 Vol.1, No.4, 97-111 (2013) Advances in Enzyme Research http://dx.doi.org/10.4236/aer.2013.14011 Structural and functional evidence for two separate oligosaccharide binding sites of Pasteurella multocida hyaluronan synthase Floor K. Kooy1,2, Hendrik H. Beeftink2*, Michel H. M. Eppink2, Johannes Tramper2, Gerrit Eggink1,2, Carmen G. Boeriu1 1Food and Biobased Research, Wageningen University and Research Center, Wageningen, The Netherlands 2Bioprocess Engineering, Wageningen University and Research Center, Wageningen, The Netherlands; *Corresponding Author: rik.beeftink@wur.nl Received 28 June 2013; revised 12 August 2013; accepted 24 August 2013 Copyright © 2013 Floor K. Kooy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Pasteurella multocida hyaluronan synthase (PmHAS) is a bi-functional glycosyltransferase, containing a β1,3-glucuronyltransferase and β1,4-N-acetylglucosaminetransferase domain. PmHAS catalyzes the elongation of hyaluronan (HA) through the sequential addition of single monosaccharides to the non-reducing end of the hyaluronan chain. Research is focused on the relation between the length of the HA oligo- saccharide and the single-step elongation ki- netics from HA4 up to HA9. It was found that the turnover number kcat increased with length to maximum values of 11 and 14 s−1 for NAc- and UA-transfer, respectively. Interestingly, the spe- cificity constant kcat/KM increased with polymer length from HA5 to HA7 to a value of 44 mM−1·s−1, indicating an oligosaccharide binding site with increasing specificity towards a heptasaccha- ride at the UA domain. The value of kcat/KM re- mained moderately constant around 8 mM−1·s−1 for HA4, HA6, and HA8, indicating a binding site with significantly lower binding specificity at the NAc domain than at the UA domain. These find- ings are further corroborated by a structural homology model of PmHAS, revealing two dis- tinct sites for binding of oligosaccharides of different sizes, one in each transferase domain. Structural alignment studies between PmHAS and glycosyltransferases of the GT-A fold showed significant similarity in the binding of the UDP-sugars and the orientation of the ac- ceptor substrate. These similarities in substrate orientation in the active site and in essential amino acid residues involved in substrate bind- ing were utilized to localize the two HA oligo- saccharide binding sites. Keywords: Pasteurella; Hyaluronan; Binding Site; Polymerization; Co-Polymers 1. INTRODUCTION Enzymatic production of glycosaminoglycans has in- creasingly attracted attention over the last two decades as these polysaccharides are applied multifold in pharmacy and cosmetics. For organ integrity and functioning, gly- cosaminoglycans are essential since they activate signal- ing pathways that control cell proliferation, differentia- tion, adhesion, and migration [1,2]. The controlled pro- duction of oligosaccharides with a defined chain length and sulfate groups would constitute a breakthrough in medical sciences with potential applications in, for ex- ample, anti-cancer therapeutics [3] and disrupting viral invasion and pathogenesis [1]. In nature, glycosami- noglycans are produced by glycosyltransferases that catalyze the transfer of an activated donor sugar to an oligosaccharide acceptor. One particular glycosaminoglycan is hyaluronan (HA), an alternating copolymer of β3-N-acetylglycosamine (GlcNAc) and β4-glucuronic acid (GlcUA). Following the initial HA isolation from animal tissues [4-7], it was ascertained that HA was also produced by a small num- ber of microbial pathogens [8-10], employing HA as a cloak in order to disguise themselves from the mammal- ian immune system. Numerous cultivation procedures have been developed to produce HA utilizing either these pathogenic microorganisms [8,11,12] or safe recombi- nant hosts [13-18], containing the hyaluronan synthases Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 98 (HAS) that synthesize HA. The quality of the product is crucial since applications of HA depend on the oligo- or polysaccharide length; consequently, the production of HA with defined lengths is required. Innovative produc- tion techniques aspire for high molecular weight HA with a moderate length distribution, or polydispersity, by inflicting stress through culture conditions [12,19-21] or by avoiding HA degradation through hyaluronidase [13, 18,21]. Recently, it was demonstrated that, for Streptococcus zooepidemicus, overexpression of genes involved in UDP-GlcNAc biosynthesis increased the molecular weight of the HA products [22], indicating that the chain length is controlled by the availability of the substrates. This is corroborated by kinetic data from HAS enzymes of different sources, demonstrating a significantly larger KMNAc value than KMUA value [23-26]. Furthermore, the hyaluronan synthase in Pasteurella multocida (PmHAS) possesses the ability to elongate HA oligosaccharides [27], which offers an additional opportunity to optimize product length and polydispersity. The addition of HA oligosaccharides to the reaction in the presence of both UDP-sugars exacerbates the polymerization rate of PmHAS and diminishes the polydispersity of the HA products compared to reactions initiated with only the UDP-sugars [28]. Although these findings have resulted in increased molecular weight products with minimal polydispersity, the kinetic elongation mechanism of HAS enzymes supporting these results remains unknown. The following is an investigation of single-step elon- gation kinetics of various HA oligosaccharide chain lengths (HA4 up to HA9). Several single-substrate models were evaluated and kinetic parameters were determined for each individual oligosaccharide. Binding affinities for the UA-transferase site were ascertained to be considera- bly higher than those for the NAc-transferase site and two physically distinct binding sites were indicated and supported by a structural homology model for PmHAS, based on the crystal structure of chondroitin polymerase K4CP [29]. Chondroitin polymerase and PmHAS have a prominent sequence identity and sequence homology (62% and 79%, respectively), resulting in a reliable structural model for PmHAS. With the support of struc- tural alignment studies, similarities in the active sites of PmHAS and other glycosyltransferases have been ascer- tained such as the location of acceptor binding sites and several conserved amino acids involved in binding the substrates. Conserved regions have been reported before for UDP-sugar binding sites, whereas, in this study, we have also determined structural similarities for the ac- ceptor binding site. To summarize, this study presents evidence for two distinct oligosaccharides binding sites within PmHAS which affects the polydispersity of the HA products. 