotolerant and thermophilic aerobic microorganisms (including bacteria, actinobacteria, yeast, moulds and various other fungi) have been extensively reported in composting and other self-heating organic materials at temperatures between 20 and 60°C [2,3].  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 125 Many factors determine the microbial communities dur- ing composting. Under aerobic conditions, temperature is a major factor determining the type of microorganisms, species diversity, and the rate of metabolic activities [3]. On the other side, waste stream from biosolids or mu- nicipal solid waste can contain elevated concentration of various metals as potential toxic elements (PTEs), and the impact of metals on compost quality and use is a major concern throughout the world [4]. In small amounts, many of these elements may be essential for plant growth; however, in higher concentrations they are likely to have a detrimental effect upon plant growth. A molecular detection procedure, using ribosomal in- tergenic spacer analysis, was tested for microbial com- munity diversity assessment. Analysis of compost mi- crobial communities is one of the challenging areas of research due to the enormous complexity of biodiversity caused by the heterogeneity of the physical and chemical structure of compost environments. Recently, a number of molecular biological methodologies have been suc- cessfully developed and introduced to reveal the biodi- versity of microbial communities in a wide range of en- vironments including soils [5,6] and solid wastes [7-9]. This diversity concerned pathogens and saprophytic mi- croorganisms useful for a good procedure and organiza- tion of the composting process. The aim of this study was to assess, in a semi-indus- trial pilot plant and under semi-arid pedo-climatic condi- tions, a new process of co-composting of municipal solid wastes and sewage sludge. So, some physico-chemical, biochemical and microbiological parameters are fol- lowed during the two steps of pre-fermentation (uncon- trolled fermentation) and of maturation (controlled fer- mentation), respectively. Also, total heavy metals con- tent as potential toxic elements (PTEs) and microbial diversity are evaluated. 2. MATERIALS AND METHODS Preparation and monitoring of windrows. The study was performed in a semi-industrial pilot plant and using two types of windrow W1 and W2. The process of com- posting proceeds in two successive separately steps: pre-fermentation (uncontrolled fermentation) and matu- ration (controlled fermentation), and with two different raw composting materials: municipal solid waste from the Erriadh city of Beja were pre-selected at source (household pre-selection with average physical-chemical characteristics, humidity = 60%, organic matter = 30% dry weight, and C/N = 32) and dry sewage sludge (stabi- lized from anaerobic digestion of the urban wastewater treatment plant of Beja was integrated in the process at the dry state (with average physical-chemical character- istics, humidity = 30%, organic matter = 65% dry weight, and C/N = 12.5) and primarily for the cover of windrow W2. During the first step, the two windrows W1 and W2 of pyramidal form (of approximately 7 × 3 × 1.5 m3, length × width × height, respectively) are constituted exclusively with raw waste materials (100% of munici- pal solid wastes) for W1 and (60% of municipal solid wastes and a superficial layer of dry sewage sludge of about 40% of dried sewage sludge) for W2, respectively, and deposed in the platform of composting, under natu- ral conditions, for 3 months of pre-fermentation. Sec- ondly, at the end of this period of pre-fermentation, the waste material of each windrow is submitted to a deep manual sorting and fermentable organic material is chipped, shred, sieved to 40-mm stitch sieve to reduce heterogeneity and subjected for approximately 2 months to a second controlled fermentation in pile of about 1.5 m of height. The pile of wastes during the first step of process is left under natural condition without any main- tenance, and on the contrary the maintaining of waste pile during the second step of composting consists to monitor temperature inside the pile in order to maintain a temperature around 25-55°C, by turning as needed (1-3 times per month), and to preserve pile moisture at the consistency of a well-wrung sponge by watering. The finished product is sieved with a 10-mm stitch sieve. Temperature and humidity were controlled daily. When the mean temperature recorded in the different depths (depths 20, 40 and 60 cm) of