 Journal of Sustainable Bioenergy Systems, 2013, 3, 250-259 Published Online December 2013 (http://www.scirp.org/journal/jsbs) http://dx.doi.org/10.4236/jsbs.2013.34034 Open Access JSBS Hydrothermal Pretreatment of Lignocellulosic Biomass and Kinetics Hanwu Lei1*, Iwona Cybulska2, James Julson2 1Bioproducts, Sciences and Engineering Laboratory, Department of Biological Systems Engineering, Washington State University, Richland, USA 2Department of Agricultural and Biosystems Engineering, South Dakota State University, Brookings, USA Email: *hlei@wsu.edu Received August 30, 2103; revised September 25, 2013; accepted October 16, 2013 Copyright © 2013 Hanwu Lei et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The study focus was an examination of the hydrothermal pretreatment method applied to the lignocellulosic substrate, represented by the prairie cord grass, and comparison between different conditions based on the yield of glucose after enzymatic hydrolysis. The treatment did not involve any chemicals usage. Enzymatic hydrolysis was performed in order to examine the amount of glucose which was released from pretreated materials. The most efficient pretreatment condi- tions were at high temperature and relatively short reaction time (210˚C and 10 min), after which the lignocellulose structure was the most available for enzymes actions which resulted in a pretreatment conversion rate of 97%. Tem- perature had a significant influence on glucose release during the hydrolysis, which was confirmed by the Micha- elis-Menten and kinetic models. Kinetic models were used to fit the inhibitors and their conversion rates were related to temperature. Keywords: Hydrothermal Pretreatment; Prairie Cord Grass; Enzymatic Hydrolysis; Kinetics 1. Introduction As the only renewable resource to be converted to liquid fuel, biomass has been recognized as one of the most significant sustainable replacements for petroleum-based fuels [1]. Lignocellulosic biomass provides a unique and sustainable resource for environmentally friendly fuels and chemicals. Biomass including wood, crop residues and energy grass is enormous and renewable energy source that can provide clean energy and help to reduce the greenhouse gas emission [2]. The conversion of lig- nocellulosic biomass to ethanol is considered one of the most important uses of biomass as an energy source and the conversion would serve a dual purpose because the product is both a fuel and a potential chemical substrate [3]. Cellulose, hemicellulose, and lignin are three major components of lignocellulosic biomass. In nature, cellu- lose is usually associated with other polysaccharides such as xylan and lignin. Cellulose is the skeletal basis of plant cell walls [4]. Lignin is a highly cross-linked phenylpropylene polymer [5]. Lignin plays an important role in cell wall structure as a permanent bonding agent among plant cells. Cellulose and hemicellulose are not directly available for bioconversion because of their in- timate association with lignin [6]. To increase the enzy- matic digestibility of lignocellulosic biomass, biomass has to be treated/degraded mechanically or chemically. Hydrolysis of lignocellulose without any pretreatment tends to achieve low efficiencies [7] due to structural properties, such as lignin content, acetylated hemicellu- lose, a limited surface area, and crystallinity [8]. The treated biomass is then enzymatically hydrolyzed to sug- ars by cellulase and hemicellulase. The resulting sugars are subsequently fermented to ethanol by yeast fermenta- tion [9]. There are a number of pretreatment methods applied to lignocellulosic biomass under extensive research. Bio- mass pretreatment is an appropriate first step of ligno- cellulosics conversions to fuels and chemicals. Pretreat- ment of lignocellulosic biomass is a common step to re- move hemicelluloses and lignin, reduce cellulose crystal- linity, and increase porosity of the lignocellulosic bio- mass [10,11]. Without pretreatment, biomass digestibility for enzymatic hydrolysis or microbial fermentation is *Corresponding author. 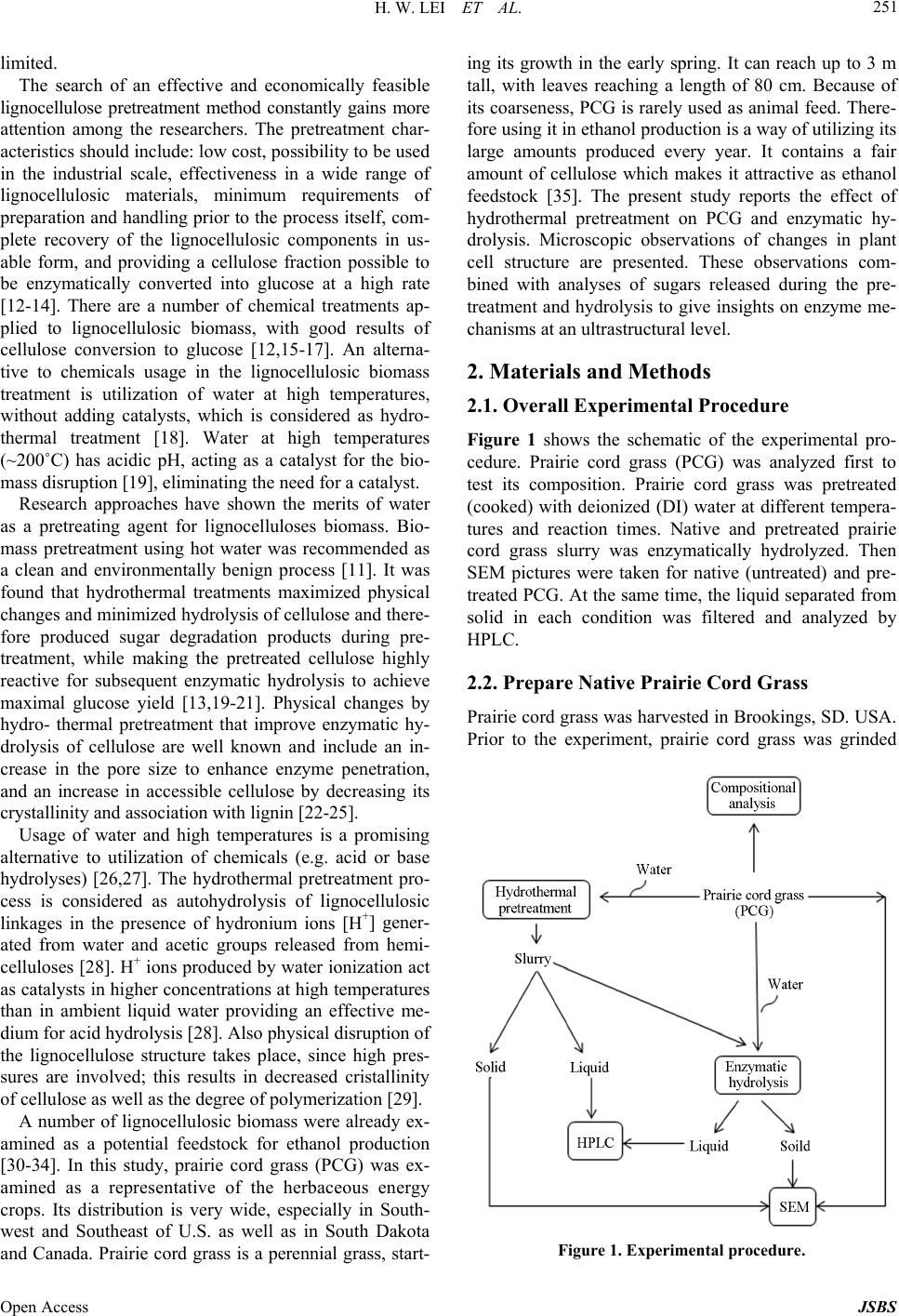 H. W. LEI ET AL. 251 limited. The search of an effective and economically feasible lignocellulose pretreatment method constantly gains more attention among the researchers. The pretreatment char- acteristics should include: low cost, possibility to be used in the industrial scale, effectiveness in a wide range of lignocellulosic materials, minimum requirements of preparation and handling prior to the process itself, com- plete recovery of the lignocellulosic components in us- able form, and providing a cellulose fraction possible to be enzymatically converted into glucose at a high rate [12-14]. There are a number of chemical treatments ap- plied to lignocellulosic biomass, with good results of cellulose conversion to glucose [12,15-17]. An alterna- tive to chemicals usage in the lignocellulosic biomass treatment is utilization of water at high temperatures, without adding catalysts, which is considered as hydro- thermal treatment [18]. Water