2. EXPERIMENTAL 2.1. Characterization of PmHAS All reagents were purchased from either Fisher or Sigma-Aldrich unless stated otherwise. Purified PmHAS was provided by Merck & Co. (formerly Organon N.V.). PmHAS represents the soluble PmHAS1-703 enzyme, as described by Jing and DeAngelis[30], cloned and ex- pressed in a pET101/D-TOPO expression vector (Invi- trogen) with an additional V5 epitope and polyhistidine (6x His) region at the C-terminal end of the enzyme. PmHAS was purified from the crude extract employing affinity chromatography on Ni-NTA columns (Qiagen). A coupled-enzyme assay, similar to assays created for other glycosyltransferases[23,31,32], was developed to measure PmHAS activity. The coupled-enzyme assay directly links the increase in the UDP by-product of the PmHAS elongation to the decrease of NADH that was spectrophotometrically measured at 340 nm. PmHAS activity was measured varying one of the reaction condi- tions, while keeping the others constant. The standard reaction buffer incorporated 5 mM MgCl2, 112.5 mM KCl, 1 M ethylene glycol, and 50 mMTris·HCl (pH 8.0) and the assay components 60 U PK/ml, 75 U LDH/ml, 2 mM PEP, and 0.4 mM NADH. Bis-Tris·HCl was used for experiments below pH 7, and Tris·HCl for the ex- periments above or at pH 7. The following reaction con- ditions were varied and measured with the coupled-en- zyme assay: pH 5.6 - 9; temperature 20˚C - 40˚C; 5 mM of either MgCl2, MnCl2, CoCl2, NiCl2, or CaCl2; MgCl2 5 - 50 mM; viscous buffer either 1 M trehalose, 1 M su- crose, or 0.1 - 2 M ethylene glycol. Concentrations of PmHAS, sugar nucleotides, and HA4 were kept constant at 50 μg/ml, 5.5 mM and 0.1 mM, respectively. In ex- periment with varying MgCl2 concentrations, substrate concentrations were 1mM for both UDP-sugars and 0.1mM for HA4. The reactions were measured at 35˚C for 20 min in 96-well150 μl UV star microplates (Greiner Bio-One, Germany) and a temperature-controlled Safire spectro- photometer (Tecan, Switzerland). Following measure- ment of the absorbance reduction, reactions were discon- tinued by 15 min of heating at 95˚C and then placed in the freezer (−20˚C) until analysis through gel electro- phoresis. PmHAS activity was also examined for 1 and 5 hours of reaction at KCl concentrations ranging from 0 to 200 mM; because KCl is needed for PK activity, this ex- periment was only analyzed through gel electrophoresis. 2.2. HA Product Analysis by Gel Electrophoresis Reaction mixtures were analyzed on 20% TBE poly- acrylamide gels (Invitrogen) by gel electrophoresis and Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 99 stained by Stains-All [33]. On the gels, Generuler DNA ladder Ultra low range (Invitrogen) was employed as a marker. 2.3. PmHAS Activity in Single-Step Elongations Initial rates were evaluated at 35˚C for each one-step elongation from HA4 up to HA 9 with the coupled-en- zyme assay with 60 u PK/ml, 75 u LDH/ml, 2 mM phosphoenolpyruvate, 0.4 mM NADH, 15 mM MgCl2, 112.5 mM KCl, 1 M ethylene glycol, 50 mMTris·HCl at pH 8.0. Reaction mixtures contained a single purified HA oligomer (obtained from Hyalose, L.L.C, USA) and one tyoe of monomer. Single-step elongations of even- numbered oligosaccharides as a substrateproceeded at saturating UDP-GlcNAc concentrations of 40 mM in the absence of UDP-GlcUA, while elongationsof odd-num- bered oligomers were performed at saturating UDP- GlcUA concentrations of 1 mM in the absence of UDP- GlcNAc. This setup ascertained reactions to be sin- gle-step elongations. After 5 min of incubation, the reac- tion was initiated by addition of 5 μg/ml PmHAS and various oligosaccharide concentrations (0.1, 0.5, 1, 2, 4, or 6 mM). Reactions were discontinued by 15 min heat- ing at 95˚C; enzymes were then removed with a Micro- con YM-30 centrifugal filter unit (Millipore). Samples were desalted with Dowex AG 50W-X8 (Bio-rad Labo- ratories) before measuring product formation by Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS). For the MALDI-TOF MS analysis, an Ultraflex work- station (BrukerDaltonics, Germany) with a 337 nm laser was employed. The mass spectrometer was operated in positive mode and calibrated with a mixture of malto- dextrins (mass range 250 - 2500 Da). The laser irradiance was adjusted between 29% and 32% of the full laser power and, following a delayed extraction period of 200 ns. Ions were accelerated by a 25 kV voltage and de- tected in the reflector mode. For data collection, 200 shots were used. Samples were diluted 10 times in a ma- trix solution prepared with solution of 10 mg of 2,5-di- hydroxybenzoic acid (DHB) in water. ; for analysis, 2 ml of the mixture was transferred to a MALDI sample plate and dried under a stream of warm air. 2.4. Analysis of Kinetic Data Three one-substrate equations were matched to the kinetic datato find the best fit. The Michaelis Menten, Hill, and substrate inhibition equations [34,35] were fit- ted in Excel with unweighted nonlinear regression: max Mich aelis Menten M vHA vKHA (1) max M Hill n nn vHA vKHA (2) max 2 Mi substrate inhibition vHA vKHAHAK (3) with v indicating the reaction rate by volume, vmax its (virtual) maximum and equal to kcatE, kcat the turnover number, E the enzyme concentration, HA the oligosac- charide concentration, KM the saturation constant, KI the inhibition constant. The uncertainties of the fits, standard deviations of the parameters and the correlation matrices were determined with the Excel SolverAid macro [36]. Goodness of fit for the three models was evaluated from graphical plots, such as residual and normal probability plots, and by analysis of the following goodness-of-fit estimators: stability of the model; the corrected Akaike criterion; Sy.x; the correlation between the estimated pa- rameters kcat, KM, n or Ki; and the standard deviation of these estimates [37,38]. 2.5. Competition Studies Competition at the oligosaccharide site was evaluated by measuring the activity of, for example, the elongation of an even-numbered oligosaccharide by UDP-GlcNAc as a sugar donor in the presence of an odd-numbered HA that cannot be extended by this donor sugar [39]. Activ- ity was measured by the coupled-enzyme assay under identical reaction conditions as used for kinetic studies but also containing 10 mM UDP-GlcUA or 20 mM UDP- GlcNAc. Reactions of HA4, HA5 and HA6 were indi- vidually monitored in the presence of a competing oli- gosaccharide with a molar ratio between the reacting and competing oligosaccharides of 1:1 or 1:10. Reactions of 0.3 mM reacting oligosaccharide with the omission of the competing oligosaccharide were taken as a reference. 2.6. Structure Homology Modeling Model building and energy minimization of PmHAS was performed with Modeler using the Accelrys Discov- ery Studio 2.1 software package with K4CP as a template structure. The protein model was validated with Pro- files-3D (Accelrys Discovery Studio 2.1), the stereo- chemical quality of the homology model was verified by PROCHECK [40], and the protein folding was assessed with PROSAII [41]. Docking studies of HA oligosaccha- rides were performed with the program AutodockVina [42]. Structural alignment was performed utilizing Dali- Lite [43]. Structural alignment of enzymes was evaluated by their root mean square deviation (RSMD) and Z- scores. Low RSMD values (below 4.0 Å) and elevated Z-scores (above 2) are an indication of a favorable structural superimposition and may indicate a conserved Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 100 fold structure. All structural images were generated with PyMOL version 0.99 (Delano Scientific LLC, San Carlos, California, USA). 2.7. Polymerization Reactions with HA4-Fluor Purified HA4 oligosaccharides were labeled with the fluorophoreanthranillic acid at the reducing end of the chain. Labeling and purification of HA4-fluor was ac- complished as described before [33]. All reactions con- tained 2 mM UDP-GlcUA, 40 mM UDP-GlcNAc, 15 mM MgCl2, 1 M ethylene glycol, and 50 mMTris·HCl at pH 8.0. The HA4-fluor concentration was maintained at saturating values (2.5 mM) or at subsaturating values (0.1 mM). Reactions were initiated by adding either 15 or 30 μg/ml PmHAS and performed in 7.5 μl of reaction volume in PCR eppendorf tubes. The reaction progress was analyzed for 130 min at 30˚C; every 5 or 10 min, the reaction of 1 sample tube was discontinued by freezing in liquid nitrogen and maintaining it at −20˚C. Following the experiments, all samples were heated for 15 min at 95˚C and analyzed on 20% TBE polyacrylamide gels; gel images were processed as described elsewhere [33]. 