the pile averaged 55°C (using a thermo-couple iron-constantan type J), the wind- row was turned and watered. These operations of turning and watering with tap water were performed almost twice monthly in considering ambient temperature. Temperature inside the pile is regularly determined (at least 1 time per week) at many different places and at two different depths of 20 and 100 cm, using a thermo- couple thermometer; iron-constantan type J and humid- ity (approximately 50 g of the waste sample was dried at 105°C in an oven for 24 h) is regularly controlled. Analytical methods. Samples of wastes are in general taken every 2 weeks and sampled as described by [10]. These samples are subjected to different determinations after screening through 12 mm (0.5 inch) mesh. Electrical conductivity (EC) and pH are measured in 10:50 (dry compost: deionized water) extracts using a standard pH-meter (LPH 230 T, Tacussel electronic, France) and a standard conductivity-meter (Orion re- search, model 150, USA). Total nitrogen (N) and total organic carbon (C) are determined by the Kjeldahl method [11] and by the wet dichromate oxidation method [12], respectively. Organic matter is determined by ashing at 550°C for 2 h in a muffle furnace. Total heavy metal contents are measured by an atomic  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 126 absorption spectrophotometer (Perkin-Elmer, Model 560) after digestion of the samples in concentrated HNO3- HClO4 (2:1) according to [13]. Biological parameters. Total indigenous viableculti- vable mesophilic and thermophilic bacteria from differ- ent suspension-dilution of waste samples are enumerated by the pour plate technique on Tryptic soy agar (Pasteur Production, Paris). Enumerations are performed after 3 days of incubation at 28 and 55°C for mesophilic and thermophilic bacteria, respectively. The count of spore- forming bacteria includes several steps from selection by applying heat to destroy vegetative bacteria (80°C for 10 min) to the indication by spreading volumes of the sam- ple into Tryptic soy agar followed by incubation at 37°C for 3 days. Spore-counting bacteria were expressed as colony-forming units per gram (CFU s/g) of dry weight of compost. The deshydrogenase activity expressed as triphenyl- formazan unit is measured according to Tabatabai [14]. Solid waste microbial biomasses C (BC) and N (BN) are evaluated using the Fumigation-Extraction method and total microbial DNA extraction method [15]. DNA was extracted and purified from equivalent dry weights of each waste materials sample (500 mg fresh materials), using the Bio 101 Fast DNA Kit for Soil (Biogene, France), according to the manufacturer in- structions. Purified DNA was quantified by spectropho- tometry (Bio-RAD Smart SpecTM Plus, France) [16]. The spectrophotometric A260/A280 and A260/A230 ratios were determined to evaluate levels of protein and humic acid impurities, respectively, in the extracted DNA [17]. These last methods recommended at first for soil micro- bial biomass analysis is used in this study for microbial biomass determination in the compost [18]. The microbial diversity in compost is evaluated by DNA extraction and polymerase chain reaction (PCR) of rRNA intergenic spacer. Total DNA extraction and puri- fication are performed on waste extracts according to [5]. Each DNA sample was used as template to amplify the intergenic spacer between the genes encoding for the large (23S) and small (16S) subunits of ribosomic RNA. Universal eubacterial primers are used. Amplification products were resolved in a 5% non-denaturing acryla- mide gel, stained with SYBR green I and photographed under UV illumination. Pearson’s correlation coefficients between selected parameters were calculated. 3. RESULTS AND DISCUSSION 3.1. Temperature Profiles Temperature profiles established during the two steps of composting (pre-fermentation and maturation) in the two windrows W1 and W2 revealed the three classical temperature steps of composting the mesophilic, ther- mophilic and cooling phases, respectively (Figure 1). The step of maturation (around 60 days) appeared less active as compared to the first step of pre-fermentation (around 90 days) since the values of temperature, re- corded during the second step of maturation, are found generally less important than those recorded during the first step. Differences in temperature averaged 15°C. 3.2. Evolution of the Physico-Chemical Parameters of Composting 3.2.1. Acidity Ideally, the pH should be in the range of 6-8 to allow the highest rates of decomposition. If the pH is outside this range, microbial activity will be compromised and decomposition will be slowed or even stopped. The pH values recorded in the two piles W1 and W2 showed in general a weak variation from the beginning until the end of pre-fermentation, and present a slight acidic com- post increase during the step of maturation (Table 1). 