L. Q. ZHU ET AL.
Copyright © 2013 SciRes. ENG
high-precision laboratory-type instrument developed for
use with strains gauges and strain gauge-based transduc-
ers to correcting for the spread in gauge resistance and
gauge factor. For overcoming the circumstances and to
compensate the thermal effect, temperature compensa-
tion circuit was designed mainly by means of two exter-
nal temperature compensating gauges.
The micro-controller unit (MCU) based closed-loop
control subsystem was built to measure and manipulate
this device. The data acquisition and control unit consists
of a multifunction single chip (SPCE061A, Sunplus Tech-
nology Company Limited) [10], which provides seven
channel 10-bit high-speed analog-to-digital converters
(ADC), and two 10-bit digital-to-analog con ve rt e r (D AC)
output channels, combined with higher processing speed
(up to 49.152 MHz) . The voltage r egulator SPY0029 was
utilized as power source to provide 3.3 V d.c. based on
the operating voltage of single chip that ranged from 2.6
to 3.6 V. The input signal comes from a microchip pro-
gram based on the DAC, it could predefine the desired
force level. In addition, the I/O channel provides output
electronically control as well as impulse wave signal. A
four phase stepper motor component is driven to deliver
and maintain a desired precise force with the uniaxial
movement. This feedback control circuit provides a real-
time visual indication on the digital display of the strain
indicator output, the signal of the strain gauge and the
contr ol si gnal woul d be compa red whil e the for ce is sensed.
3. System Test
The loading system must be calibrated by verifying the
accuracy and precision of linearity of the displacement
sensor. To verify this device, the protoplasts isolated from
the wild-type Arabidopsis thaliana (ecotype Columbia
Col-0) rosette leaves were retained as experimental ma-
terial, Protoplasts were then immobilized by gently swirl-
ing them into low-melting-point agar in MS medium sup-
plemented with 0.4 mol∙l−1 mannitol, 0.1% MES, sucrose,
2,4-dichlorophenoxyacetic acid, and self-conditioned me-
dium harvested from suspension cultured Arabidopsis
cells [11]. Then the loaded test specimen which embed-
ded with living cells was removed and chipped into slices
of approximately 2.0 - 5.0 mm thick, and it was placed
onto the chamber of the mechanical loading apparatus
described above.