3. RESULTS AND DISCUSSION 3.1. Kinetic Characteristics A combination of kinetic characterization and struc- tural modeling was employed in order to study the po- lymerization of hyaluronan by Pasteurella multocida hyaluronan synthase (PmHAS), focusing on the influ- ence of the oligosaccharide length on the turnover num- ber (kcat) and the specificity constant (kcat/KM). Kinetic results are first introduced that characterize the optimal conditions for PmHAS activity. To accomplish this, sin- gle-step elongation kinetics were investigated, including the influence of competing oligosaccharides on the po- lymerization rate. Subsequently, a structural homology model of PmHAS is considered, and structural relations to other glycosyltransferases are then submitted. Utiliz- ing the determined kinetic parameters, we demonstrate that - due to two oligosaccharide binding sites - product polydispersity increases at sub-saturating HA concentra- tions. 3.1.1. Characterization of PmHAS The polymerization activity of PmHAS was measured to study optimal conditions for elongation with HA4 as template and equimolar amounts of both UDP sugar sub- strates. The reaction conditions that were varied included: pH, temperature, the nature of the divalent ions and the stabilizing buffer components. PmHAS activity has a pH optimum between 7.5 and 9 (Figure 1(A)), with highest activity at pH 8. Activity increased with increasing tem- perature (Figure 1(B)) to 35˚C, after which activity de- creases, probably due to enzyme inactivation. At optimal pH and temperature, elevated molecular weight products were obtained with a narrow product range (Figures 1(A) and (B)). The nature of the divalent metal ions signify- cantly affected the PmHAS activity; most efficient was Mg2+, whereas the activity decreased to 70% with Mn2+ or Co2+, to 28% for Ni2+ and to 22% for Ca2+ (Figure 1(C)). PmHAS activity was optimal at 15 mM of MgCl2. Bufferviscogens, such as ethylene glycol or trehalose, not only influenced PmHAS activity, but also affected product polydispersity (Figure 1(D)). Significantly lower polydispersity and a high activity were found at 1 M of ethylene glycol. Varying the ethylene glycol concentra- tion from 0.1 up to 2 M resulted in minor changes in PmHAS activity; activity was highest at 1 M ethylene glycol (not shown). The following conditions were therefore selected for kinetic experiments: pH 8.0, 35˚C, 15 mM Mg2+ and 1 M ethylene glycol. PmHAS activity in cell membrane preparations has been previously described with a maximum between pH 6.8 and 7.6 and a 2 - 3 fold higher activity withMn2+ than with Mg2+ [44]. Differences in optimal conditions are possibly a result of the use of isolated PmHAS in our studies. Similar pH and temperature optima were re- ported for hyaluronan synthases from Streptococcus equisimilis (SeHAS) and Xenopuslaevis (XlHAS) [24, 45]. The metal ion preference of PmHAS is also compa- rable with that observed for XlHAS. The most effective divalent metal ion for XlHAS was Mg2+ with a 4- to 10-fold reduction with Mn2+, Ni2+ or Co2+ [24]. Al- though the metal ion preference was not reported for other HAS enzymes, the corresponding activity assays included 15 - 20 mM of MgCl2 [25,26,46,47], suggesting that Mg2+ is the preferred ion for HAS enzymes in gen- eral. In addition, viscous compounds increased SeHAS activity as well as PmHAS activity when employing eth- ylene glycol and sucrose below 0.5 M; however, when increasing the concentration of these viscogens, SeHAS activity exhibited inhibition [45]. 3.1.2. Influence of Oligosaccharide Length on kcat and KM HAn oligosaccharides, (with n ranging from 4 to 9), were individually elongated in single-step reactions with the corresponding sugar nucleotide in excess (UDP- GlcNAc for even-numbered and UDP-GlcUA for odd- numbered oligosaccharides). The non-reducing end, where elongation occurs, contains a GlcUA sugar for the even-numbered oligosaccharides, and, mutatis mutandis, a GlcNAc sugar for the odd-numbered oligosaccharides. The reaction progress was analyzed employing the cou- led enzyme assay described in the Experimental section. p Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 Copyright © 2013 SciRes. 101 Figure 1. Specific activity of PmHAS under different reaction conditions. (A) pH dependency of PmHAS ac- tivity; reaction products at pH 5.6, 7, 8 and 9 were analyzed on gel; most abundant and longest products were observed at p H8. M indicates the Generuler DNA ladder; bars demonstrate the corresponding duplicates. (B) Temperature dependency of PmHAS activity; reactions products at 20˚C, 25˚C, 35˚C and 40˚C were analyzed on gel. Product size and amount concur with activity measurements; (C) Effect of divalent ions on PmHAS activity. Best results were obtained with 15 mM Mg2+. (D) Effect of viscogens on PmHAS activity; sucrose (S), trehalose (T) and ethylene glycol (E) were compared to a viscogen-less buffer as negative control (N.C.). Gel analysis of reaction products show ethylene glycol to stimulate low polydispersity. MALDITOF-MS confirmed the formation of the ex- pected products HA(n + 1) in all reactions (data not shown). Since the corresponding UDP-sugar is in excess, the elongation can be considered as a one-substrate reaction [34]. Three models for one substrate kinetics were used for fitting the data by nonlinear regression: the Micha- elis-Menten equation, the substrate-inhibition equation and the Hill equation (see the Experimental section). These models were selected to determine if PmHAS elongates HA through classical Michaelis kinetics or if the elongation was regulated by other mechanisms, such as cooperativity or substrate inhibition. Goodness-of-fit was judged statistically by examina- tion of residual and normal probability plots, by evalua- tion of standard deviations of the estimated parameters kcat and KM, and by statistical tests such as the corrected Akaike criterion. These statistical tests demonstrated that all three models fitted well (Ta bl e 1). However, the Hill equation was found to reduce to the Michaelis Menten equation, since the estimated Hill number n equaled unity for every reaction. In addition, the substrate inhibi- tion model did not allow accurate estimation of kcat and KM. The substrate inhibition model resulted in substantial standard deviations for these parameters, and a very strong correlation between kcat and KM varying from 0.90 to 0.99, which was not observed for the other models. In summary, the Michaelis Menten equation was superior for the present concentration range, although substrate inhibition may possibly become relevant at elevated oli- gosaccharide concentrations. Experimental data and their Michaelis Menten fit are depicted in Figure 2; the resulting values for kcat and KM are given in Ta ble 2. To the best of our knowledge, it is the first time that these kinetic parameters are reported for individual oligosaccharides for any of the HAS en- zymes. Globally, the value of the turnover number kcat is seen to increase with oligosaccharide length; this in- crease levels off at higher lengths. In addition, even- numbered HA polymers seem to feature a lower kcat than the next larger odd-numbered ones. Figure 3 illustrates this behavior and shows a general increase with length as well as (considering the relatively small standard devia- tions) the difference in kcat values between odd-numbered and even-numbered oligosaccharides. Since no literature values for kcat are available as a reference, we use spe- cific activity values for comparison. The UA-transferase specific activity of approximately 450 μmol/mg·hr ob- served for 1 mM HA7 or HA9 at 1 mM UDP-GlcUA (Figure 2) are virtually equal to the reported value of 484 