3.2.2. Conductivity The change in value of electrical conductivity (EC), used as chemical indicators and as an indirect measure of the soluble salts in a growing medium, for the two wind- rows may be due to the characteristics and the amounts of the materials used for constructing the pile of wastes. The electrical conductivity showed no major variation for the two windrows during the two steps of composting, and limit values averaged 60 ms/m (Table 1). The addi- tion of dry sewage sludge to windrow W2 seems have no apparent impact on the conductivity value of the compost. 3.2.3. Organic Matter The degradation rate of organic matter during com- posting is importantly used to evaluate the compost ma- turity. The organic matter decreased notably over time during the first step of pre-fermentation in the two windrows W1 and W2, 20.9 to13.5 and 20.44 to 13.60%, respectively (Table 1), pointing to active degradation of the organic materials during this step. On the other side, organic matter degradation appeared no so active during the second step of maturation in the two windrows, 12.24 to 10.8 and 12.4 to 11.45%, respectively. 3.2.4. C/N Ratio At the beginning of the first step of pre-fermentation, the C/N ratio values averaged 30 and these values de- creased remarkably at the end of this step to 23.22 and 20.2 for windrows W1 and W2, respectively. These re- sults, both valid for the two piles of wastes, indicate a 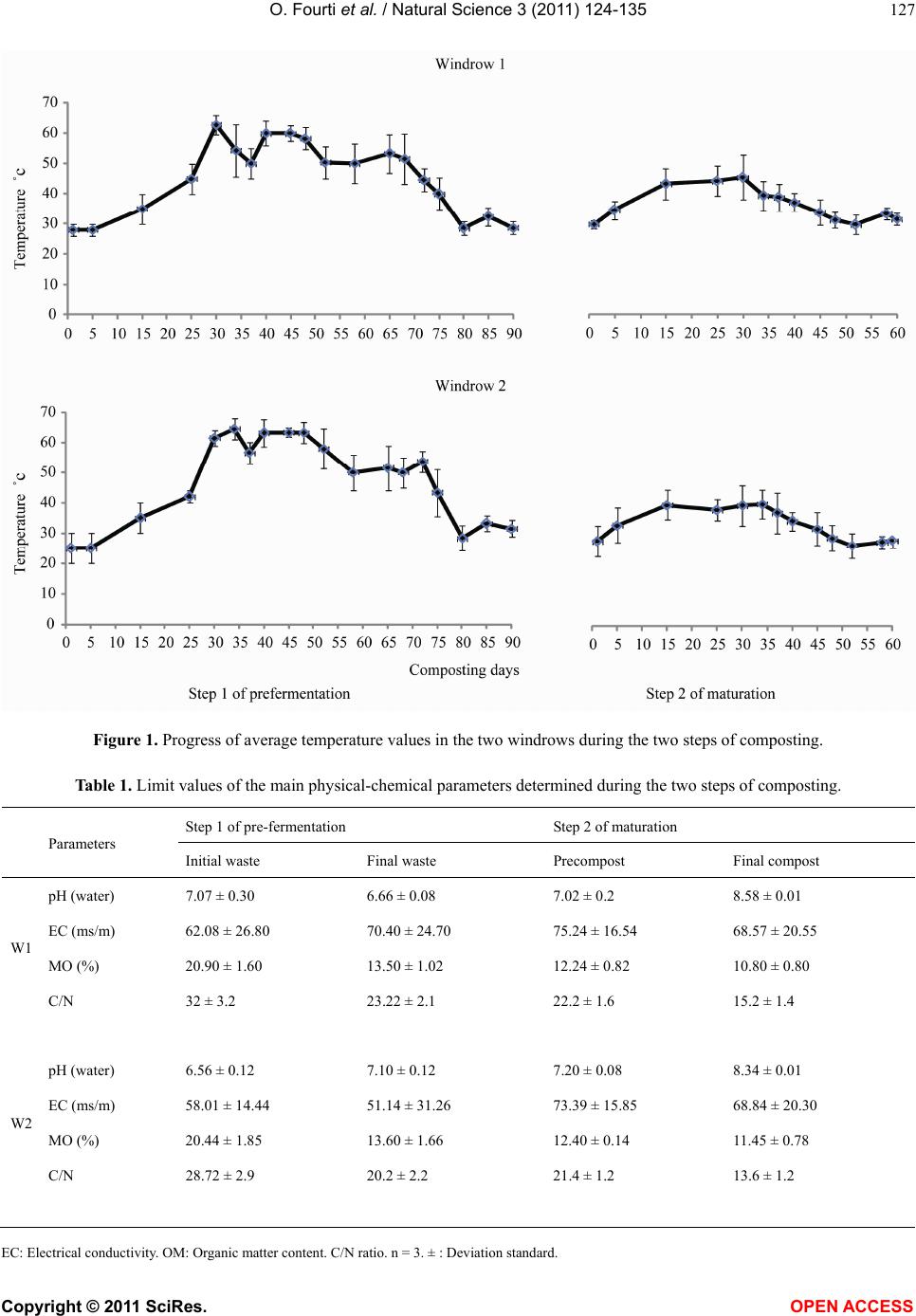 O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 127 Figure 1. Progress of average temperature values in the two windrows during the two steps of composting. Table 1. Limit values of the main physical-chemical parameters determined during the two steps of composting. Step 1 of pre-fermentation Step 2 of maturation Parameters Initial waste Final waste Precompost Final compost pH (water) 7.07 ± 0.30 6.66 ± 0.08 7.02 ± 0.2 8.58 ± 0.01 EC (ms/m) 62.08 ± 26.80 70.40 ± 24.70 75.24 ± 16.54 68.57 ± 20.55 MO (%) 20.90 ± 1.60 13.50 ± 1.02 12.24 ± 0.82 10.80 ± 0.80 W1 C/N 32 ± 3.2 23.22 ± 2.1 22.2 ± 1.6 15.2 ± 1.4 pH (water) 6.56 ± 0.12 7.10 ± 0.12 7.20 ± 0.08 8.34 ± 0.01 EC (ms/m) 58.01 ± 14.44 51.14 ± 31.26 73.39 ± 15.85 68.84 ± 20.30 MO (%) 20.44 ± 1.85 13.60 ± 1.66 12.40 ± 0.14 11.45 ± 0.78 W2 C/N 28.72 ± 2.9 20.2 ± 2.2 21.4 ± 1.2 13.6 ± 1.2 EC: Electrical conductivity. OM: Organic matter content. C/N ratio. n = 3. ± : Deviation standard.  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 128 high rate of biological activities. The similar tendency of result is observed during the second step of maturation since there is a net decrease of C/N ratio between the beginning and the end of this step of maturation, 22.2 to 15.2 and 21.4 to 13.6 in the two piles of wastes, respec- tively. The C/N ratio values determined in the pile of wastes free of sewage sludge (W1) are generally slightly higher than those observed in the pile added with dry sewage sludge (W2). This result is certainly due to the special composition of sewage sludge used (around 30% of the total composting wastes) in the windrow W2. This sub- strate is therefore loaded in degradable carbon and compost must lead to a very steady and slowly biode- gradable compound (low C/N). 3.2.5. Heavy Metals Table 2 illustrates the total concentration of metals (Zn, Pb, Cu, Ni, Cr, Cd) during the two steps of com- posting. The amount of total metal appeared very het- erogeneous and showed a large variation. This tendency is valid for the two piles of wastes, and could be ex- plained by the high bio-physical-chemical complexity of this environment; since there will be a loss by leaching and a concentration of metals by adsorption. The order of total metal content in the composted material and fi- nal compost was Zn > Pb > Ni > Cu > Cr > Cd. The use of sewage sludge in the waste of pile W2 showed gener- ally no apparent impact on the whole amount of total heavy metals recorded in the finished product (Table 2). 3.2.6. Microbial Biomass BC/BN ratio values showed usually a net increase dur- ing the thermophilic phase, 30-70 and 20-50 days, for the steps of pre-fermentation and maturation, respec- tively (Figure 2). The BC/BN ratio values are on average around 4-6 and 3-4 during the first and second steps of composting, respectively. 