at high temperatures (~200˚C) has acidic pH, acting as a catalyst for the bio- mass disruption [19], eliminating the need for a catalyst. Research approaches have shown the merits of water as a pretreating agent for lignocelluloses biomass. Bio- mass pretreatment using hot water was recommended as a clean and environmentally benign process [11]. It was found that hydrothermal treatments maximized physical changes and minimized hydrolysis of cellulose and there- fore produced sugar degradation products during pre- treatment, while making the pretreated cellulose highly reactive for subsequent enzymatic hydrolysis to achieve maximal glucose yield [13,19-21]. Physical changes by hydro- thermal pretreatment that improve enzymatic hy- drolysis of cellulose are well known and include an in- crease in the pore size to enhance enzyme penetration, and an increase in accessible cellulose by decreasing its crystallinity and association with lignin [22-25]. Usage of water and high temperatures is a promising alternative to utilization of chemicals (e.g. acid or base hydrolyses) [26,27]. The hydrothermal pretreatment pro- cess is considered as autohydrolysis of lignocellulosic linkages in the presence of hydronium ions [H+] gener- ated from water and acetic groups released from hemi- celluloses [28]. H+ ions produced by water ionization act as catalysts in higher concentrations at high temperatures than in ambient liquid water providing an effective me- dium for acid hydrolysis [28]. Also physical disruption of the lignocellulose structure takes place, since high pres- sures are involved; this results in decreased cristallinity of cellulose as well as the degree of polymerization [29]. A number of lignocellulosic biomass were already ex- amined as a potential feedstock for ethanol production [30-34]. In this study, prairie cord grass (PCG) was ex- amined as a representative of the herbaceous energy crops. Its distribution is very wide, especially in South- west and Southeast of U.S. as well as in South Dakota and Canada. Prairie cord grass is a perennial grass, start- ing its growth in the early spring. It can reach up to 3 m tall, with leaves reaching a length of 80 cm. Because of its coarseness, PCG is rarely used as animal feed. There- fore using it in ethanol production is a way of utilizing its large amounts produced every year. It contains a fair amount of cellulose which makes it attractive as ethanol feedstock [35]. The present study reports the effect of hydrothermal pretreatment on PCG and enzymatic hy- drolysis. Microscopic observations of changes in plant cell structure are presented. These observations com- bined with analyses of sugars released during the pre- treatment and hydrolysis to give insights on enzyme me- chanisms at an ultrastructural level. 2. Materials and Methods 2.1. Overall Experimental Procedure Figure 1 shows the schematic of the experimental pro- cedure. Prairie cord grass (PCG) was analyzed first to test its composition. Prairie cord grass was pretreated (cooked) with deionized (DI) water at different tempera- tures and reaction times. Native and pretreated prairie cord grass slurry was enzymatically hydrolyzed. Then SEM pictures were taken for native (untreated) and pre- treated PCG. At the same time, the liquid separated from solid in each condition was filtered and analyzed by HPLC. 2.2. Prepare Native Prairie Cord Grass Prairie cord grass was harvested in Brookings, SD. USA. Prior to the experiment, prairie cord grass was grinded Figure 1. Experimental procedure. Open Access JSBS 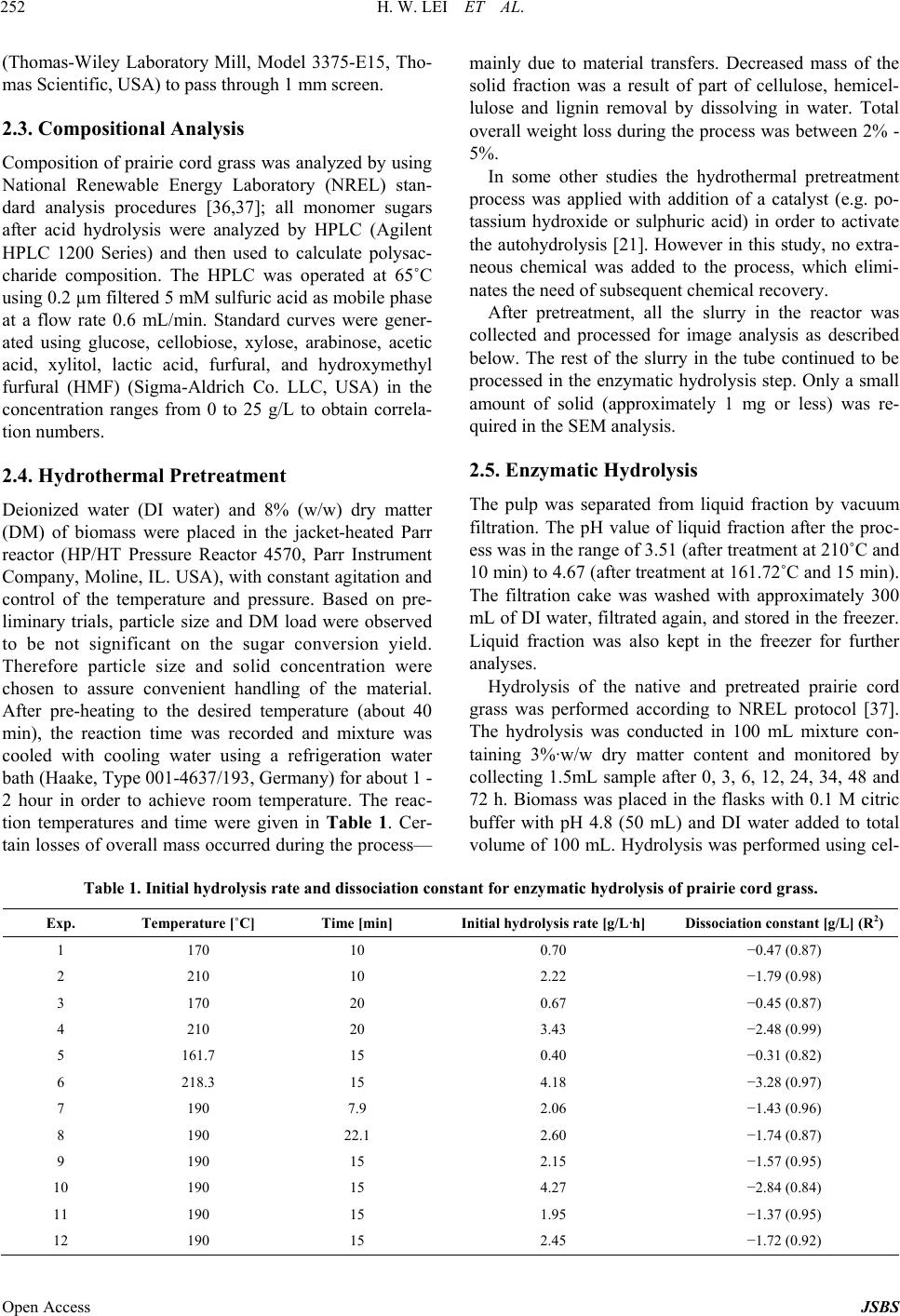 H. W. LEI ET AL. Open Access JSBS 252 (Thomas-Wiley Laboratory Mill, Model 3375-E15, Tho- mas Scientific, USA) to pass through 1 mm screen. 2.3. Compositional Analysis Composition of prairie cord grass was analyzed by using National Renewable Energy Laboratory (NREL) stan- dard analysis procedures [36,37]; all monomer sugars after acid hydrolysis were analyzed by HPLC (Agilent HPLC 1200 Series) and then used to calculate polysac- charide composition. The HPLC was operated at 65˚C using 0.2 µm filtered 5 mM sulfuric acid as mobile phase at a flow rate 0.6 mL/min. Standard curves were gener- ated using glucose, cellobiose, xylose, arabinose, acetic acid, xylitol, lactic acid, furfural, and hydroxymethyl furfural (HMF) (Sigma-Aldrich Co. LLC, USA) in the concentration ranges from 0 to 25 g/L to obtain correla- tion numbers. 