B. Y. LIU ET AL.
Copyright © 2013 SciRes. ENG
399
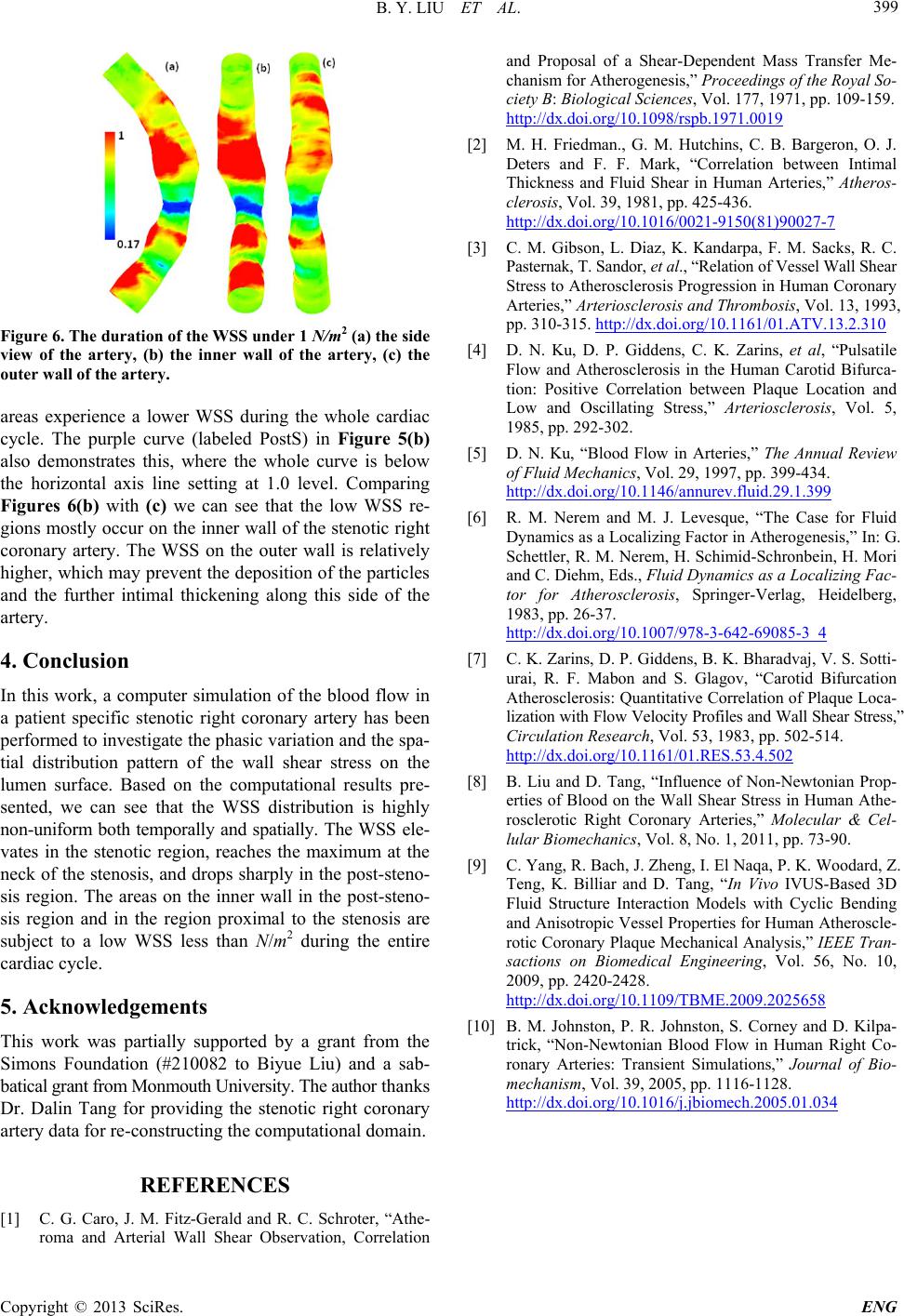
Figure 6. The duration of the WSS under 1 N/m2 (a) the side
view of the artery, (b) the inner wall of the artery, (c) the
outer wall of the artery.
areas experience a lower WSS during the whole cardiac
cycle. The purple curve (labeled PostS) in Figure 5(b)
also demonstrates this, where the whole curve is below
the horizontal axis line setting at 1.0 level. Comparing
Figures 6(b) with (c) we can see that the low WSS re-
gions mostly occur on the inner wall of the stenotic right
coronary artery. The WSS on the outer wall is relatively
higher, which may prevent the deposition of the particles
and the further intimal thickening along this side of the
artery.