3.2.7. Deshydrogenase Activity The deshydrogenase activity generally showed a net increase between 20-70 and 15-35 days through the pre- fermentation and maturation steps, respectively. A good positive correlation is observed between the values of deshydrogenase and of temperature recorded (r = 0.96, P = 0.01). These values of deshydrogenase activity fluctu- ated between 3.2-5.84 and 1.7-5.66 triphenylformazan (TPF)/g of waste dry weight during the step of prefer- mentation in the two windrows W1 and W2, respectively (Figure 2). By the same, these values varied between 3.6-4.9 and 1.96-3.22 TPF/g of waste dry weight during the step of maturation in the two windrows W1 and W2, respectively. So variation of the deshydrogenase activity appeared very narrow (around 1.2 TPF/g of waste dry weight on average for W1 and W2) during the second step of composting and indicated a certain degree of homogeneity of the waste materials used in this second step of maturation; and in the opposite, a high heteroge- neity of the waste used during the first step of pre-fer- mentation since variation of deshydrogenase values re- corded were relatively large (around 2.64 and 4 TPF/g of waste dry weight for W1 and W2, respectively). On the other hand, this activity appeared slightly higher in the windrow free of sludge than in the one with sewage sludge. 3.3. Biological Parameters of Composting 3.3.1. Total Viable-Cultivable Mesophilic, Thermophilic and Spore-Forming Bacteria The changes in main microbial population, total viable- cultivable mesophilic, thermophilic and spore-forming bacteria, in the two piles of composting materials during the two steps of process are presented in Figure 3. The number of mesophilic microorganisms during the first step of pre-fermentation varied, and during the first weeks of this step the number of these microorganisms remained stable around 8.5 Ulog10 CFUs/g of compost dry weight in the two windrows W1 and W2. The lowest level of mesophilic bacteria in compost materials was recorded between 65 and 75 days of pre-fermentation (around 5.5 Ulog10 CFUs/g of compost dry weight). From that moment, the number of mesophilic bacteria began to increase and remained at the level of 7 Ulog10 CFUs/g of compost dry weight for the two windrows. In the case of step of maturation, the number of mesophilic bacteria did not varied remarkably and it fluctuated on average around 6 Ulog10 CFU s/g of compost dry weight. On the other side, the number of thermophilic bacteria started to increase, from 4 Ulog10 CFUs/g of compost dry weight, since the first weeks of pre-fermentation step, and it remained constant around 6 Ulog10 CFUs/g of compost dry weight from the days 35 to 85. At the end of this step of pre-fermentation, the number of thermo- philic bacteria reached 4 Ulog10 CFUs/g of compost dry weight for the two windrows. For the second step of maturation, the number of thermophilic bacteria showed low variation, particularly in the case of windrow W2, and a little increase is observed between 25-48 days of maturation (around 6 Ulog10 CFUs/g of compost dry weight). For the number of spore-forming bacteria, the variation appeared very weak (around 4.5 Ulog10 CFUs/g of compost dry weight) during the two steps of composting for the two windrows W1 and W2, and a little increase is always remarked specially during the  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 129 Table 2. Heavy metal contents obtained during the two steps of composting and the metal concentration limits for composts in sev- eral countries. Step 1 of pre-fermentation Step 2 of maturation Canadian nor- malization French nor- malization German nor- malization Spanish nor- malization US nor- malization Initial waste content Final waste content Precompost content Final compost content Limit values Class AClass B (mg kg-1 dry wt) Cu 24,54 ± 5,18 42,83 ± 7,37 28,06 ± 9,7343,25 ± 2,66100 757 100 100 450 100 Cd 4,97 ± 2,28 1,75 ± 0,50 2,78 ± 1,8 3,6 ± 1,5 3 20 8 1,5 10 2 Cr 53,26 ± 14,7 24,15 ± 5,76 17,41 ± 3,0526,68 ± 1,44210 1060100 100 400 100 Pb 90,47 ± 27,80 66,19 ± 25,54 80,01 ± 56,94102,98 ± 15,40150 500 800 150 300 150 Zn 195,60 ± 20,30 237,82 ± 19,68 160,30 ± 30,65188,40 ± 11,40500 1850500 400 1100 400 W1 Ni 64,34 ± 20,30 75,80 ± 4,70 37,47 ± 11,5060,10 ± 10,1062 180 200 50 120 50 Cu 42,32 ± 9,60 76,31 ± 24,52 71 ± 12,54 97,39 ± 17,5100 757 100 100 450 100 Cd 4,80 ± 2,78 3,98 ± 2,08 3,04 ± 2,08 1,07 ± 0,14 3 20 8 1,5 10 2 Cr 30,15 ± 7,98 31,92 ± 5,82 26,73 ± 11,6923,2 ± 2,07 210 1060100 100 400 100 Pb 112,53 ± 28,40 82,20 ± 17,42 64,28 ± 34,3092,16 ± 27,75150 500 800 150 300 150 Zn 141,90 ± 23,69 191,29 ± 32,12 203,08 ± 17,70229,15 ± 36,50500 1850500 100 1100 400 W2 Ni 44,91 ± 10,11 62,85 ± 16,56 60,80 ± 20,5574,0 ± 5,10 62 180 200 50 120 50 Values for Class A of composts ‘‘which have no restrictions in use’’ and Class B of compost ‘‘which can be used on forest lands and road sides and for other landscaping purposes’’, according to Canadian normalization [19]; n = 3, ± : Deviation standard. days 34-35 and 25-48 of pre-fermentation and matura- tion steps, respectively (around 5 Ulog10 CFUs/g of compost dry weight). 