2.4. Hydrothermal Pretreatment Deionized water (DI water) and 8% (w/w) dry matter (DM) of biomass were placed in the jacket-heated Parr reactor (HP/HT Pressure Reactor 4570, Parr Instrument Company, Moline, IL. USA), with constant agitation and control of the temperature and pressure. Based on pre- liminary trials, particle size and DM load were observed to be not significant on the sugar conversion yield. Therefore particle size and solid concentration were chosen to assure convenient handling of the material. After pre-heating to the desired temperature (about 40 min), the reaction time was recorded and mixture was cooled with cooling water using a refrigeration water bath (Haake, Type 001-4637/193, Germany) for about 1 - 2 hour in order to achieve room temperature. The reac- tion temperatures and time were given in Table 1. Cer- tain losses of overall mass occurred during the process— mainly due to material transfers. Decreased mass of the solid fraction was a result of part of cellulose, hemicel- lulose and lignin removal by dissolving in water. Total overall weight loss during the process was between 2% - 5%. In some other studies the hydrothermal pretreatment process was applied with addition of a catalyst (e.g. po- tassium hydroxide or sulphuric acid) in order to activate the autohydrolysis [21]. However in this study, no extra- neous chemical was added to the process, which elimi- nates the need of subsequent chemical recovery. After pretreatment, all the slurry in the reactor was collected and processed for image analysis as described below. The rest of the slurry in the tube continued to be processed in the enzymatic hydrolysis step. Only a small amount of solid (approximately 1 mg or less) was re- quired in the SEM analysis. 2.5. Enzymatic Hydrolysis The pulp was separated from liquid fraction by vacuum filtration. The pH value of liquid fraction after the proc- ess was in the range of 3.51 (after treatment at 210˚C and 10 min) to 4.67 (after treatment at 161.72˚C and 15 min). The filtration cake was washed with approximately 300 mL of DI water, filtrated again, and stored in the freezer. Liquid fraction was also kept in the freezer for further analyses. Hydrolysis of the native and pretreated prairie cord grass was performed according to NREL protocol [37]. The hydrolysis was conducted in 100 mL mixture con- taining 3%·w/w dry matter content and monitored by collecting 1.5mL sample after 0, 3, 6, 12, 24, 34, 48 and 72 h. Biomass was placed in the flasks with 0.1 M citric buffer with pH 4.8 (50 mL) and DI water added to total volume of 100 mL. Hydrolysis was performed using cel- Table 1. Initial hydrolysis rate and dissociation c onstant for enzymatic hydrolysis of prairie cord grass. Exp. Temperature [˚C] Time [min] Initial hydrolysis rate [g/L·h] Dissociation constant [g/L] (R2) 1 170 10 0.70 −0.47 (0.87) 2 210 10 2.22 −1.79 (0.98) 3 170 20 0.67 −0.45 (0.87) 4 210 20 3.43 −2.48 (0.99) 5 161.7 15 0.40 −0.31 (0.82) 6 218.3 15 4.18 −3.28 (0.97) 7 190 7.9 2.06 −1.43 (0.96) 8 190 22.1 2.60 −1.74 (0.87) 9 190 15 2.15 −1.57 (0.95) 10 190 15 4.27 −2.84 (0.84) 11 190 15 1.95 −1.37 (0.95) 12 190 15 2.45 −1.72 (0.92)  H. W. LEI ET AL. 253 lulase (Novozymes, NS50013) and β-glucosidase (No- vozymes, NS50010), added in amounts 15 FPU/gDM and 60 CBU/gDM respectively. Samples were then in- cubated at 50˚C and shaken at 180 rpm in an Environ- mental Incubator Shaker (New Brunswick Scientific CO., Inc., Edison, NJ). Hydrolysis was performed in dupli- cates. Concentrations of sugars and by-products were mea- sured on High Performance Liquid Chromatography (Agilent HPLC 1200 Series) instrument and samples were prepared according to LAP 013 [38] and LAP 015 [39]. 2.6. Scanning Electron Microscope Analysis The type of instrument used was a Hitachi 3500 Scan- ning Electron Microscope, operated at 30 kV, 33 mm. Samples were prepared by mounting them on specimen stubs using double-coated tape. Excess material was gen- tly blown off before SEM measurement. The difference in lignocellulosic structure of prairie cord grass before and after the hydrothermal pretreatment was measured by Scanning Electron Microscope. Low magnification pic- tures were taken first to obtain the information on the shape distribution of particles in the observation area. Then a higher magnification was applied, focusing on typical particle surfaces. The SEM images show how the raw structure can be opened during the treatment which enhanced surface area available for the enzymes. Pictures were taken at 30.0 kV and magnifications between ×350 and ×2.3 k. 2.7. Conversion Analysis In order to compare the efficiency among the pretreat- ment conditions as well as the enzymatic hydrolysis itself (to assess the availability of cellulose structure for en- zymes), cellulose into glucose conversion rates was cal- culated. Conversion rate represents the ratio of the amount of glucose which can be recovered from the pre- treated material to the amount of glucose in the material fed to the process [30]. Glucose conversion was defined as the percentage of cellulose pretreated or enzymatically converted to glucose, which is based on glucose concen- tration measured by HPLC and is calculated as follow- ing: Hydrolysis conver cos 100% co si s on Glueamountafter hydrolysis Glue amount in raw material (1) cos co Pre-treat s100% c ment conversi os on Glue insolidGlue infiltrate Glueamountin raw material (2) 2.8. Experimental Design The pretreatment trials were based on central composite experimental design (CCD) with application of statistical software (Design Expert version 8.0.1.0). The 22-facto- rial central composite design with four replications at the center point was used (Table 1) giving 12 experiments overall. Kinetics equations were developed to describe the relationship between independent variables and re- sponse variables, such as concentration of glucose, acetic acid, etc. The pretreatment process variables included temperature (˚C) and time (min), and response variables including conversion rates. 