4. Conclusion
In this work, a computer simulation of the blood flow in
a patient specific stenotic right coronary artery has been
performed to investigate the phasic variation and the spa-
tial distribution pattern of the wall shear stress on the
lumen surface. Based on the computational results pre-
sented, we can see that the WSS distribution is highly
non-uniform both temporally and spatially. The WSS ele-
vates in the stenotic region, reaches the maximum at the
neck of the stenosis, and drops sharply in the post-steno-
sis region. The areas on the inner wall in the post-steno-
sis region and in the region proximal to the stenosis are
subject to a low WSS less than N/m2 during the entire
cardiac cycle.
5. Acknowledgements
This work was partially supported by a grant from the
Simons Foundation (#210082 to Biyue Liu) and a sab-
batic al gra nt f rom Monm o uth Uni ve rsi ty. The author thanks
Dr. Dalin Tang for providing the stenotic right coronary
artery data for re-constructing the computational domain.
REFERENCES
[1] C. G. Caro, J. M. Fitz-Gerald and R. C. Schroter, “Athe-
roma and Arterial Wall Shear Observation, Correlation
and Proposal of a Shear-Dependent Mass Transfer Me-
chanism for Atherogenesis,” Proceedings of the Royal So-
ciety B: Biological Sciences, Vol. 177, 1971, pp. 109-159.
http://dx.doi.org/10.1098/rspb.1971.0019
[2] M. H. Friedman., G. M. Hutchins, C. B. Bargeron, O. J.
Deters and F. F. Mark, “Correlation between Intimal
Thickness and Fluid Shear in Human Arteries,” Atheros-
clerosis, Vol. 39, 1981, pp. 425-436.
http://dx.doi.org/10.1016/0021-9150(81)90027-7
[3] C. M. Gibson, L. Diaz, K. Kandarpa, F. M. Sacks, R. C.
Pasternak, T. Sandor, et al. , “Rel a t ion of Ve ssel Wall Shear
Stress to Atherosclerosis Progression in Human Coronary
Arteries,” Arteriosc le rosis and Thrombos is, Vol. 13, 1993,
pp. 310-315. http://dx.doi.org/10.1161/01.ATV.13.2.310
[4] D. N. Ku, D. P. Giddens, C. K. Zarins, et al, “Pulsatile
Flow and Atherosclerosis in the Human Carotid Bifurca-
tion: Positive Correlation between Plaque Location and
Low and Oscillating Stress,” Arteriosclerosis, Vol. 5,
1985, pp. 292-302.
[5] D. N. Ku, “Blood Flow in Arteries,” The Annual Review
of Fluid Mechanics, Vol. 29, 1997, pp. 399-434.
http://dx.doi.org/10.1146/annurev.fluid.29.1.399
[6] R. M. Nerem and M. J. Levesque, “The Case for Fluid
Dynamics as a Localizing Factor in Atherogenesis,” In: G.
Schettler, R. M. Nerem, H. Schimid-Schronbein, H. Mori
and C. Diehm, Eds., Fluid Dynamics as a Localizing Fac-
tor for Atherosclerosis, Springer-Verlag, Heidelberg,
1983, pp. 26-37.
http://dx.doi.org/10.1007/978-3-642-69085-3_4
[7] C. K. Zarins, D. P. Giddens, B. K. Bharadvaj, V. S. Sotti-
urai, R. F. Mabon and S. Glagov, “Carotid Bifurcation
Atherosclerosis: Quantitative Correlation of Plaque Loca-
lization with Flow Velocity Profiles and Wall Shear Stress,”
Circulation Research, Vol. 53, 1983, pp. 502-514.
http://dx.doi.org/10.1161/01.RES.53.4.502
[8] B. Liu and D. Tang, “Influence of Non-Newtonian Prop-
erties of Blood on the Wall Shear Stress in Human Athe -
rosclerotic Right Coronary Arteries,” Molecular & Cel-
lular Biomechanics, Vol. 8, No. 1, 2011, pp. 73-90.
[9] C. Yang, R. Bach, J. Zheng, I. El Naqa, P. K. Woodard, Z.
Teng, K. Billiar and D. Tang, “In Vivo IVUS-Based 3D
Fluid Structure Interaction Models with Cyclic Bending
and Anisotropic Vessel Properties for Human Atheroscle-
rotic Coronary Plaque Mechanical Analysis,” IEEE Tran-
sactions on Biomedical Engineering, Vol. 56, No. 10,
2009, pp. 2420-2428.
http://dx.doi.org/10.1109/TBME.2009.2025658
[10] B. M. Johnston, P. R. Johnston, S. Corney and D. Kilpa-
trick, “Non-Newtonian Blood Flow in Human Right Co-
ronary Arteries: Transient Simulations,” Journal of Bio-
mechanism, Vol. 39, 2005, pp. 1116-1128.
http://dx.doi.org/10.1016/j.jbiomech.2005.01.034