3.3.2. Microbial DNA Concentration Microbial total DNA extracted from composting ma- terials and followed during all the two steps of compost- ing process showed a net variation over time, and re- vealed a good parallel increase with the temperature pro- gress inside the waste materials (Figure 4). This increase varied between 13.2 to 26.1 µg of total DNA per g dry weight. The lowest values of DNA are observed at the start and the end of each steps of process (around 13.2 µg of total DNA per g dry weight) and the highest values are always recorded during each thermophilic phase (around 26 µg of total DNA per g dry weight). The de- termination of A260/A230 and A260/A280 ratios for compost DNA showed a significantly lower values (0.96 and 1.2) than those for DNA solutions of pure cultures (1.57 and 1.89). 3.3.3. Microbial Diversity Changes in microbial diversity are assessed during the two steps of process by DNA extraction and polymerase chain reaction (PCR) of rRNA intergenic spacer. The RISA analysis of microbial communities change and diversity in compost showed an equivalent diversity at the start of the first step of composting process in the two windrows W1 and W2 (Figure 5, Lanes 1 and 2). During the thermophilic phases of the first step of proc- ess, there is a net variation in microbial diversity, this diversity appeared very rich and obvious in case of windrow W2 added with sewage sludge (Lanes 3 and 4). At the end of this first step of composting (Lanes 5 and 6), the RISA profile appeared equivalent. The start of the second step of composting showed a varied profile (Lanes 7 and 8) and diversity is richer also in case of windrow W2. The end of step of maturation exhibit a high microbial diversity materialized in the RISA profile by a various big number of bands (Lanes 9 and 10). Generally, this microbial diversity is much nuanced in the case of windrow W2 added with dry sewage sludge as compared to that observed in the case of windrow W1 free of sewage sludge. Also, it is important to mention that there were a temporal dynamics of the microbial communities with modifications of the RISA profile  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 130 Figure 2. Progress of the deshydrogénase activity and BC/BN ratio values in the two windrows during the two steps of composting. over time of composting for the same type of composts. unity structures as follow: Lane 1, Composting raw materials of W2; Lane 2, Composting raw materials of W1. Lane 3, Composting materials of W2 during ther- mophilic step; Lane 4, Composting materials of W1 during thermophilic step; Lane 5, Composting materials of W2 at the end of step of pre-fermentation; Lane 6, Composting materials of W1 at the end of step of pre-fermentation; Lane 7, Composting materials of W2 at the start of step of maturation; Lane 8, Composting materials of W1 at the start of step of maturation; Lane 9, Composting materials of W2 at the end of step of matu- ration; Lane 10, Composting materials of W1 at the end of step of maturation. 3.4. Relationship between Some Selected Parameters of Composting A correlation matrix (Table 3) showed some signifi- cant relationships between the temperature, biomasses C and N, deshydrogenase activity and DNA concentration. There was a good positive linear relationship between ambient temperature recorded inside the waste heap and deshydrogenase activity and BC/BN ratio (r = 0.93, r = 0.96, r = 0.87, r = 0.71, P = 0.01, respectively). So, in- crease of temperature during each thermophilic phase is equivalent to a good deshydrogenase activity and a lot of active microbial biomass. Also, BC/BN ratio is well correlated to the extracted DNA concentration only in the case of windrow W2 (r = 0.63, P = 0.01). The values of DNA concentration recorded in the case of windrow W1 is well correlated to those observed in case of wind- row W2 (r = 0.61, P = 0.02). This result is valid for the two steps of composting, pre-fermentation and matura- tion. Moreover, stability of the temperature values inside the mass of waste means the stability of the microbial activity during the process (low deshydrogenase activity and BC/BN ratio), and this fact could be exploited as an indicator of maturity of the compost. 