Hamant has reported that microtubule orientation in
the shoot apical meristem was found to follow the orien-
tation of stress patterns in the organ [12], this conclusion
supports the prior viewpoint that th e lo c al microinduction
of expansin expression and resulting cell wall softening
is sufficient to induce morphogenetic processes, leading
to the initiation of leaf structures from the shoot apical
meristem [13]. Continuous uniaxial compressive force (at
approximately 50 - 300 mN) was imposed on the oppo-
site sides of agar block, time ranged from 60 seconds to
1200 seconds, this allowed a microscope to be brought
close to the cell under test. Our device could play a role
in the relative research field. The applying baffle dis-
placement and the cell deformation could be observed,
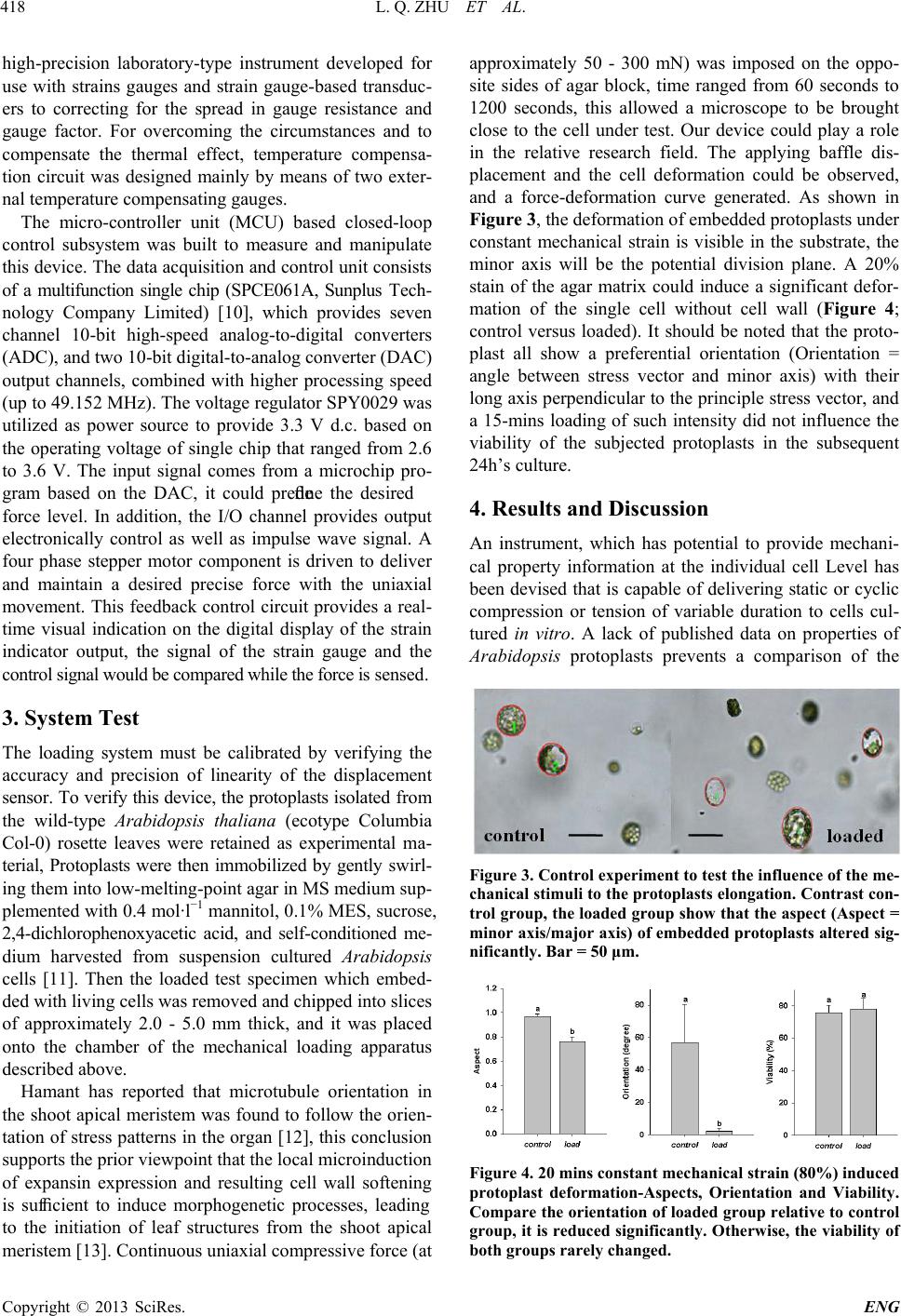
and a force-deformation curve generated. As shown in
Figure 3, the deformation of embedded protoplasts under
constant mechanical strain is visible in the substrate, the
minor axis will be the potential division plane. A 20%
stain of the agar matrix could induce a significant defor-
mation of the single cell without cell wall (Figure 4;
control versus loaded). It should be noted that the proto-
plast all show a preferential orientation (Orientation =
angle between stress vector and minor axis) with their
long axis perpendicular to the principle stress vector, and
a 15-mins loading of such intensity did not influence the
viability of the subjected protoplasts in the subsequent
24h’s culture.
4. Results and Discussion
An instrument, which has potential to provide mechani-
cal property information at the individual cell Level has
been devised that is capable of delivering static or cyclic
compression or tension of variable duration to cells cul-
tured in vitro. A lack of published data on properties of
Arabidopsis protoplasts prevents a comparison of the
Figure 3. Control experiment to test the influence of t he me -
chanical stimuli to the protoplasts elongation. Contrast con-
trol group, the loaded group show that the aspect (Aspect =
minor axis/major axis) of embedded protoplasts altered sig-
nificantly. Bar = 50 μm.
Figure 4. 20 mins constant mechanical strain (80%) induced
protoplast deformation-Aspects, Orientation and Viability.
Compare the orientation of loaded group relative to control
group, it is reduc ed significantly. Ot herwise, the viability of
both groups rarely changed.