μmol/mg·hr for HA21 [39]. This indicates that the maximal rate at the UA-transferase domain is attained utilizing a heptasaccharide or longer. The NAc-trans- ferase specific activities with values of 175 μmol/mg·hr observed in our study for 1 mM HA8 at 1 mM UDP-GlcNAc is seven times higher than reported [39]. Maximal NAc-transferase rates were only reached in our studies at elevated UDP-GlcNAc concentration of 40 M; which is considerably higher than the value of m OPEN ACCESS 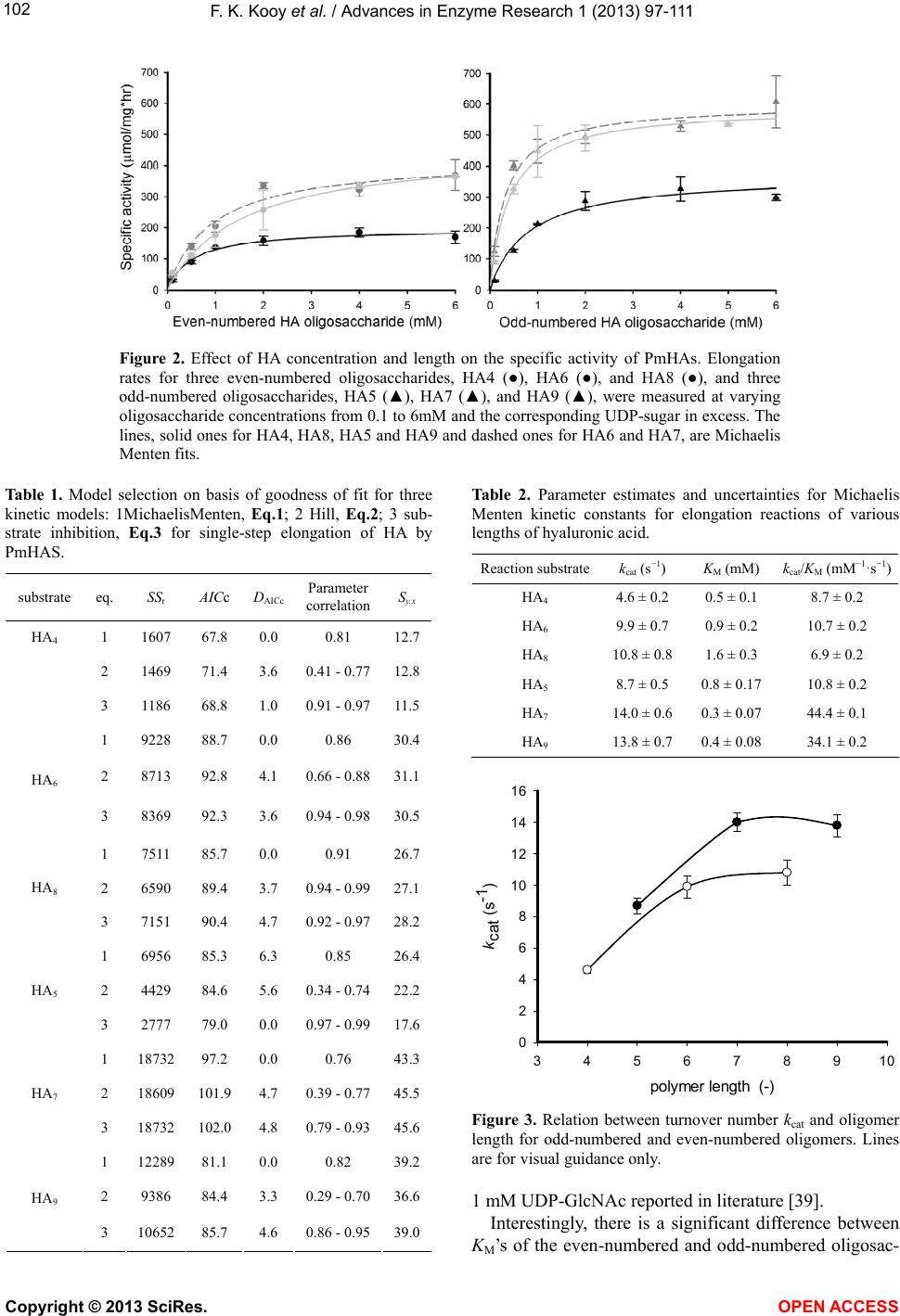 F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 102 Figure 2. Effect of HA concentration and length on the specific activity of PmHAs. Elongation rates for three even-numbered oligosaccharides, HA4 (●), HA6 (●), and HA8 (●), and three odd-numbered oligosaccharides, HA5 (▲), HA7 (▲), and HA9 (▲), were measured at varying oligosaccharide concentrations from 0.1 to 6mM and the corresponding UDP-sugar in excess. The lines, solid ones for HA4, HA8, HA5 and HA9 and dashed ones for HA6 and HA7, are Michaelis Menten fits. Ta bl e 1. Model selection on basis of goodness of fit for three kinetic models: 1MichaelisMenten, Eq.1; 2 Hill, Eq.2; 3 sub- strate inhibition, Eq.3 for single-step elongation of HA by PmHAS. substrate eq. SSr AICcDAICc Parameter correlation Sy.x HA4 1 1607 67.8 0.0 0.81 12.7 2 1469 71.4 3.6 0.41 - 0.7712.8 3 1186 68.8 1.0 0.91 - 0.9711.5 1 9228 88.7 0.0 0.86 30.4 2 8713 92.8 4.1 0.66 - 0.8831.1 HA6 3 8369 92.3 3.6 0.94 - 0.9830.5 1 7511 85.7 0.0 0.91 26.7 2 6590 89.4 3.7 0.94 - 0.9927.1 HA8 3 7151 90.4 4.7 0.92 - 0.9728.2 1 6956 85.3 6.3 0.85 26.4 2 4429 84.6 5.6 0.34 - 0.7422.2HA5 3 2777 79.0 0.0 0.97 - 0.9917.6 1 18732 97.2 0.0 0.76 43.3 2 18609 101.94.7 0.39 - 0.7745.5 HA7 3 18732 102.04.8 0.79 - 0.9345.6 1 12289 81.1 0.0 0.82 39.2 2 9386 84.4 3.3 0.29 - 0.7036.6 HA9 3 10652 85.7 4.6 0.86 - 0.9539.0 Table 2. Parameter estimates and uncertainties for Michaelis Menten kinetic constants for elongation reactions of various lengths of hyaluronic acid. Reaction substratekcat (s−1) KM (mM) kcat/KM (mM−1·s−1) HA4 4.6 ± 0.2 0.5 ± 0.1 8.7 ± 0.2 HA6 9.9 ± 0.7 0.9 ± 0.2 10.7 ± 0.2 HA8 10.8 ± 0.8 1.6 ± 0.3 6.9 ± 0.2 HA5 8.7 ± 0.5 0.8 ± 0.17 10.8 ± 0.2 HA7 14.0 ± 0.6 0.3 ± 0.07 44.4 ± 0.1 HA9 13.8 ± 0.7 0.4 ± 0.08 34.1 ± 0.2 polymer length (-) 3456789 kcat (s-1) 10 0 2 4 6 8 10 12 14 16 Figure 3. Relation between turnover number kcat and oligomer length for odd-numbered and even-numbered oligomers. Lines are for visual guidance only. 1 mM UDP-GlcNAc reported in literature [39]. Interestingly, there is a significant difference between KM’s of the even-numbered and odd-numbered oligosac- Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 103 charides. The KM’s of the odd-numbered oligosaccha- rides are decreasing with the increase of the chain length, while, on the contrary, those for the even-numbered in- crease. Understanding these trends is accomplished by assessing the differences in the specificity constant kcat/KM between even-numbered and odd-numbered oli- gosaccharides. The specificity constant kcat/KM contains the affinity 1/KM of the enzyme towards substrates [34,48] and is seen to increases (Table 2) from 10.8 to 44.4 mM−1·s−1 for HA5 and HA7; after that, a slight decrease is observed to 34.1 mM−1·s−1for HA9. This suggests that the two sugar residues at the reducing end of HA7 (GlcUA2-GlcNAc1), compared to HA5, enhance the binding to the oligosaccharide site by interacting more strongly. The specificity constant of HA9 does not in- crease further; apparently, it does not develop additional interactions with the binding site. By investigating initial polymerization rates of HA acceptor analogs up to hex- asaccharides, Williams et al. [39] demonstrated that the minimal oligosaccharide length for high-efficiency elon- gation is comprised of, at the least, a trisaccharide with two glucuronic acid residues. This indicates that the oli- gosaccharide binding site at the UA-transferase site has the capacity to bind a minimum of three saccharide resi- dues, as suggested by Williams [39], and a maximum of seven saccharide residues, as proposed in this study. Contradictorily, the kcat/KM value for even-numbered oligosaccharides is moderately constant ranging from 6.9 to 10.7 mM−1·s−1. The variance in binding specificity of PmHAS for odd and even-numbered oligosaccharides would be difficult to understand if there was only one oligosaccharide binding site in PmHAS at which both elongations (NAc and UA) would occur. These differ- ences can only be explicated by two separate oligosac- charide binding sites for odd and even-numbered oligo- saccharides. A long oligosaccharide binding site at the UA-transferase site could explain the increased specific- ity constant for HA7, whereas the moderately constant specificity constant of HA4, HA6 and HA8 can be ex- plained by a short oligosaccharide binding site interact- ing with the first four sugar residues of HA4, HA6 and HA8 at the NAc-transferase site. 3.1.3. Competition Studies To further examine the difference in binding specificity for odd-numbered and even-numbered oligosaccharides, we conducted competition studies to investigate 1) the influence of HA5 as an inhibitor of HA4 elongation with UDP-GlcNAc, 2) the influence of HA4 as an inhibitor of HA5 elongation with UDP-GlcUA, and 3) the competi- tion between HA4and HA6 for NAc-transferase activity. For each individual step elongation, enzyme activity in the absence of the competing oligosaccharide was meas- ured as a reference. Two reactions were conducted with a molar ratio between the reacting and competing oligo- saccharide of 1:1 or 1:10 (Figure 4). Theoretically, if only a single oligosaccharide binding site was evident, the reacting and competing oligosaccharides would con- currently bind, and are therefore compete for the site. If two oligosaccharide binding sites were evident, a com- peting oligosaccharide (HA4 or HA5) might still bind non-productively at the elongation site, since these com- petitors only differ from the reacting oligosaccharide in the last sugar at the non-reducing end. In both cases, the competing oligosaccharide binds at the reacting site, and the measured activity should decrease compared to the reference reaction. The results in Figure 4(A) show that HA5 does not in- fluence the reaction between HA4 with UDP-GlcNAc, nor does HA4 create variation with the rates of the reac- tion between HA5 with UDP-GlcUA. The absence of competition confirms that there are two separate oligo- saccharide binding sites within PmHAS, one for each transferase activity, and that these binding sites are very specific for their substrates. Previously, Williams et al. [39] had also observed absence of competition for a par- ticular class of longer oligosaccharides (HA14 and HA15). Both HA4 and HA6 oligosaccharides can react with UDP-GlcNAc at the same site, and this is confirmed by the experimental outcomes. The reaction rate measured in experiments with both substrates simultaneously equals the sum of the individual elongation rates of HA4 and HA6 (Figure 4(B)). The specificity constants of HA4 and HA6 are generally the same, which is the result of approximately twice as high KM and kcat values for HA6 compared to the KM and kcat values of HA4 (see Table 2). In the reactions with 3 mM HA4, i.e. the equivalent of 6·KM of HA4, the kcat is almost attained for the HA4 reac- tions, corresponding to a specific activity of 177 μmol/ mg·hr (see Figure 4 and the last two white bars in Fig- ure 4(B)). The specific activity (see second to last white bar in Figure 4(B)) increased, moreover, to 213 μmol/ mg·hr by the addition of 0.3 mM HA6. On the contrary, the kcat of HA6 is not attained, since 3 mM HA6, the equivalent of ~3*KM of HA6, is not sufficient to achieve saturating concentrations (last two grey bars in Figure 4(B)). The results from the competition studies between HA4 and HA6 thus demonstrate that elongation of these two oligosaccharides is dependent on their concentration levels and that, under the current conditions, the com- bined rate is the sum of the individual rates. 