4. DISCUSSION AND CONCLUSIONS The present study investigates, in a semi-industrial pilot plant for compost production and under semi-arid  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 131 Figure 3. Progress of the number of mesophilic, thermophilic and spore-forming bacteria in the two windrows during the two steps of composting. Tunisian pedo-climatic condition, a new and a sim- pleprocess of co-composting of municipal solid wastes and sewage sludge, and some physical-chemical and biological parameters are followed during two succes- sive steps of pre-fermentation and of maturation using two types of pyramidal windrows W1 (100% of munici- pal solid wastes) and W2 (60% of municipal solid wastes and 40% of sewage sludge in weight). Results revealed that temperature profiles established during the two steps of composting in the two windrows W1 and W2 showed the three classical temperature steps of composting the mesophilic, thermophilic and cooling phases, respectively. The optimal duration of pre-fermentation was evalu- ated to three months on average. Consequently, a maxi- mum organic material recovery is ensured during a minimum time interval. The second step of process of controlled fermentation  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 132 Figure 4. Progress of the total compost DNA concentrations and agarose gel electrophoresis of total DNA extracted from com- posting materials (W1) during the two steps of composting. (step of maturation) is characterized by a degradation of the organic materials by microorganisms. The purpose of management of this second step of composting is to ensure favorable conditions in order to force the micro- biological activity. The procedure of microbes during this second step of composting appeared on average less active than the one recorded during the first step of pre- fermentation. All parameters considered in the present study are in favour of this conclusion. We could explain this result by several factors mainly by the decrease or exhaustion and depletion of readly degradable compounds in the mass of waste materials. This study confirm amongst others that temperature is the main parameter to consider in composting. This  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 133 1 2 3 4 5 6 7 8 9 10 Figure 5. Example of the ARISA analysis of microbial communities change and diversity in compost of wind- row 1. important factor condition the funtionning of all the others parameters, specifically the microbial activity. As a result, the level of temperature condition and depends on the type of microorganisms operating during composting. So mesophilic microbes dominate at the beginning and at the end of each step of composting process; on the other hand, thermophilic microbes control the important step of composting, the thermophilic phase. The values of temperature recorded during the two steps of composting revealed a good relashionship of this parameter with others studied here such as the deshydrogenase activity, the BC/BN ratio and the total extracted DNA. In fact, statiscal analysis showed a good positive correlation of all these last parameters with tem- perature values recorded inisde the pile of waste. Also, the preliminaly molecular study using RISA method confirmed the high complexity of the microbial com- munauties acting during the two steps of composting in the two windrows W1 and W2 and underlined by several authors [20-23]. In addition, this preliminaly molecular study demonstrated a temporal dynamics of the micro- bial communities according to the modifications of the RISA profile over time of composting for the same type of composts. It appeared also from the result of this study that the microbial diversity is much nuanced in the case of windrow W2 added with sewage sludge as com- pared to that observed in the case of windrow W1 free of sewage sludge. Furthermore, contaminants in feedstock can impact the quality, marketability, and use of finished composts. Overuse and persistence of some heavy metals, herbi- cides and insecticides could result in heavy metals and pesticide contamination of compost. The viability of composting depends very much on the quality and con- sistency of compost produced. Heavy metals content is of primary concern, and generally