2.9. Kinetics Analysis Kinetic modeling plays an important role in the design, development, and operation of many chemical processes. Kinetic data are also important in the design and evalua- tion of processes to hydrolyze cellulosic materials to glucose for fermentation into ethanol or a variety of other chemical intermediates. During pretreatment polysaccha- rides are being decomposed to oligomers and monomers, while part of monomers (hexoses pyranosidic structures and pentoses furanosidic structures) are converted into hydroxymethylfurfural (HMF) and furfural. These com- pounds are considered as inhibitors for the fermentation, therefore should be controlled. Besides compounds men- tioned above, several other by-products are being formed during the pretreatment. These include: acetic acid (formed during breaking off the acetic groups from he- micellulose), furfural (can be degraded to formic acid) and HMF (can be degraded to formic and levulinic acids). Reaction rate Kc of acetic acid, furfural, and HMF were modeled according to the following kinetic model [40]: a KcAHEXPE RT (3) where [H+]a = molal hydrogen-ion concentration, A = constant, a = constant, E = activation energy, R = gas constant, and T = temperature. For hydrothermal pretreatment without adding any chemicals, a constant molal hydrogen-ion concentration was assumed; the following expression can be obtained: H KcAEXPE RT (4) where AH = constant. 3. Results and Discussion 3.1. Compositional Analysis Compositional analysis of the prairie cord grass was performed by acid hydrolysis according to Hames et al., 2008; and Selig et al., 2008 [36,37], with results given in Table 2. These results show that carbohydrate and lignin contents of prairie cord grass had a similar composition Open Access JSBS 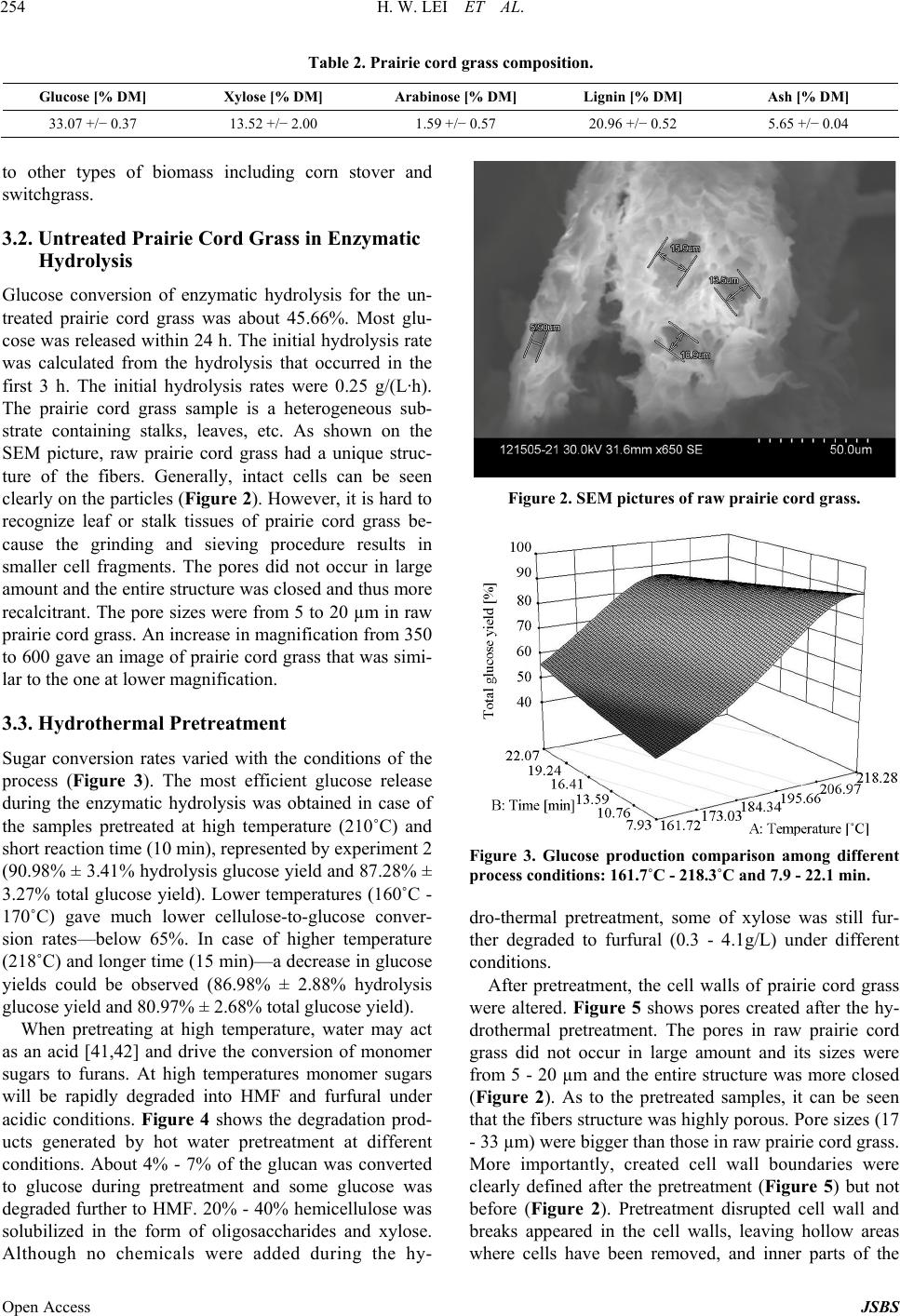 H. W. LEI ET AL. 254 Table 2. Prairie cord grass composition. Glucose [% DM] Xylose [% DM] Arabinose [% DM] Lignin [% DM] Ash [% DM] 33.07 +/− 0.37 13.52 +/− 2.00 1.59 +/− 0.57 20.96 +/− 0.52 5.65 +/− 0.04 to other types of biomass including corn stover and switchgrass. 3.2. Untreated Prairie Cord Grass in Enzymatic Hydrolysis Glucose conversion of enzymatic hydrolysis for the un- treated prairie cord grass was about 45.66%. Most glu- cose was released within 24 h. The initial hydrolysis rate was calculated from the hydrolysis that occurred in the first 3 h. The initial hydrolysis rates were 0.25 g/(L·h). The prairie cord grass sample is a heterogeneous sub- strate containing stalks, leaves, etc. As shown on the SEM picture, raw prairie cord grass had a unique struc- ture of the fibers. Generally, intact cells can be seen clearly on the particles (Figure 2). However, it is hard to recognize leaf or stalk tissues of prairie cord grass be- cause the grinding and sieving procedure results in smaller cell fragments. The pores did not occur in large amount and the entire structure was closed and thus more recalcitrant. The pore sizes were from 5 to 20 µm in raw prairie cord grass. An increase in magnification from 350 to 600 gave an image of prairie cord grass that was simi- lar to the one at lower magnification. 3.3. Hydrothermal Pretreatment Sugar conversion rates varied with the conditions of the process (Figure 3). The most efficient glucose release during the enzymatic hydrolysis was obtained in case of the samples pretreated at high temperature (210˚C) and short reaction time (10 min), represented by experiment 2 (90.98% ± 3.41% hydrolysis glucose yield and 87.28% ± 3.27% total glucose yield). Lower temperatures (160˚C - 170˚C) gave much lower cellulose-to-glucose conver- sion rates—below 65%. In case of higher temperature (218˚C) and longer time (15 min)—a decrease in glucose yields could be observed (86.98% ± 2.88% hydrolysis glucose yield and 80.97% ± 2.68% total glucose yield). When pretreating at high temperature, water may act as an acid [41,42] and drive the conversion of monomer sugars to furans. At high temperatures monomer sugars will be rapidly degraded into HMF and furfural under acidic conditions. Figure 4 shows the degradation prod- ucts generated by hot water pretreatment at different conditions. About 4% - 7% of the glucan was converted to glucose during pretreatment and some glucose was degraded further to HMF. 20% - 40% hemicellulose was solubilized in the form of oligosaccharides and xylose. Although no chemicals were added during the hy- Figure 2. SEM pictures of raw prairie cord grass. Figure 3. Glucose production comparison among different process conditions: 161.7˚C - 218.3˚C and 7.9 - 22.1 min. dro-thermal pretreatment, some of xylose was still fur- ther degraded to furfural (0.3 - 4.1g/L) under different conditions. After pretreatment, the cell walls of prairie cord grass were