3.2. Structural Characteristics A three-dimensional model of PmHAS was con- structed based on the recently obtained crystal structure of K4CP chondroitin polymerase [29], which exhibits a sequence identity of 62% and sequence homology of 79% compared to PmHAS. The Ramachandran plot ob- Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 Copyright © 2013 SciRes. 104 Figure 4. Influence of the competing oligosaccharide on the PmHAS elongation reaction. (A) The light grey bars show the results of HA5 as competitor to the elongation reaction of HA4 with UDP-GlcNAc, whereas the dark grey depicts the HA5 elongation reaction with UDP-GlcUA and competing oligosaccharide HA4; (B) The results of HA6 as “competing” oligosaccharide are shown in grey, and for HA4 in white. tained from PROCHECK demonstrated that the stereo- chemical quality of the model was very valuable. Only two residues, Ala310 and Asn411, show slightly deviating Phi and Psi angles that do not project consequences on the overall structure; both of these residues are in flexible loops. The structure of PmHAS residues 72 to 688 is almost identical to the K4CP structure (cf. the overlay of the two structures, Figure 5(A)); only the last 15 resi- dues (689 - 703) could not be modeled. This structural model thus allows localization of the two oligosaccharide binding sites that were inferred from the kinetic studies. PmHAS consists of three domains (Figure 5(B)): an N-terminal domain (residues 72 to 134); the NAc-trans- ferase-domain (residues 135 to 426); and the UA-trans- ferase domain (residues 427 - 688). The N-terminal do- main consists of a random coil and two α-helices, and the first 71 residues are missing as no alignment could be discerned for this N-terminal region. The NAc-trans- ferase domain of PmHAS contains 13 α-helices and 12 β-strands. The UA-transferase domain consists of 10 α-helices and 12 β-strands as with the structure of K4CP. Both the NAc-transferase and the UA-transferase do- mains of PmHAS adopt the GT-A fold, which consists of an α/β/α sandwich and is one of the fold types in glycol- syltransferases [49] (Figure 5). The orientation of the two active sites and the substantial distance of over 60 Å make a single oligosaccharide binding site within PmHAS unlikely (Figure 5). The PmHAS model was further analyzed for structural alignment with GT-A folded glycosyltransferases to identify conserved regions in structure and sequence. Conserved regions, such as amino acid residues involved in binding substrates, are frequently important in enzyme functionality. Below, similarities in substrate binding and substrate orientation are employed to ascertain binding sites of HA oligosaccharides in PmHAS. Various GT-A folded glycosyltransferases were structurally aligned with PmHAS (abbreviations in parentheses): UDP- GalNAc:polypeptide α-N-acetylgalactosaminyl-transferase T2 (hT2, [50]), β1,3-glucuronosyltransferase (GlcAT1, [51]), β1,3-glucuronosyltransferase (GlcAT-P, [52]), β1, 4-galactosyltransferase (β4Gal-T1, [53]), α1,4-N-ace- tylhexosaminlytransferase (EXTL2, [54]), α1,3-galac- tosyltransferase (α3GT, [55]), blood group A α1,3-N- galactosaminyltransferase (hGTA, [56]), α1,4-galacto- syltransferase (LgtC, [57]). 3.2.1. UDP-Sugar Binding Site Although the structurally aligned enzymes represent a broad spectrum of glycosyltransferase reactions, regions that bind the UDP-sugars are conserved, resulting in strong similarities such as the orientation of the UDP- sugar in the site (Figure 6). The DXD motif [58,59] is extremely conserved within GT-A folded glycosyltrans- ferases and forms a complex with divalent ions, such as Mn2+ and Mg2+, which are crucial for UDP-sugar binding. The DXD motif in PmHAS is defined as Asp247, Cys248 and Asp249 within the NAc-transferase site and Asp527, Ser528, and Asp529 within the UA-transferase site. Muta- tion studies within PmHAS have exhibited that varying any of these Asp residues deactivates the transferase site containing that DXD motif [60]. In addition, the DGS motif in PmHAS contains an Asp residue that is con- erved in certain aligned glycosyltransferases as well. s OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 105 (A) (B) Figure 5. Structural homology model of PmHAS. (A) Superimposition of the crystal structure of K4CP chondroitin polymerase (green) and the PmHAS homology model (blue) with RMSD value and Z-score of 0.5Å and 53.4, respectively. Substrates UDP (orange) and UDP-GalNAc (yellow), depicted as sticks, and Mn2+, as red dots, were originally present in the crystal structure. Secondary structural elements are indi- cated by ribbons for α-helices, and arrows for β-strands. (B) Overall structure of PmHAS with UDP-GlcUA (green) and UDP-GlcNAc (yellow) in sticks and Mn2+ as red dots. The structure is divided in three domains: the N-terminal region (residues 72 - 134) in green, the UA-transferase (427 - 688) in orange and the NAc- transferase (135 - 426) in blue. This Asp residue interacts with the N3 of the uracyl group within the UDP moiety. Mutation of Asp196 or Asp477 in PmHAS resulted in loss of NAc- or UA-trans- ferase activity [30], respectively, which emphasizes the importance of these residues. 3.2.2. Acceptor Binding Site Since PmHAS contains β1,3-glucuronyltransferase and β1,4-N-acetylglucosaminyltransferase domains, the aligned glycosyltransferases were selected on the basis of the ability to elongate the acceptor at the 3OH or 4OH group with the donor sugar. The structural alignments indicate that the acceptor has distinct orientations to- wards the UDP-sugar, depending on the type of linkage formed following the sugar moiety transfer (Figure 6). The α-configuration of the C1 in the sugar moiety at- tached to UDP is preserved in the product after the con- veyance by retaining glycosyltransferases, whereas in- verting glycosyltransferases convert this into a β-linked product. Consequently, the attacking OH group in the acceptor in retaining enzymes is arranged sequentially to the C1 of the sugar moiety, ready to form the α-linkage (Figures 6(B) and (D)). Similarly, the attacking OH group in inverting glycosyltransferases is positioned op- posite to the α-linked UDP (Figures 6(A) and (C)). This is emphasized by the reported hydrogen bonds in retain- ing glycosyltransferases between the attacking OH group in the acceptor and an oxygen atom of the β-phosphate group in UDP [55-57,64]. These hydrogen bonds were not evident in inverting enzymes, where the distance between UDP and the acceptor is too extensive. In addition to the similarities in acceptor orientation, aromatic hydrophobic residues Phe, Trp, and Tyr are lo- cated near every catalytic center. Their roles in the ac- ceptor binding site appear to depend on their location and orientation within the active site. Mutation studies in the aligned glycosyltransferases illustrate that aromatic hy- drophobic residues exhibit specific affinity towards the acceptor [65,66], perform as a stabilizer of the transition state [66], or assist in the orientation of the acceptor into the correct position [53]. 