K. M. XU
Copyright © 2013 SciRes. ENG
and is the limiting step or bottleneck in charge transfer
along the DNA strands [6,9]. However, at long (+T:A−)n
sequences the size of the bottleneck remains the same so
that the sequence distance dependence vanishes [9]. Be-
cause +G:C− has a relatively high capacitance, it is easy
to trap charges, so it is likely to become the end point of
a charge transport [6,9]. In this regard, the circuit model
prediction agrees with experimental resu lts completely.
Oscillatory current through the double helix is likely to
have physiological significance. For example, if a base
pair is mismatched at a certain position, then the oscilla-
tory rhythms would be broken. Proofreading enzyme that
scans the DNA sequence continually might easily locate
the trouble point by the abnormal electric signal. Fur-
thermore, bio molecules are inherentl y unstable. Only con-
stant flow of energy prevents them from being disorga-
nized. It stands to reason that the incessant charge vibra-
tions in the genetic substance are vital for living organ-
isms to maintain the integrity of the gene sequence.
From organic chemistry perspective, the base pair is
capable of storing considerable amount of charges in ei-
ther polarity by at least two conceivable mechanisms.
First, both pyrimidine and purine are heterocyclic rings
composed of carbon and nitrogen atoms. On the one hand,
nitrogen is more electronegative than carbon for attract-
ing higher electron density in covalent bonds with carbon.
On the other hand, nitrogen atom has lone pair electrons
to share with carbon under electron deficiency. The com-
bination imparts great flexibility to the nucleotide bases
for either holding or releasing electrons. Second, the he-
terocyclic rings are aromatic that possess diamagnetic
ring current. In the circuit, aromatic ring current may re-
duce the charge saturation of the nitrogenous base through
electromagnetic effect, and as a result increase the elec-
tric capacitance of the base pair. Both properties enable a
base pair to be a good bipolar capacitor. The recognition
of a base pair as a valid capacitor provides a sharp insight
into molecular electronics.
A base pair is an electric capacitor. Alternate current
passes through th e hydrogen bonds. There are oscillating
waves between each pair of nitrogenous bases within
DNA nucleotides. Hydrogen bonding is a dynamic elec-
tronic action that delivers messages between the two
bases, helping to balance the strain in a DNA double he-
lix. Hydrogen bonds serve as the gateways to deliver
messages. In this way, we describe the hydrogen bonds
as a portal between the pair of bases. This dynamic in-
terpretation is contrary to traditional static model of hy-