altered. Figure 5 shows pores created after the hy- drothermal pretreatment. The pores in raw prairie cord grass did not occur in large amount and its sizes were from 5 - 20 µm and the entire structure was more closed (Figure 2). As to the pretreated samples, it can be seen that the fibers structure was highly porous. Pore sizes (17 - 33 µm) were bigger than those in raw prairie cord grass. More importantly, created cell wall boundaries were clearly defined after the pretreatment (Figure 5) but not before (Figure 2). Pretreatment disrupted cell wall and breaks appeared in the cell walls, leaving hollow areas where cells have been removed, and inner parts of the Open Access JSBS 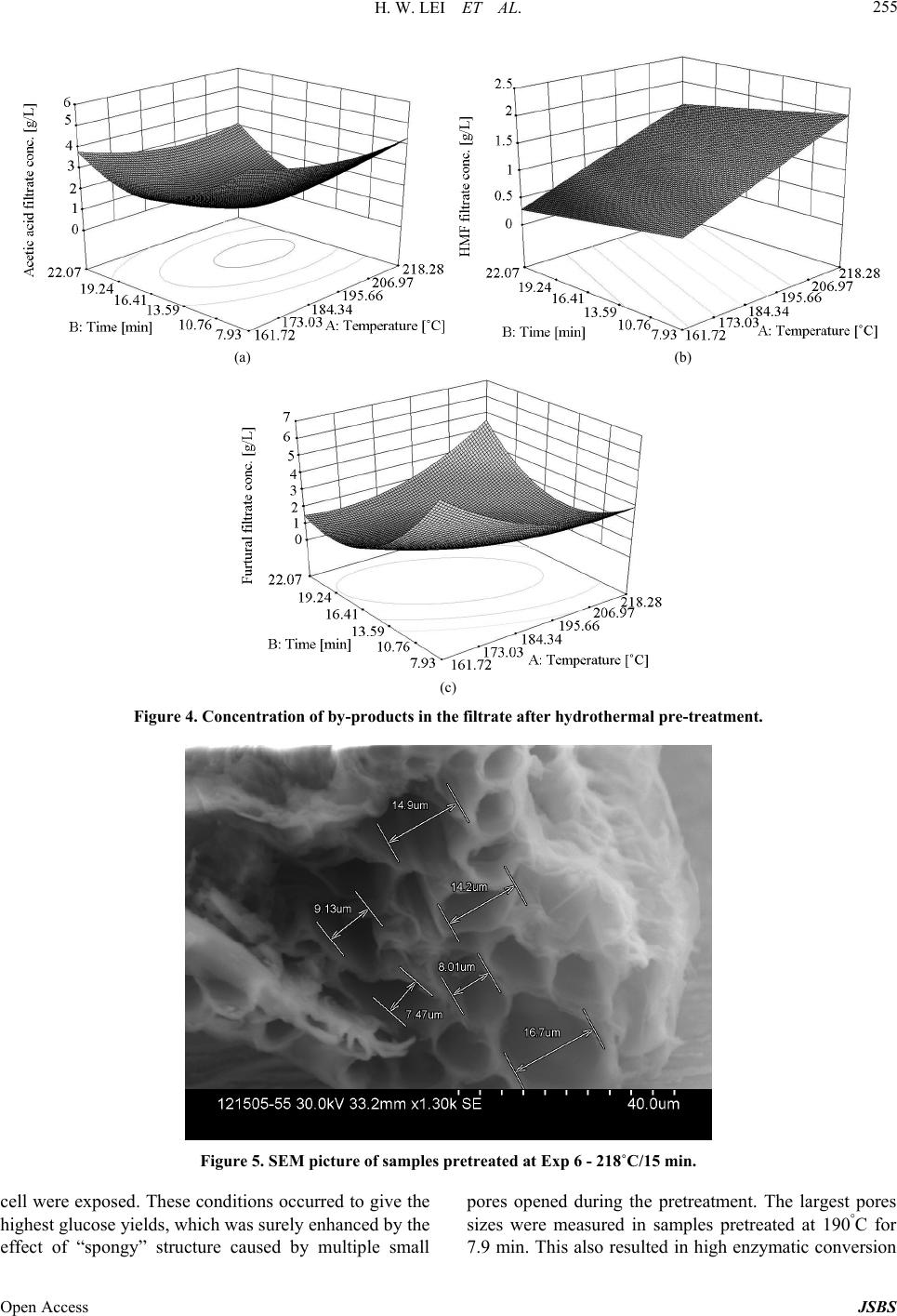 H. W. LEI ET AL. 255 (a) (b) (c) Figure 4. Concentration of by-products in the filtrate after hydrothermal pre-treatment. Figure 5. SEM picture of samples pretreated at Exp 6 - 218˚C/15 min. cell were exposed. These conditions occurred to give the highest glucose yields, which was surely enhanced by the effect of “spongy” structure caused by multiple small pores opened during the pretreatment. The largest pores sizes were measured in samples pretreated at 190°C for 7.9 min. This also resulted in high enzymatic conversion Open Access JSBS  H. W. LEI ET AL. 256 (~90%). 3.4. Pretreatment Effect on Enzymatic Hydrolysis As it can be seen (Figure 3), sugars production varied with the conditions of the process. The most efficient glucose production from the lignocellulosic structure during the hydrolysis was obtained in case of the material pretreated at high temperature (210˚C) and short reaction time (10 min). The lowest process efficiency was ob- served in case of applying relatively low temperature (170°C) and short reaction time (10 min). Comparison among different conditions of the hydrothermal pre- treatment in terms of by-products generation during at-line monitored hydrolysis was also studied. By-prod- ucts generation was not significant during the hydrolysis. This was a result of a thorough washing of the cellulose fraction after the pretreatment. Also lack of significant lactic acid production proved that no bacterial infection occurred during the process. Acetic acid production was observed to be the highest in case of either low tempera- ture or time of reaction, and the lowest in case of high temperature application. Acetic acid was produced by decomposition of hemicellulose during enzymatic (or chemical) hydrolysis. Its generation can be avoided by effective transfer of hemicellulose to the liquid fraction during pretreatment. It can be seen that in case of high temperature application, hemicellulose was removed most effectively, resulting in low acetic acid and xylose pro- duction during the hydrolysis. However, most of the xy- lose was converted into furfural during the pretreatment, resulting in high concentration of this inhibitor in the liquid fraction. As mentioned above, hemicellulose and products of its degradation were removed to the filtrate after hydro- thermal pretreatment. The filtrate was also analyzed for the presence of sugars and inhibitors (without any post-treatment). To be able to use hemicellulose sugars in the hydrolysis and further in the fermentation process, liquid fraction needs to be detoxified, which is a labori- ous and expensive procedure. Moreover, the sugars pre- sent in the filtrate are mostly pentoses, which do not have a feasible application in fermentation process currently. Instead, C-5 sugars could be utilized in cattle feed pro- duction [18]. In case of the filtrate, time change did not seem to be significant for glucose and xylose production, however it did influence arabinose and cellobiose release. Tempera- ture had a major influence on all the sugars production. By-products release into the filtrate depended strongly on temperature (increasing with its increase), but not on time change. Temperature had a significant effect on both conversion rates of pretreatment and enzymatic hy- drolysis, unlike time change, which influenced only the pretreatment conversion rate since in this calculation filtrate was taken into account. 3.5. Conversion Rates and Hydrolysis Kinetics The highest conversion rates in the enzymatic hydrolysis (94.53%) as well as during the pretreatment (97.96%) were assigned to the following conditions: 210°C and 10 min. In case of higher temperature (218°C) and longer time (15 min)—about 8% decrease in glucose conversion rate was observed. Lower temperatures (160˚C - 170°C) gave much lower glucose conversion rates—below 70%. However, even cooking at relatively low temperatures (161.72 °C) gave conversion rate of the hydrolysis higher than the non-treated sample (control). Before pretreatment, the initial hydrolysis rate was 0.25 g/(L·h). After pretreatment initial hydrolysis rates were significantly increased up to 4.2 g/(L·h) for prairie cord grass under different pretreatment conditions. A possible explanation for this phenomenon can be found by examining the kinetics of hydrolysis. Initial hydroly- sis rate can be expressed as dG/dt = −k[G0], where [G0] is exposed cellulose expressed as concentration of mo- nomer units (g/L), and it is a function of surface area, and k is pretreatment conditions related constant (Table 1). Hydrolysis data were fit to a Michaelis-Menten model [43] to determine the kinetic constant: dissociation con- stant, Km. High initial hydrolysis rate shows more rapid dissociation of the sugar in the hydrolysis and faster production of the product glucose, whereas large Km shows lower affinity of the enzyme for the cellulose in the hydrolysis. The kinetic model equation is shown below: mm vVSK S (5) where, v: Rate of reaction (g/L·hr) Vm: initial rate of reaction (g/L·hr) S: Substrate/Product concentration (g/L) Km: dissociation constant (g/L) A typical data fitting using Michaelis-Menten model (Figure 6, experiment #4) was applied to determine dis- sociation constant (Km) for enzymatic hydrolysis of prai- rie cord grass. Values of R2 showed that the models for each response variable explain the hydrolysis process relationships well (Table 1 and Figure 6). The higher the dissociation constant, the lower the affinity of the en- zyme to the pretreated prairie cord grass. The dissocia- tion constant, Km in this study was a good representation of the affinity to the pretreated prairie cord grass. 3.6. Kinetic Evaluation of Hydrothermal Pretreatment Prairie cord grass is mainly comprised of cellulose, he- micellulose, and lignin. Prairie cord grass is a complex Open Access JSBS 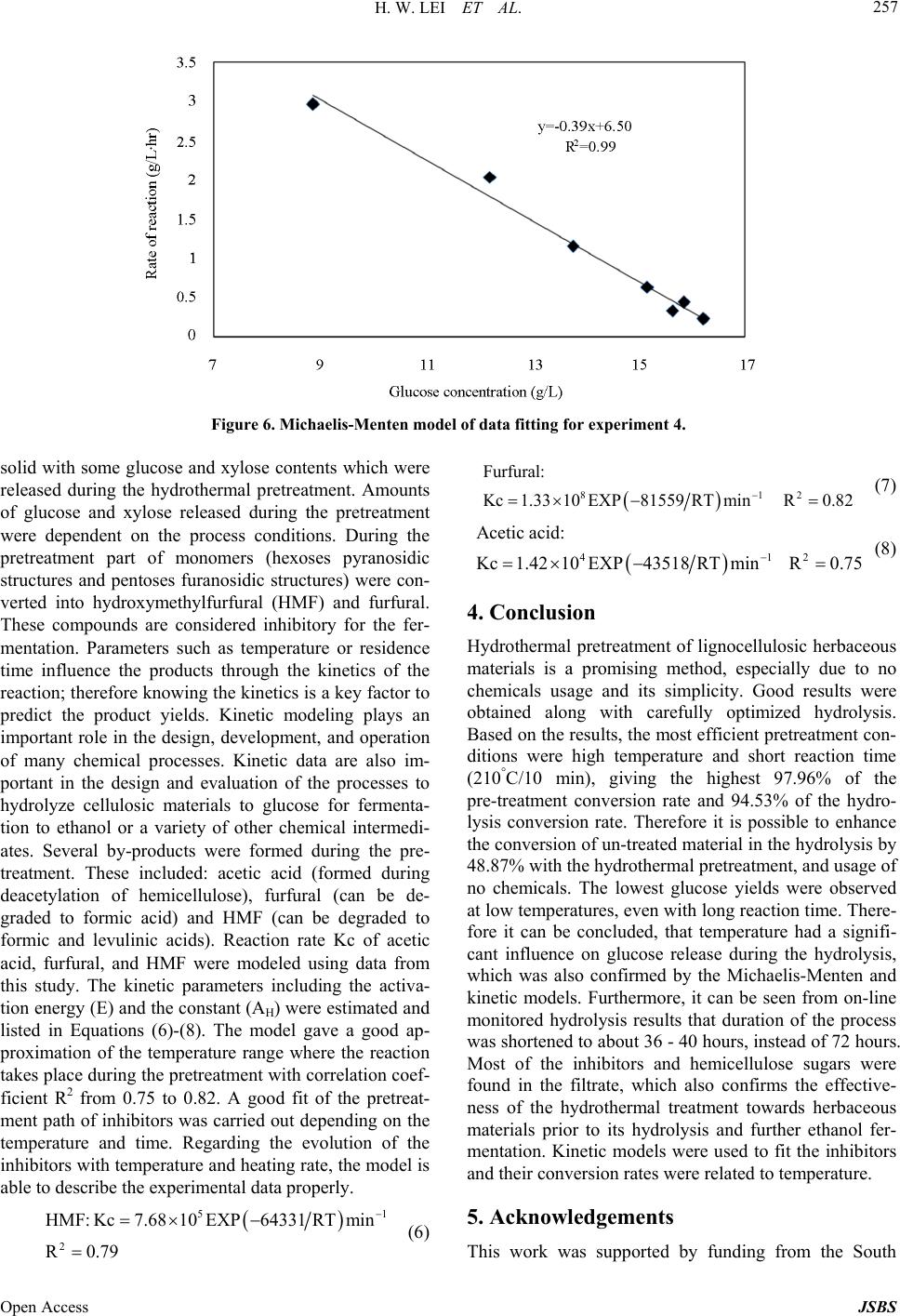 H. W. LEI ET AL. Open Access JSBS 257 Figure 6. Michaelis-Menten model of data fitting for experiment 4. solid with some glucose and xylose contents which were released during the hydrothermal pretreatment. Amounts of glucose and xylose released during the pretreatment were dependent on the process conditions. During the pretreatment part of monomers (hexoses pyranosidic structures and pentoses furanosidic structures) were con- verted into hydroxymethylfurfural (HMF) and furfural. These compounds are considered inhibitory for the fer- mentation. Parameters such as temperature or residence time influence the products through the kinetics of the reaction; therefore knowing the kinetics is a key factor to predict the product yields. Kinetic modeling plays an important role in the design, development, and operation of many chemical processes. Kinetic data are also im- portant in the design and evaluation of the processes to hydrolyze cellulosic materials to glucose for fermenta- tion to ethanol or a variety of other chemical intermedi- ates. Several by-products were formed during the pre- treatment. These included: acetic acid (formed during deacetylation of hemicellulose), furfural (can be de- graded to formic acid) and HMF (can be degraded to formic and levulinic acids). Reaction rate Kc of acetic acid, furfural, and HMF were modeled using data from this study. The kinetic parameters including the activa- tion energy (E) and the constant (AH) were estimated and listed in Equations (6)-(8). The model gave a good ap- proximation of the temperature range where the reaction takes place during the pretreatment with correlation coef- ficient R2 from 0.75 to 0.82. A good fit of the pretreat- ment path of inhibitors was carried out depending on the temperature and time. Regarding the evolution of the inhibitors with temperature and heating rate, the model is able to describe the experimental data properly. 81 Furfural: Kc1.3310EXP81559RTmi n8 0R. 2 2 (7) 41 Acetic acid: Kc1.4210EXP43518 RTmin0.5 R7 2 (8) 4. Conclusion Hydrothermal pretreatment of lignocellulosic herbaceous materials is a promising method, especially due to no chemicals usage and its simplicity. Good results were obtained along with carefully optimized hydrolysis. Based on the results, the most efficient pretreatment con- ditions were high temperature and short reaction time (210°C/10 min), giving the highest 97.96% of the pre-treatment conversion rate and 94.53% of the hydro- lysis conversion rate. Therefore it is possible to enhance the conversion of un-treated material in the hydrolysis by 48.87% with the hydrothermal pretreatment, and usage of no chemicals. The lowest glucose yields were observed at low temperatures, even with long reaction time. There- fore it can be concluded, that temperature had a signifi- cant influence on glucose release during the hydrolysis, which was also confirmed by the Michaelis-Menten and kinetic models. Furthermore, it can be seen from on-line monitored hydrolysis results that duration of the process was shortened to about 36 - 40 hours, instead of 72 hours. Most of the inhibitors and hemicellulose sugars were found in the filtrate, which also confirms the effective- ness of the hydrothermal treatment towards herbaceous materials prior to its hydrolysis and further ethanol fer- mentation. Kinetic models were used to fit the inhibitors and their conversion rates were related to temperature. 