3.2.3. Oligosaccharide Positioning Structural similarities among PmHAS and other gly- cosyltransferases demonstrated that the HA oligosaccha- ride should be positioned underneath the C1 of the sugar moiety to form β-linkages, since PmHAS has two in- verting transferase domains. AutodockVina [42] was employed to model HA6 and HA7 into the NAc-trans- ferase and UA-transferase sites (Figure 7), respectively. The orientations of HA6 and HA7 (Figures 7(A) and (B)) concur with the results from Figure 6. Additionally, the amino acids that are likely to interact with the substrates are depicted as sticks (Figure 7). Kinetic results indicated that there is a difference in binding specificity between the NAc-transferase and UA-transferase sites and that in the UA-transferase site he specificity constant strongly increases upon elongation t Copyright © 2013 SciRes. OPEN ACCESS 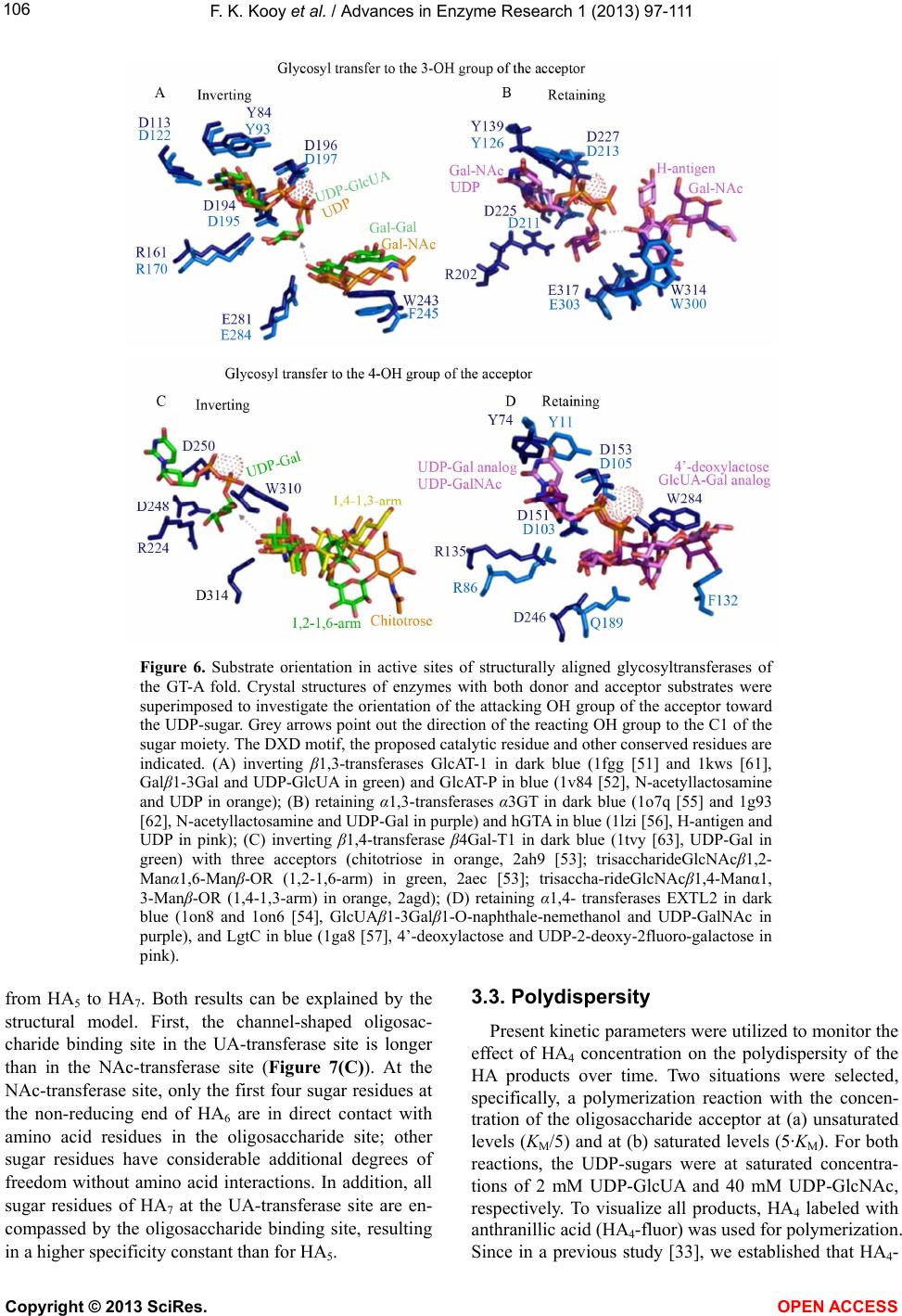 F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 106 Figure 6. Substrate orientation in active sites of structurally aligned glycosyltransferases of the GT-A fold. Crystal structures of enzymes with both donor and acceptor substrates were superimposed to investigate the orientation of the attacking OH group of the acceptor toward the UDP-sugar. Grey arrows point out the direction of the reacting OH group to the C1 of the sugar moiety. The DXD motif, the proposed catalytic residue and other conserved residues are indicated. (A) inverting β1,3-transferases GlcAT-1 in dark blue (1fgg [51] and 1kws [61], Galβ1-3Gal and UDP-GlcUA in green) and GlcAT-P in blue (1v84 [52], N-acetyllactosamine and UDP in orange); (B) retaining α1,3-transferases α3GT in dark blue (1o7q [55] and 1g93 [62], N-acetyllactosamine and UDP-Gal in purple) and hGTA in blue (1lzi [56], H-antigen and UDP in pink); (C) inverting β1,4-transferase β4Gal-T1 in dark blue (1tvy [63], UDP-Gal in green) with three acceptors (chitotriose in orange, 2ah9 [53]; trisaccharideGlcNAcβ1,2- Manα1,6-Manβ-OR (1,2-1,6-arm) in green, 2aec [53]; trisaccha-rideGlcNAcβ1,4-Manα1, 3-Manβ-OR (1,4-1,3-arm) in orange, 2agd); (D) retaining α1,4- transferases EXTL2 in dark blue (1on8 and 1on6 [54], GlcUAβ1-3Galβ1-O-naphthale-nemethanol and UDP-GalNAc in purple), and LgtC in blue (1ga8 [57], 4’-deoxylactose and UDP-2-deoxy-2fluoro-galactose in pink). from HA5 to HA7. Both results can be explained by the structural model. First, the channel-shaped oligosac- charide binding site in the UA-transferase site is longer than in the NAc-transferase site (Figure 7(C)). At the NAc-transferase site, only the first four sugar residues at the non-reducing end of HA6 are in direct contact with amino acid residues in the oligosaccharide site; other sugar residues have considerable additional degrees of freedom without amino acid interactions. In addition, all sugar residues of HA7 at the UA-transferase site are en- compassed by the oligosaccharide binding site, resulting in a higher specificity constant than for HA5. 3.3. Polydispersity Present kinetic parameters were utilized to monitor the effect of HA4 concentration on the polydispersity of the HA products over time. Two situations were selected, specifically, a polymerization reaction with the concen- tration of the oligosaccharide acceptor at (a) unsaturated levels (KM/5) and at (b) saturated levels (5·KM). For both reactions, the UDP-sugars were at saturated concentra- tions of 2 mM UDP-GlcUA and 40 mM UDP-GlcNAc, respectively. To visualize all products, HA4 labeled with anthranillic acid (HA4-fluor) was used for polymerization. Since in a previous study [33], we established that HA4- Copyright © 2013 SciRes. OPEN ACCESS 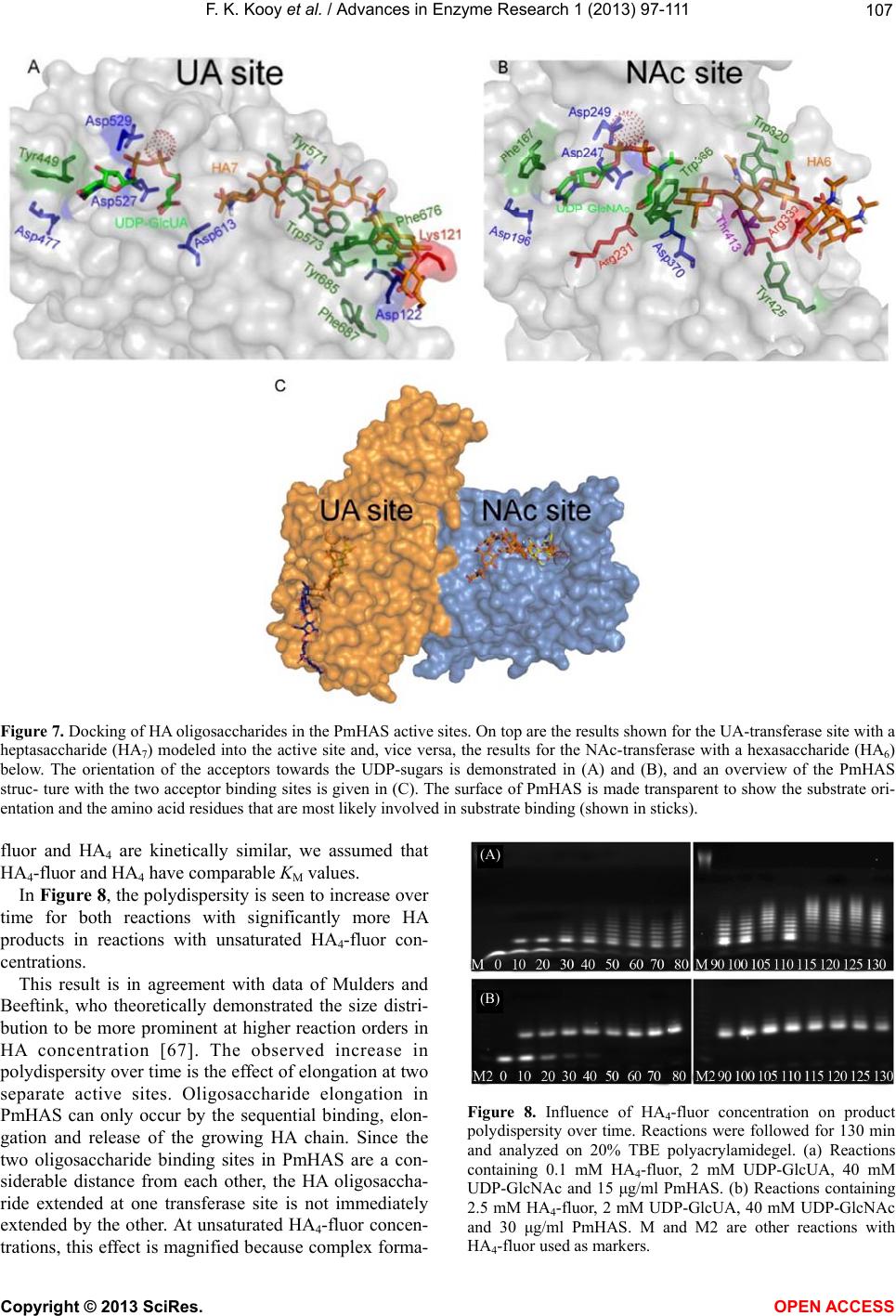 F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 107 Figure 7. Docking of HA oligosaccharides in the PmHAS active sites. On top are the results shown for the UA-transferase site with a heptasaccharide (HA7) modeled into the active site and, vice versa, the results for the NAc-transferase with a hexasaccharide (HA6) below. The orientation of the acceptors towards the UDP-sugars is demonstrated in (A) and (B), and an overview of the PmHAS struc- ture with the two acceptor binding sites is given in (C). The surface of PmHAS is made transparent to show the substrate ori- entation and the amino acid residues that are most likely involved in substrate binding (shown in sticks). fluor and HA4 are kinetically similar, we assumed that HA4-fluor and HA4 have comparable KM values. In Figure 8, the polydispersity is seen to increase over time for both reactions with significantly more HA products in reactions with unsaturated HA4-fluor con- centrations. This result is in agreement with data of Mulders and Beeftink, who theoretically demonstrated the size distri- bution to be more prominent at higher reaction orders in HA concentration [67]. The observed increase in polydispersity over time is the effect of elongation at two separate active sites. Oligosaccharide elongation in PmHAS can only occur by the sequential binding, elon- gation and release of the growing HA chain. Since the two oligosaccharide binding sites in PmHAS are a con- siderable distance from each other, the HA oligosaccha- ride extended at one transferase site is not immediately extended by the other. At unsaturated HA4-fluor concen- trations, this effect is magnified because complex forma- (A) (B) Figure 8. Influence of HA4-fluor concentration on product polydispersity over time. Reactions were followed for 130 min and analyzed on 20% TBE polyacrylamidegel. (a) Reactions containing 0.1 mM HA4-fluor, 2 mM UDP-GlcUA, 40 mM UDP-GlcNAc and 15 μg/ml PmHAS. (b) Reactions containing 2.5 mM HA4-fluor, 2 mM UDP-GlcUA, 40 mM UDP-GlcNAc and 30 μg/ml PmHAS. M and M2 are other reactions with HA4-fluor used as markers. Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 108 tion between PmHAS and HA4-fluor is decelerated, re- sulting in an increased polydispersity. Polydispersity of HA products can be decreased by controlling the initial concentrations of HA oligosaccharide and UDP-sugars. 4. CONCLUSION Evidence is presented for two separate oligosaccharide binding sites within PmHAS, one in each transferase domain. Based on kinetic and structural analysis, we demonstrate that the two transferase domains act as two independent enzymes, where GlcNAc is transferred at the NAc-transferase domain and GlcUA at the UA- transferase domain. The transferase domains in PmHAS indicate structural similarity with glycosyltransferases of the GT-A fold. With structural alignment of these en- zymes, we identified general characteristics of acceptor binding sites in these enzymes that are useful in locating oligosaccharide binding sites in other GT-A folded gly- cosyltransferases. We propose two locations for these oligosaccharide binding sites that are considerably dis- tant from each other. As a result of this extensive dis- tance, the number of products will increase over time in a polymerization reaction unless single-step reactions are imposed. The molecular weight and polydispersity of the product can be controlled by the availability of the sub- strates. 