the values recorded during the two steps of composting are usually lower than the metal concentration limits imposed by several countries. So, it appeared from this study that the level of heavy metals contamination of the finished product of compost is satisfactory and suitable for soil application and enrichment. These low values of heavy metal con- tent are related to many factors, mainly the low level of metal contaminants in feedstock used as raw composting materials in this plant as compared to those usually men- tioned in several developed countries. This low level of heavy metal contaminants of the finished product is con- firmed by the study of [18] in the same plant. Compost is often contaminated by heavy metals due to inadequate separation of biodegradable fractions from non-degradable or inert materials. Composting can con- centrate or dilute heavy metals present in waste material by adsorption and leaching, respectively. Lowering the amounts of heavy metal present depends on metal loss through leaching. The increase of metal level is due to weight loss in the course of composting following or- ganic matter decomposition, release of carbon dioxide and water and mineralization processes [24,25]. As a consequence, potential utilisation of compost in agricul- ture might be restricted, and therefore, it is essential to monitor the magnitude of heavy metal contamination in soils, which urgently requires detailed chemical analysis of the soil. Finally, this study reviews a new and simple com- posting process tested and used in a pilot plant for com- post production under semi-arid Tunisian pedo-climatic condition, and some important chemical, physical and biological monitoring parameters are investigated in order to understand and to master the process of com- posting of municipal solid waste. 5. ACKNOWLEDGMENTS This study was supported by the Japan International Cooperation Agency (JICA) within the framework of a research program. We thank the staff of a semi-industrial composting plant, Mr K. Ben Khedija and Ms Tounsi N. at ANPE, for their assistance, cooperation and par- ticipation in this study. We are most grateful to Pr H.W. Ackermann of  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 134 Table 3. Pearson’s correlation coefficients between the main parameters studied during the two steps of composting. T°C. W1 T°C. W2 Deh. W1 Deh. W2 BC/BN W1BC/BN W2 DNA W1 DNA W2 Temp. W1 r 1.000 0.924** 0.930** 0.963** 0.873** 0.718** 0.100 0.509* S 0.000 0.000 0.000 0.000 0.001 0.724 0.053 Co 96.053 112.217 11.136 8.969 11.247 9.394 0.996 4.416 Temp. W2 r 1.000 0.999** 0.938** 0.862** 0.593** 0.008 0.494 S . 0.000 0.000 0.000 0.007 0.977 0.061 Co 153.619 15.116 11.042 14.035 9.818 0,098 5.267 Dehy. W1 r 1.000 0.943** 0.875** 0.593** 0.003 0.480 S 0.000 0.000 0.007 0.992 0.070 Co 1.492 1.095 1.404 0.967 0,003 0.503 Dehy. W2 r 1.000 0.830** 0.639** 0.047 0.503 S 0.000 0.003 0.867 0.056 Co 0.903 1.036 0.811 0,046 0.427 BC/BN W1 r 1.000 0.638** 0.080 0.424 S 0.003 0.776 0.115 Co 1.727 1.120 0,098 0.450 BC/B W2 r 1.000 0.472 0.711** S 0.076 0.003 Co 1.782 0.640 0.837 DNA W1 r 1.000 0.610* S 0.016 Co 0.907 0.480 DNA W2 r 1.000 S Co 0.683 ** Correlation is significant at level 0.01 (bilateral). * Correlation is significant level at 0.05 (bilateral). r: Pearson correlation, Co : Covariance, S: Sig. (bilat- eral), Microbial C biomass (BC), Microbial N biomass (BN), DNA: DNA concentration, Deh: Deshydrogenase activity, W1 and W2 : Windrows 1 and 2. the Department of Microbiology, Faculty of Medicine, Laval University in Quebec, Canada, for helpful discussion and critical review of the manuscript. REFERENCES [1] Ouatmane, A., Provenzano, M.R., Hafidi, M. and Senesi N. (2000). Compost maturity assessment using calo- rimetry, spectroscopy and chemical analysis. Compost Science & Utilization, 8, 124-134. [2] de Bertoldi, M., Vallini, G. and Pera, A. (1985) Practices Composting of agricultural wastes and other wastes. In: Gasser, J. K. R. (Ed.). [3] Hassen, A., Belguith, K., Jedidi, N., Cherif, A., Cherif, M. and Boudabous, A. (2001) Microbial characterization during composting of municipal solid waste. Bioresource Technology, 80, 185-192. doi:10.1016/S0960-8524(01)00065-7 [4] Whittle, A.J. and Dyson, A.J. (2002) The fate of heavy metals in green waste composting. The Environmentalist, 22, 13-21. doi:10.1007/BF00505887 [5] Ranjard, L., Poly, F., Combrisson, J., Gourbiere, F., Richaume, A., Thioulouse, J. and Nazaret, S. (2000) Heterogeneous cell density and genetic structure of bac- terial pools associated with various soil