5 2 HMF:Kc7.6810EXP64331RTin 0.79 m R 1 (6) 5. Acknowledgements This work was supported by funding from the South  H. W. LEI ET AL. 258 Dakota Research and Commercialization Council and Washington State University. REFERENCES [1] Z. Tang, Q. Lu, Y. Zhang, X. F. Zhu and Q. X. Guo, “One Step Bio-Oil Upgrading through Hydrotreatment, Esterification, and Cracking”, Industrial & Engineering Chemistry Research, Vol. 48, No. 15, 2009, pp. 6923- 6929. http://dx.doi.org/10.1021/ie900108d [2] M. Wang, M. Wu and H. Huo, “Life-Cycle Energy and Greenhouse Gas Emission Impacts of Different Corn Etha- nol Plant Types,” Environmental Research Letters, Vol. 2, No. 2, 2007, p. 24. http://dx.doi.org/10.1088/1748-9326/2/2/024001 [3] C. Colin, “The Coming Oil Crisis,” Multi-Science Pub- lishing Company, New York, 2003. [4] M. T. Holtzapple, “Cellulose,” In: R. Macrae, R. K. Rob- inson and M. J. Sadler, Eds., Encyclopedia of Food Sci- ence, Food Technology and Nutrition, Academic Press, London, 1993, pp. 2731-2738. [5] M. T. Holtzapple, “Lignin,” In: R. Macrae, R. K. Robin- son and M. J. Sadler, Eds., Encyclopedia of Food Science, Food Technology and Nutrition, Academic Press, London, 1993, pp. 758-767. [6] A. G. Williams and I. M. Morrison, “Studies on the Pro- duction of Saccharinic Acids by the Alkaline Treatment of Young Grass and Their Effectiveness as Substrates for Mixed Rumen Microorganisms in Vitro,” Journal of the Science of Food and Agriculture, Vol. 33, No. 1, 1982, pp. 21-29. http://dx.doi.org/10.1002/jsfa.2740330106 [7] M. H. Thomsen, J. B. Holm-Nielsen, P. Oleskowicz-Popiel and A. B. Thomsen, “Pretreatment of Whole-Crop Har- vested, Ensiled Maize for Ethanol Production,” Applied Biochemistry and Biotechnology, Vol. 148, No. 1-3, 2008, pp. 23-33. http://dx.doi.org/10.1007/s12010-008-8134-2 [8] V. S. Chang and M. T. Holtzapple, “Fundamental Factors Affecting Biomass Enzymatic Reactivity,” Applied Bio- chemistry and Biotechnology, Vol. 84-86, No. 1-9, 2000, pp. 5-37. http://dx.doi.org/10.1385/ABAB:84-86:1-9:5 [9] H. B. Hahn, “Ethanolic Fermentation of Lignocellulose Hydrolysates: A Minireview,” Applied Biochemistry and Biotechnology, Vol. 57-58, No. 1, 1996, pp. 195-199. http://dx.doi.org/10.1007/BF02941700 [10] T. E. Amidon, C. D. Wood, A. M. Shupe, Y. Wang, M. Graves and S. Liu, “Biorefinery: Conversion of Woody Biomass to Chemicals, Energy and Materials,” Journal of Biobased Materials and Bioenergy, Vol. 2, No. 2, 2008, pp. 100-120. http://dx.doi.org/10.1166/jbmb.2008.302 [11] P. Alvira, E. Tomás-Pejó, M. Ballesteros and M. J. Negro, “Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review,” Bioresource Technology, Vol. 101, No. 13, 2010, pp. 4851-4861. http://dx.doi.org/10.1016/j.biortech.2009.11.093 [12] T. I. Georgieva, X. Hou, T. Hilstrom and B. K. Ahring, “Enzymatic Hydrolysis and Ethanol Fermentation of High Dry Matter Wet-Exploded Wheat Straw at Low En- zyme Loading,” Applied Biochemistry and Biotechnology, Vol. 148, No. 1-3, 2008, pp. 35-44. http://dx.doi.org/10.1007/s12010-007-8085-z [13] H. Lei, K. Hennessey, Y. Liu, X. Lin, Y. Wan and R. Ruan R, “Optimization of Hydrothermal Pretreatment of Corn Stover,” 2008 ASABE Annual International Meeting, Providence, 29 June-2 July 2008. [14] I. Cybulska, H. Lei and J. Julson, “Hydrothermal Pre- treatment and Enzymatic Hydrolysis of Prairie Cord Grass,” Energy and Fuels, Vol. 24, No. 1, 2009, pp. 718- 727. http://dx.doi.org/10.1021/ef9009179 [15] D. J. Schell, J. Farmer, M. Newman and J. D. McMillan, “Dilute-Sulfuric Acid Pretreatment of Corn Stover in Pi- lot-Scale Reactor,” Applied Biochemistry and Biotech- nology, Vol. 105-108, 2003, pp. 69-85. [16] H. Alizadeh, F. Teymouri, T. I. Gilbert and B. E. Dale, “Pretreatment of Switchgrass by Ammonia Fiber Explo- sion (AFEX),” Applied Biochemistry and Biotechnology, Vol. 124, No. 1-3, 2005, pp. 1-3. http://dx.doi.org/10.1385/ABAB:124:1-3:1133 [17] A. S. Schmidt and A. B. Thomsen, “Optimization of Wet Oxidation Pretreatment of Wheat Straw,” Bioresource Technology, Vol. 64, No. 2, 1998. pp. 139-151. http://dx.doi.org/10.1016/S0960-8524(97)00164-8 [18] J. Larsen, M. Oestegaard Petersen, L. Thirup, L. H. Wen, and F. Krogh Iversen, “The IBUS Process—Lignocellu- losic Bioethanol Close to a Commercial Reality,” Che- mical Engineering & Technology, Vol. 31, No. 5, 2008, pp. 765-772. http://dx.doi.org/10.1002/ceat.200800048 [19] N. Mosier, R. Hendrickson, N. Ho, M. Sedlak and M. R. Ladisch, “Optimization of pH Controlled Liquid Hot Water Pretreatment of Corn Stover,” Bioresource Tech- nology, Vol. 96, No. 18, 2005, pp. 1986-1993. http://dx.doi.org/10.1016/j.biortech.2005.01.013 [20] K. Kohlmann, P. Westgate, J. Weil and M. R. Ladisch, “Biological Based Systems for Waste Processing,” Pro- ceedings of 1993 ICES Meetingm, SAE Technical Paper Series 932251, 1993. [21] J. R. Weil, M. A. Brewer, R. L. Hendrickson, A. Sarikaya and M. R. Ladisch, “Pretreatment of Corn Fiber by Pre- ssure Cooking in Water,” Applied Biochemistry and Bio- technology, Vol. 73, No. 1, 1998, pp. 1-17. [22] E. Walch, A. Zemann, F. Schinner, G. Bonn and O. Bo- bleter, “Enzymatic Saccharification of Hemicellulose Ob- tained from Hydrothermally Pretreated Sugar Cane Baga- sse and