5. ACKNOWLEDGEMENTS The authors thank Jan Springer and Aukje Zimmerman for the pro- duction of PmHAS; Martinus van Boekel for helpful discussions on statistical analysis; and Lambertus van den Broek for technical assis- tance with MALDI-TOF MS experiments. The project is a collaboration with Merck & Co. (formerly Organon N.V.) and financially supported by the Netherlands Ministry of Eco- nomic Affairs and B-Basic partners (www.b-basic.nl) through B-Basic, a public-private NWO-ACTS program (ACTS = Advanced Chemical Technologies for Sustainability). REFERENCES [1] Gandhi, N.S. and Mancera, R.L. (2008) The structure of glycosaminoglycans and their interactions with proteins. Chemical Biology & Drug Design, 72, 455-482. http://dx.doi.or g/ 10.1111/j.1747-0285.2008.00741.x [2] Schaefer, L. and Schaefer, R.M. (2010) Proteoglycans: From structural compounds to signaling molecules. Cell and Tissue Research, 339, 237-246. http://dx.doi.org/10.1007/s00441-009-0821-y [3] Stern, R. (2008) Association between cancer and “acid mucopolysaccharides”: An old concept comes of age, finally. Seminars in Cancer Biology, 18, 238-243. http://dx.doi.org/10.1016/j.semcancer.2008.03.014 [4] Meyer, K. and Palmer, J.W. (1934) The polysaccharide of the vitreous humor. Journal of Biological Chemistry, 107, 629-634. [5] Boas, N.F. (1949) Isolation of hyaluronic acid from the cock’s comb. Journal of Biological Chemistry, 181, 573- 575. [6] Meyer, K. and Chaffee, E. (1941) The mucopolysac- charides of skin. Journal of Biological Chemistry, 138, 491-499. [7] Chain, E. and Duthie, E.S. (1940) Identity of hyal- uronidase and spreading factor. British Journal of Experimental Pathology, 21, 324-338. [8] Kendall, F.E., Heidelberger, M. and Dawson, M.H. (1937) A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic streptococcus. Journal of Biological Chemistry, 118, 61-69. [9] Carter, G.R. and Annau, E. (1953) Isolation of capsular polysaccharides for colonial variants of Pasteurella mul- tocida. American Journal of Veterinary Research, 14, 475-478. [10] MacLennan, A.P. (1956) The production of capsules, hyaluronic acid and hyaluronidase by 25 strains of group C streptococci. Journal of General Microbiology, 15, 485-491. http://dx.doi.org/10.1099/00221287-15-3-485 [11] Thonard, J.C., Migliore, S.A. and Blustein, R. (1964) Isolation of hyaluronic acid from broth cultures of strep- tococci. Journal of Biological Chemistry, 239, 726-728. [12] Armstrong, D.C. and Johns, M.R. (1997) Culture con- ditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepide- micus. Applied & Environmental Microbiology, 63, 2759- 2764. [13] Widner, B., et al. (2005) Hyaluronic acid production in Bacillus subtilis. Applied & Environmental Microbiology, 71, 3747-3752. http://dx.doi.org/10.1128/AEM.71.7.3747-3752.2005 [14] DeAngelis, P.L., Papaconstantinou, J. and Weigel, P.H. (1993) Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. Journal of Biological Che- mistry, 268, 14568-14571. [15] Chien, L.-J. and Lee, C.-K. (2007) Hyaluronic acid production by recombinant Lactococcus lactis. Applied Microbiology and Biotechnology, 77, 339-346. http://dx.doi.org/10.1007/s00253-007-1153-z [16] Yu, H. and Stephanopoulos, G. (2008) Metabolic en- gineering of Escherichia coli for biosynthesis of hy- aluronic acid. Metabolic Engineering, 10, 24-32. http://dx.doi.org/10.1016/j.ymben.2007.09.001 [17] Mao, Z. and Chen, R.R. (2007) Recombinant synthesis of hyaluronan by agrobacterium sp. Biotechnology Progress, 23, 1038-1042. [18] Mao, Z., Shin, H.-D. and Chen, R. (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Applied Microbiology and Biotechnology, 84, 63-69. http://dx.doi.org/10.1007/s00253-009-1963-2 [19] Johns, M.R., Goh, L.-T. and Oeggerli, A. (1994) Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus. Biotechnology Letters, 16, 507-512. http://dx.doi.org/10.1007/BF01023334 Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 109 [20] Huang, W.-C., Chen, S.-J. and Chen, T.-L. (2006) The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochemical Engineering Journal, 32, 239-243. http://dx.doi.org/10.1016/j.bej.2006.10.011 [21] Kim, J.-H., et al. (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme and Microbial Technology, 19, 440-445. http://dx.doi.org/10.1016/S0141-0229(96)00019-1 [22] Chen, W.Y., et al. (2009) Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. Journal of Biological Che- mistry, 284, 18007-18014. http://dx.doi.org/10.1074/jbc.M109.011999 [23] Krupa, J.C., et al. (2007) Quantitative continuous assay for hyaluronan synthase. Analytical Biochemistry, 361, 218-225. http://dx.doi.org/10.1016/j.ab.2006.11.011 [24] Pummill, P.E., Achyuthan, A.M. and DeAngelis, P.L. (1998) Enzymological characterization of recombinant Xenopus DG42, a vertebrate hyaluronan synthase. Jour- nal of Biological Chemistry, 273, 4976-4981. http://dx.doi.org/10.1074/jbc.273.9.4976 [25] Itano, N., et al. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. Journal of Biological Chemistry, 274, 25085-25092. http://dx.doi.org/10.1074/jbc.274.35.25085 [26] Tlapak-Simmons, V.L., et al. (1999) Kinetic character- ization of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis. Journal of Biological Chemistry, 274, 4246-4253. http://dx.doi.org/10.1074/jbc.274.7.4246 [27] DeAngelis, P.L. (1999) Molecular directionality of poly- saccharide polymerization by the Pasteurella multocida hyaluronan synthase. Journal of Biological Chemistry, 274, 26557-26562. http://dx.doi.org/10.1074/jbc.274.37.26557 [28] Jing, W. and DeAngelis, P.L. (2004) Synchronized che- moenzymatic synthesis of monodisperse hyaluronan po- lymers. Journal of Biological Chemistry, 279, 42345- 42349. http://dx.doi.org/10.1074/jbc.M402744200 [29] Osawa, T., et al. (2009) Crystal structure of chondroitin polymerase from Escherichia coli K4. Biochemical and Biophysical Research Communications, 378, 10-14. http://dx.doi.org/10.1016/j.bbrc.2008.08.121 [30] Jing, W. and DeAngelis, P.L. (2000) Dissection of the two transferase activities of the Pasteurella multocida hy- aluronan synthase: Two active sites exist in one po- lypeptide. Glycobiology, 10, 883-889. http://dx.doi.org/10.1093/glycob/10.9.883 [31] Fitzgerald, D.K., et al. (1970) Enzymic assay for gala- ctosyl transferase activity of lactose synthetase and [alpha]- lactalbumin in purified and crude systems. Analytical Biochemistry, 36, 43-61. http://dx.doi.org/10.1016/0003-2697(70)90330-1 [32] Gosselin, S., et al. (1994) A continuous spectro- photometric assay for glycosyltransferases. Analytical Biochemistry, 220, 92-97. http://dx.doi.org/10.1006/abio.1994.1303 [33] Kooy, F.K., et al. (2009) Quantification and character- ization of enzymatically produced hyaluronan with fluorophore-assisted carbohydrate electrophoresis. Analy- tical Biochemistry, 384, 329-336. http://dx.doi.org/10.1016/j.ab.2008.09.042 [34] Cornish-Bowden, A. (1995) Fundamentals of enzyme kinetics. Portland Press Ltd., London. [35] Cook, P.F. and Cleland, W.W. (2007) Enzyme kinetics and mechanism. Garland Science, London. [36] De Levie, R. (2004) Macros for least-squares & for the propagation of imprecision, in advanced excel for scien- tific data analysis. Oxford University Press, New York. [37] Van Boekel, M.A.J.S. (2010) Kinetic modeling of reac- tions in foods. CRC Press, Boca Raton. [38] Motulsky, H. and Christopoulos, A. (2003) Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. GraphPad Software Inc., San Diego. [39] Williams, K.J., Halkes, K.M., Kamerling, J.P. and DeAn- gelis, P.L. (2006) Critical elements of oligosaccharide acceptor substrates for the Pasteurella multocida hyaluro- nan synthase. Journal of Biological Chemistry, 281, 5391- 5397. http://dx.doi.org/10.1074/jbc.M510439200 [40] Laskowski, R.A., MacArthur, M.W., Moss, D.S. and Tho- rnton, J.M. (1993) PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystalography, 26, 283-291. http://dx.doi.org/10.1107/S0021889892009944 [41] Sippl, M.J. (1993) Recognition of errors in three-dimen- sional structures of proteins. Proteins: Structure, Func- tion, and Genetics, 17, 355-362. http://dx.doi.org/10.1002/prot.340170404 [42] Trott, O. and Olson, A.J. (2009) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Jour- nal of Computational Chemistry, 31, 455-461. [43] Holm, L. and Sander, C. (1996) Mapping the protein uni- verse. Science, 273, 595-602. http://dx.doi.org/10.1126/science.273.5275.595 [44] DeAngelis, P.L. (1996) Enzymological characterization of the Pasteurella multocida hyaluronic