microenviron- ments as determined by enumeration and DNA finger- printing approach (RISA). Microbial Ecology, 39, 263- 272. [6] Seishi, I., David, M.C.L., Roberts, W.K.N. and Ytow, N. (2004) Microbial community analyses using a simple, rapid detection method for DNA fingerprints with a fluorescence scanner. Journal of Bioscience and Bioen-  O. Fourti et al. / Natural Science 3 (2011) 124-135 Copyright © 2011 SciRes. OPEN ACCESS 135 gineering, 98, 500-503. [7] Lehtokari, M., Nikkola, P. and Paatero, J. (1983) Deter- mination of ATP from compost using the firefly biolumi- nescence technique. European Journal of Applied Micro- biology and Biotechnology, 17, 187-192. doi:10.1007/BF00505887 [8] Hiraishi, A., Iwasaki, M., and Shinjo, H. (2000) Terminal restriction pattern analysis of 16S rRNA genes for the characterization of bacterial communities of activated sludge. Journal of Bioscience and Bioengineering, 90, 148-156. [9] Czaczyk, K., Trojanowska, K., Stachowiak, B. and Dubisz, H. (2001) Changes in Cell Number and the ATP Content during the Composting Process. Polish Journal of Environmental Studies, 10, 149-153. [10] Gillet, R. (1986). Industrial treatment of urban and as- similated wastes. Organization and management of a ser- vice. Management treaty of solid wastes and its applica- tion to under developed countries. WHO Regional Office for Europe, Copenhagen, 2, 538. [11] Bremmer, J.M. and Mulvaney, C.S. (1982) Total nitrogen. In: Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, Ed., C. A. Bluck. American Society of Agronomy, Madison, WI, 1179-1239. [12] Nelson, D.W. and Sommers, L.E. (1982) Total carbon, organic carbon, and organic matter. In: Methods of Soil Analysis. Part 2. Agronomy Monographs, Eds. A. L. Page et al. American Society of Agronomy, Madison, WI, 539-579. [13] Hassen, A., Jedidi, N., Cherif, M., M’Hiri, A., Boud- abous, A. and Cleemput, O.V. (1998) Mineralization of nitrogen in a clayey loamy-soil amended with organic wastes enriched with Zn, Cu and Cd. Bioresource Tech- nology, 64, 39-45. doi:10.1016/S0960-8524(97)00153-3 [14] Tabatabai, M.A. (1982) Soil enzymes. Agronomy, 9, 903-947. [15] Bouzaiane, O., Cherif, H., Saidi, N., Jedidi, N. and Has- sen, A. (2007) Effects of municipal solid waste compost application on the microbial biomass of cultivated and non-cultivated soil in a semi-arid zone. Waste Manage- ment & Research, 25, 334-342. doi:10.1177/0734242X07078287 [16] Leckie, S.E., Prescott, C.E., Grayston, S.J., Neufeld, J.D. and Mohn, W.W. (2004) Comparison of chloroform fu- migation-extraction, phospholipid fatty acid, and DNA methods to determine microbial biomass in forest humus. Soil Biology and Biochemistry, 36, 529-532. doi:10.1016/j.soilbio.2003.10.014 [17] Steffan, R.J., Goksoyr, J., Bej, A.K. and Atlas, R.M. (1988) Recovery of DNA from soils and sediments. Ap- plied and Environmental Microbiology, 54, 2908-2915. [18] Ben, A.L., Hassen, A., Jedidi, N., Saidi, N., Bouzaiane, O. and Murano, M. (2006) Microbial C and N dynamics during composting process of urban solid waste. Waste Management & Research, 25, 24-29. [19] CCME: Canadian Council of Ministers of the Environ- ment, 1995. Proposed compost standards for Canada, 1993. Cited in the Composting Council of Canada, Com- posting Technologies and Practices. [20] Mustin, M. (1987) Le compost “gestion de la matière organique”. Editions François Dubusc- Paris, 954. [21] Pedro, M.S., Haruta, S., Hazaka, M., Shimada, R., Yo- shida, C., Hiura, K., Ishii, M. and Igarashi, Y. (2001) Denaturing gradient gel electrophoresis analyses of mi- crobial community from field-scale composter. Journal of Bioscience and Bioengineering, 91, 159-165. doi:10.1263/jbb.91.159 [22] Marshall, M.N., Cocolin, L., Mills, D.A. and Van- derGheynst, J.S. (2003) Evaluation of PCR primers for denaturing gradient gel electrophoresis analysis of fungal communities in compost. Journal of Applied Microbiol- ogy, 95, 934-948. doi:10.1046/j.1365-2672.2 003. 02062.x [23] Poulsen, P.H.B., Møller, J. and Magid, J. (2008) Deter- mination of a relationship between chitinase activity and microbial diversity in chitin amended compost. Biore- source Technology, 99, 4355-4359. doi:10.1016/j.biortech.2007.08.042 [24] Wagner, D.J., Bacon, G.D., Knocke, W.R. and Switzenbaum, M.S. (1990) Changes and variability in concentration of heavy metals in sewage sludge during composting. Environmental Technology, 11, 949-960. doi:10.1080/09593339009384947 [25] Canaruttto, S., Petruzzelli, G., Lubrano, L. and Guidi, G.V. (1991) How composting affects heavy metal content. BioCycle, 32, 48-50.
|