drogen bonds. Since the e xpressi on of any forces r equires
message exchanges, it is the wave information conveyed
between pairs of bases that makes hydrogen bonds strong
enough to hold the double helix of DNA together. With-
out the wave message, the atoms on both sides of hydro-
gen bonds would be detached and the organization of
DNA woul d start to u nravel.
In the stepwise oscillatory circuit, a capacitor must be
charged up to a threshold potential for positive charges to
overflow throug h the ether bond of the deoxyr ibose. And
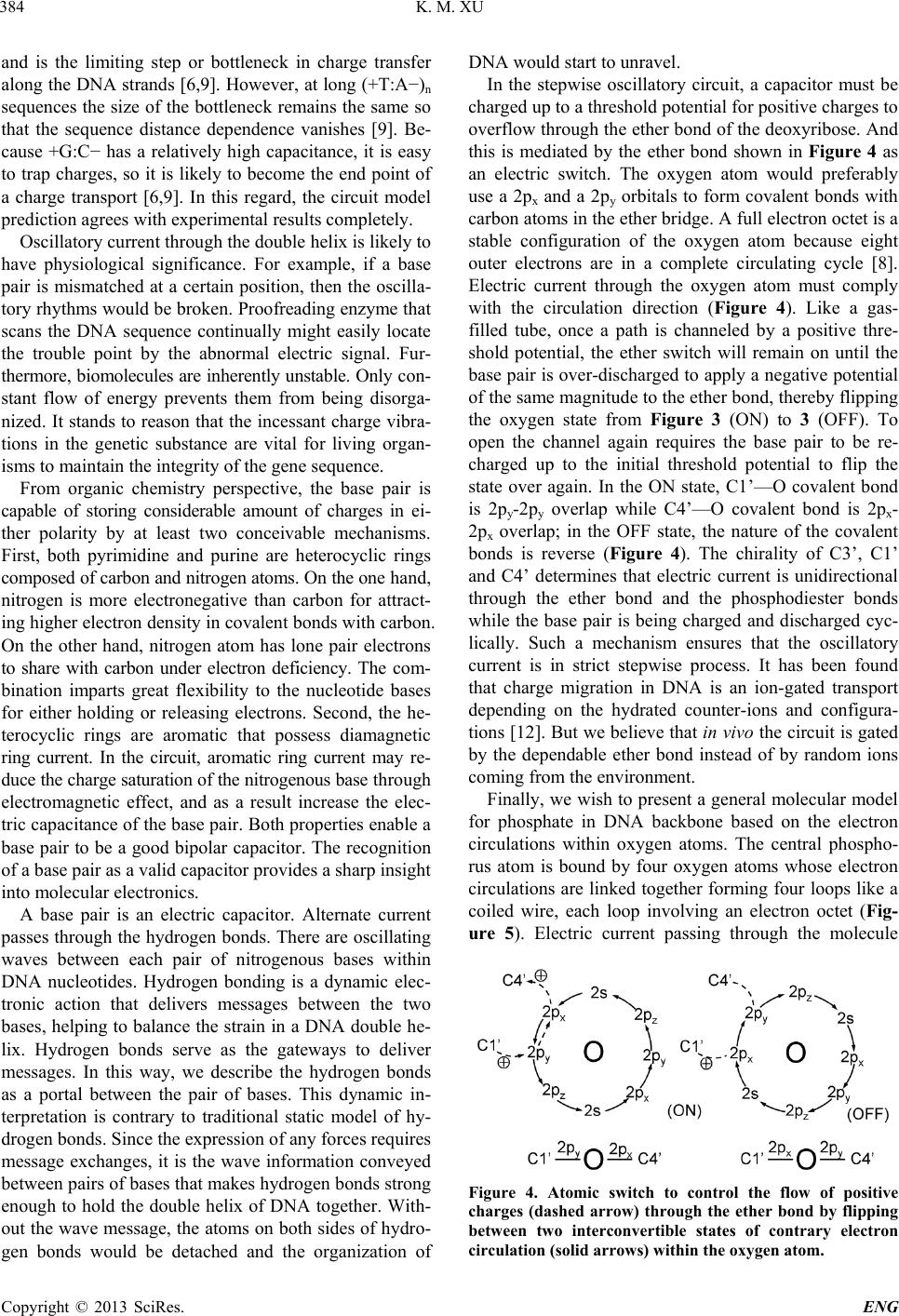
this is mediated by the ether bond shown in Figure 4 as
an electric switch. The oxygen atom would preferably
use a 2px and a 2py orbitals to form covalent bonds with
carbon atoms in the ether bridge. A full electron octet is a
stable configuration of the oxygen atom because eight
outer electrons are in a complete circulating cycle [8].
Electric current through the oxygen atom must comply
with the circulation direction (Figure 4). Like a gas-
filled tube, once a path is channeled by a positive thre-
shold potential, the ether switch will remain on until the
base pair is over-discharged to apply a negative potential
of the same magnitude to the ether bond, thereby flipping
the oxygen state from Figure 3 (ON) to 3 (OFF). To
open the channel again requires the base pair to be re-
charged up to the initial threshold potential to flip the
state over again. In the ON state, C1’—O covalent bond
is 2py-2py overlap while C4’—O covalent bond is 2px-
2px overlap; in the OFF state, the nature of the covalent
bonds is reverse (Figure 4). The chirality of C3’, C1’
and C4’ determines that electric current is unidirectional
through the ether bond and the phosphodiester bonds
while the base pair is being charged and discharged cyc-
lically. Such a mechanism ensures that the oscillatory
current is in strict stepwise process. It has been found
that charge migration in DNA is an ion-gated transport
depending on the hydrated counter-ions and configura-
tions [12]. But we believe that in vivo the circuit is gated
by the dependable ether bond instead of by random ions
coming from the environment.
Finally, we wish to present a general molecular model
for phosphate in DNA backbone based on the electron
circulations within oxygen atoms. The central phospho-
rus atom is bound by four oxygen atoms whose electron
circulations are linked together forming four loops like a
coiled wire, each loop involving an electron octet (Fig-
ure 5). Electric current passing through the molecule
Figure 4. Atomic switch to control the flow of positive
charges (dashed arrow) through the ether bond by flipping
between two interconvertible states of contrary electron
circulation (solid arrows) within the oxygen atom.