acid synthase. Bi o- chemistry, 35, 9768-9771. http://dx.doi.org/10.1021/bi960154k [45] Tlapak-Simmons, V.L., Baron, C.A. and Weigel, P.H. (2004) Characterization of the purified hyaluronan synthase from Streptococcus equisimilis. Biochemistry, 43, 9234-9242. http://dx.doi.org/10.1021/bi049468v [46] Yoshida, M., Itano, N., Yamada, Y. and Kimata, K. (2000) In Vitro synthesis of hyaluronan by a single protein derived from mouse HAS1 Gene and characterization of amino acid residues essential for the activity. Journal of Biological Chemistry, 275, 497-506. http://dx.doi.org/10.1074/jbc.275.1.497 [47] Kumari, K. and Weigel, P.H. (1997) Molecular cloning, expression, and characterization of the authentic hyalu- ronan synthase from Group C Streptococcus equisimilis. Copyright © 2013 SciRes. OPEN ACCESS  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 Copyright © 2013 SciRes. OPEN ACCESS 110 Journal of Biological Chemistry, 272, 32539-32546. http://dx.doi.org/10.1074/jbc.272.51.32539 [48] Eisenthal, R., Danson, M.J. and Hough, D.W. (2007) Ca- talytic efficiency and kcat/KM: A useful comparator? Trends in Biotechnology, 25, 247-249. http://dx.doi.org/10.1016/j.tibtech.2007.03.010 [49] Breton, C., Snajdrová, L., Jeanneau, C., Koca, J. and Im- berty, A. (2006) Structures and mechanisms of glycosyl- transferases. Glycobiology, 16, 29R-37R. http://dx.doi.org/10.1093/glycob/cwj016 [50] Fritz, T.A., Raman, J. and Tabak, L.A. (2006) Dynamic association between the catalytic and lectin domains of human UDP-GalNAc: Polypeptide a-N-acetylgalactosami- nyltransferase-2. Journal of Biological Chemistry, 281, 8613-8619. http://dx.doi.org/10.1074/jbc.M513590200 [51] Pedersen, L.C., Tsuchida, K., Kitagawa, H., Sugahara, K., Darden, T.A. and Negishi, M. (2000) Heparan/Chondroi- tin sulfate biosynthesis. Structure and mechanism of human glucuronyltransferase I. Journal of Biological Chemistry, 275, 34580-34585. http://dx.doi.org/10.1074/jbc.M007399200 [52] Kakuda, S., Shiba, T., Ishiguro, M., Tagawa, H., Oka, S., Kajihara, Y., Kawasaki, T., Wakatsuki, S. and Kato, R. (2004) Structural basis for acceptor substrate recognition of a human glucuronyltransferase, GlcAT-P, an enzyme critical in the biosynthesis of the carbohydrate epitope HNK-1. Journal of Biological Chemistry, 279, 22693- 22703. http://dx.doi.org/10.1074/jbc.M400622200 [53] Ramasamy, V., Ramakrishnana, B., Boeggeman, E., Ratner, D.M., Seeberger, P.H. and Qasba, P.K. (2005) Oligosac- charide preferences of β1,4-galactosyltransferase-I: Crystal structures of Met340His mutant of human β1,4-galac- tosyltransferase-I with a pentasaccharide and trisaccha- rides of the N-glycan moiety. Journal of Molecular Bio- logy, 353, 53-67. http://dx.doi.org/10.1016/j.jmb.2005.07.050 [54] Pedersen, L.C., Dong, J., Taniguchi, F., Kitagawa, H., Krahn, J.M., Pedersen, L.G., Sugahara, K. and Negishi, M. (2003) Crystal structure of an 1,4-N-acetylhexosami- nyltransferase (EXTL2), a member of the exostosin gene family involved in heparan sulfate biosynthesis. Journal of Biological Chemistry, 278, 14420-14428. http://dx.doi.org/10.1074/jbc.M210532200 [55] Zhang, Y., Swaminathan, G.J., Deshpande, A., Boix, E., Natesh, R., Xie, Z.H., Acharya, K.R. and Brew, K. (2003) Roles of individual enzyme—Substrate interactions by α-1,3-galactosyltransferase in catalysis and specificity. Biochemistry, 42, 13512-13521. http://dx.doi.org/10.1021/bi035430r [56] Patenaude, S.I., Seto, N.O., Borisova, S.N., Szpacenko, A., Marcus, S.L., Palcic, M.M. and Evans, S.V. (2002) The structural basis for specificity in human ABO(H) blood group biosynthesis. Nature Structural Biology, 9, 685-690. http://dx.doi.org/10.1038/nsb832 [57] Persson, K., Ly, H.D., Dieckelmann, M., Wakarchuk, W.W., Withers, S.G. and Strynadka, N.C.J. (2001) Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and accep- tor sugar analogs. Nature Structural Biology, 8, 166-175. http://dx.doi.org/10.1038/84168 [58] Breton, C., Bettler, E., Joziasse, D.H., Geremia, R.A. and Imberty, A. (1998) Sequence-Function relationships of prokaryotic and eukaryotic galactosyltransferases. Jour- nal of Biochemistry, 123, 1000-1009. http://dx.doi.org/10.1093/oxfordjournals.jbchem.a022035 [59] Tarbouriech, N., Charnock, S.J. and Davies, G.J. (2001) Three-Dimensional structures of the Mn and Mg dTDP complexes of the family GT-2 glycosyltransferase SpsA: A comparison with related NDP-sugar glycosyltransfe- rases. Journal of Molecular Biology, 314, 655-661. http://dx.doi.org/10.1006/jmbi.2001.5159 [60] Jing, W. and DeAngelis, P.L. (2003) Analysis of the two active sites of the hyaluronan synthase and the chondroi- tin synthase of Pasteurella multocida. Glycobiology, 13, 661-671. http://dx.doi.org/10.1093/glycob/cwg085 [61] Pedersen, L.C., Darden, T.A. and Negishi. M. (2002) Crystal structure of b1,3-glucuronyltransferase I in com- plex with active donor substrate UDP-GlcUA. Journal of Biological Chemistry, 277, 21869-21873. http://dx.doi.org/10.1074/jbc.M112343200 [62] Gastinel, L.N., Bignon, C., Misra, A.K., Hindsgaul, O., Shaper, J.H. and Joziass, D.H. (2001), Bovine a1,3-gala- cto-syltransferase catalytic domain structure and its rela- tionship with ABO histo-blood group and glycosphin- golipid glycosyltransferases. EMBO Journal, 20, 638-649. http://dx.doi.org/10.1093/emboj/20.4.638 [63] Ramakrishnan, B., Boeggeman, E. and Qasba, P.K. (2004) Effect of the Met344His mutation on the conformational dynamics of bovine b-1,4-galactosyltransferase: Crystal structure of the Met344His mutant in complex with chito- biose. Biochemistry, 43, 12513-12522. [64] Negishi, M., Donga, J., Dardenb, T.A., Pedersenb, L.G. and Pedersen, L.C. (2003) Glucosaminylglycan biosyn- thesis: What we can learn from the X-ray crystal struc- tures of glycosyltransferases GlcAT1 and EXTL2. Bio- chemical and Biophysical Research Communications, 303, 393-398. http://dx.doi.org/10.1016/S0006-291X(03)00356-5 [65] Fondeur-Gelinotte, M., et al. (2007) Molecular basis for acceptor substrate specificity of the human β1,3-glucu- ronosyltransferases GlcAT-I and GlcAT-P involved in gly- cosaminoglycan and HNK-1 carbohydrate epitope bio- synthesis, respectively. Glycobiology, 17, 857-867. http://dx.doi.org/10.1093/glycob/cwm055 [66] Zhang, Y., Deshpande, A., Xie, Z.H., Natesh, R., Acharya, K.R. and Brew, K. (2004) Roles of active site tryptophans in substrate binding and catalysis by α-1,3 galactosyl- transferase. Glycobiology, 14, 1295-1302. http://dx.doi.org/10.1093/glycob/cwh119 [67] Mulders, K.J.M. and Beeftink, H.H. (2013) Chain length distribution and kinetic characteristics of an enzymatic- cally produced polymer. e-Polymers, 24, 1-12.  F. K. Kooy et al. / Advances in Enzyme Research 1 (2013) 97-111 111 ABBREVIATIONS α3GT: α1,3-galactosyltransferase; β4GAlT1: β1,4-galactosyltransferase; EXTL2: α1,4-N-acetylhexosaminlytransferase; GlcAT1: β1,3-glucuronosyltransferase; GlcAT-P: β1,3-glucuronosyltransferase; GlcNAc: N-acetylglucosamine; GlcUA: glucuronic acid; HA: hyaluronan; HA4: hyaluronantetrasaccharide; hGTA: blood group A α1,3-N-galactosaminyltransferase; hT2: UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltranserase T2; K4CP: K4CP chondroitin polymerase; LDH: lactate dehydrogenase; LgtC: α1,4-galactosyltransferase; MALDI-TOF MS: Matrix-Assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry; NAc-transferase domain: domain in PmHAS that elongates UDP-GlcNAc to the oligosaccharide; PmHAS: Pasteurella multocida hyaluronan synthase; PK: pyruvate kinase; SeHAS: Streptococcus equisimilishyaluronan synthase; UA-transferase domain: domain in PmHAS that elon- gates UDP-GlcUA to the oligosaccharide; XlHAS: Xenopuslaevis hyaluronan synthase. Copyright © 2013 SciRes. OPEN ACCESS
|