Beech Bark,” Bioresource Technology, Vol. 39, No. 2, 1992, pp. 173-177. http://dx.doi.org/10.1016/0960-8524(92)90137-M [23] W. S. Mok and M. J. Antal Jr., “Uncatalyzed Solvolysis of Whole Biomass by Hot Compressed Liquid Water,” Industrial & Engineering Chemistry Research, Vol. 31, No. 4, 1992, pp. 1157-1161. http://dx.doi.org/10.1021/ie00004a026 [24] H. E. Grethlein, “The Effect of Pore Size Distribution on the Rate of Enzymatic Hydrolysis of Cellulosic Sub- strates,” Nature Biotechnology, Vol. 3, 1985, pp. 155-160. http://dx.doi.org/10.1038/nbt0285-155 [25] M. R. Ladisch, K. W. Lin, M. Voloch and G. T. Tsao, Open Access JSBS  H. W. LEI ET AL. Open Access JSBS 259 “Process Considerations in the Enzymatic Hydrolysis of Biomass,” Enzyme and Microbial Technology, Vol. 5, No. 2, 1983, pp. 82-102. http://dx.doi.org/10.1016/0141-0229(83)90042-X [26] Y. Y. Lee, Q. Xiang, T. H. Kim and J. Kim, “Enhance- ment of Dilute-Acid Total-Hydrolysis Process for High- Yield Saccharification of Cellulosic Biomass,” Depart- ment of Chemical Engineering, Auburn University, Au- burn 2000. [27] S. Zhu, Y. Wu, Z. Yu, X. Zhang, C. Wang, F. Yu and S. Jin, “Production of Ethanol from Microwave-Assisted Alkali Pretreated Wheat Straw,” Process Biochemistry, Vol. 41, No. 4, 2006, pp. 869-873. http://dx.doi.org/10.1016/j.procbio.2005.10.024 [28] N. Akiya and P. E. Savage, “Roles of Water for Chemical Reactions in High-Temperature Water,” Chemical Re- views, Vol. 102, No. 8, 2002, pp. 2725-2750. http://dx.doi.org/10.1021/cr000668w [29] G. Garrote, H. Dominguez and J. C. Parajo, “Hydro- thermal Processing of Lignocellulosic Materials,” Holz als Roh- und Werkstoff, Vol. 57, No. 3, 1999, pp. 191-202. http://dx.doi.org/10.1007/s001070050039 [30] M. H. Thomsen, J. B. Holm-Nielsen, P. Oleskowicz- Popiel and A. B. Thomsen, “Pretreatment of Whole-Crop Harvested, Ensiled Maize for Ethanol Production,” Appl- ied Biochemistry and Biotechnology, Vol. 148, No. 1-3, 2008, pp. 23-33. http://dx.doi.org/10.1007/s12010-008-8134-2 [31] J. M. Negro, P. Manzanares, I. Ballesteros, J. M. Oliva, A. Cabanas and M. Ballesteros, “Hydrothermal Pretreatment Conditions to Enhance Ethanol Production from Poplar Biomass,” Applied Biochemistry and Biotechnology, 2003, Vol. 105, No. 1-3, pp. 87-100. [32] C. Cara, I. Romero, J. M. Oliva, F. Saez and E. Castro, “Liquid Hot Water Pretreatment of Olive Tree Pruning Residues,” Applied Biochemistry and Biotechnology, Vol. 137, No. 1-12, 2007, pp. 379-394. [33] M. H. Thomsen, A. Thygesen, H. Joergensen, J. Larsen, B. Holm Christensen and A. Thomsen, “Preliminary Re- sults on Optimization of Pilot Scale Pretreatment of Wheat Straw Used in Coproduction of Bioethanol and Electricity,” Applied Biochemistry and Biotechnology, Vol. 129, 2006, pp.448-460. [34] M. Bollok, K. Reczey and G. Zacchi, “Simultaneous Saccharification and Fermentation of Steam-Pretreated Spruce to Ethanol,” Applied Biochemistry and Biotech- nology, Vol. 84, 2000, pp. 69-80. [35] L. Brown, “Grasslands,” Knopf, New York, 1985. [36] B. Hames, R. Ruiz, C. Scarlata, A. Sluiter, J. Sluiter and D. Templeton, “Preparation of Samples for Composi- tional Analysis,” Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory, Golden, 2008. [37] M. Selig, “Enzymatic Saccharification of Lignocellulosic Biomass, Laboratory Analytical Procedure,” National Re- newable Energy Laboratory, Golden, 2008. [38] R. Ruiz and T. Ehrman, “HPLC Analysis of Liquid Frac- tions of Process Samples for Monomeric Sugars and Cel- lobiose,” Laboratory Analytical Procedure (LAP 013), National Renewable Energy Laboratory, Golden, 1996. [39] R. Ruiz and T. Ehrman, “HPLC Analysis of Liquid Frac- tions of Process Samples for Byproducts and Degradation Products,” Laboratory Analytical Procedure (LAP 015), National Renewable Energy Laboratory, Golden,1996. [40] A. H. Conner, B. F. Wood, C. G. Hill and J. F. Harris, “Ki-Netic Model for the Dilute Sulfuric Acid Sacchari- fication of Lignocelluloses,” Journal of Wood Chemistry and Technology, Vol. 5, No. 4, 1985, pp. 461-489. http://dx.doi.org/10.1080/02773818508085207 [41] K. L. Kohlmann, P. J. Westgate, A. Sarikaya, A. Vela- yudhan, J. Weil, R. L. Hendrickson and M. R. Ladisch, “Enhanced Enzyme Activities on Hydrated Lignocellu- losic Substrate,” In: J. N. Saddler and M. H. Penner, Eds., ACS Symposium Series 618 (Enzymatic Degradation of Insoluble Carbohydrates), American Chemical Society, Washington DC, 1995, pp. 237-255. [42] K. L. Kohlmann, P. J. Westgate, A. Velayudhan, J. Weil, A. Sarikaya, M. A. Brewer, R. L. Hendrickson and M. R. Ladisch, “Enzyme Conversion of Lignocellulosic Plant Materials for Resource Recovery in a Controlled Ecolo- gical Life Support System,” Advances in Space Research, Vol. 18, No. 1-2, 1996, pp. 251-265. http://dx.doi.org/10.1016/0273-1177(95)00815-V [43] A. Goldbeter and D. E. Koshland, “An Amplified Sensi- tivity Arising from Covalent Modification in Biological Systems,” Proceedings of National Academy of Sciences of the United States of America, Vol. 78, No. 11, 1981, pp. 6840-6844. http://dx.doi.org/10.1073/pnas.78.11.6840
|