 Vol.1, No.1, 4-10 (2013) Occupational Diseases and Environmental Medicine http://dx.doi.org/10.4236/odem.2013.11002 Relationship between acute high altitude response, cardiac function injury, and high altitude de-adaptation response after returning to lower altitude* Shengyue Yang1, Qiquan Zhou2,3#, Zifu Shi4, Enzhi Feng1, Ziqiang Yan1, Zhongxin Tian1, He Yin1, Yong Fan2,3 1Center of Respiratory Medicine, The 4th Hospital, Lanzhou Command, PLA, Xining, China 2Department of High Altitude Diseases, College of High Altitude Military Medicine, Third Military Medical University, Chongqing, China; #Corresponding Author: zhouqq9918@163.com 3Key Laboratory of High Altitude Medicine of Ministry of Education and Key Laboratory of High Altitude Medicine of PLA, Chongqing, China 4The 68303 Troop Hospital of People’s Liberation Army, Wuwei, China Received 20 September 2013; revised 25 October 2013; accepted 8 November 2013 Copyright © 2013 Shengyue Yang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Shengyue Yang et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian. ABSTRACT The relationship between acute high altitude response (AHAR), cardiac function injury, and high altitude de-adaptation response (HADAR) was assessed. Cardiac function indicators were assessed for 96 men (18 - 35 years old) dep- loyed into a high altitude (3700 - 4800 m) envi- ronment requiring intense physical activity. The subjects were divided into 3 groups based on AHAR at high altitude: severe AHAR (n = 24), mild to moderate AHAR (Group B, n = 47) and non-AHAR (Group C, 25); and based on HADAR: severe HADAR (Group E, n = 19), mild to mod- erate HADAR (Group F, n = 40) and non-HADAR (Group G, n = 37) after return to lower altitude (1,500 m). Cardiac function indicators were measured after 50 days at high altitude and at 12 h, 15 days, and 30 days after return to lower al- titude. Controls were 50 healthy volunteers (Group D, n = 50) at 1500 m. Significant differ- ences were observed in cardiac function indi- cators among groups A, B, C, and D. AHAR score was positively correlated with HADAR score (r = 0.863, P < 0.001). Significant differ- ences were also observed in cardiac function indicators among groups D, E, F, and G, 12 h and 15 days after return to lower altitude. There were no significant differences in cardiac function indicators among the groups, 30 days after re- turn to lower altitude, compared to group D. The results indicated that the severity of HADAR is associated with the severity of AHAR and car- diac injury, and prolonged recovery. Keywords: Acute High Altitude Response; Ca rdiac Function; Cardiac Structure; Myocardial Enzyme; Return to Lower Altitude; High Altitude De-Adaptation 1. INTRODUCTION People sometimes live in high-altitude, hypoxic envi- ronments and this requires the body to make a complex series of compensatory changes in neurohumoral regula- tion, involving physiological, biochemical, and morpho- logical changes to achieve a balance between the internal and external environment. When an individual returns to the normal oxygen environment, the body undergoes a series of corresponding changes resulting in the gradual elimination of the adaptive changes previously made. Many people undergoing this process experience symp- toms such as dizziness, palpitations, chest tightness, drowsiness, precordial region pain, general fatigue, and weakness. This response is termed a high altitude *The authors declare that no conflicts of interest exist. Copyright © 2013 SciRes. OPEN ACCESS 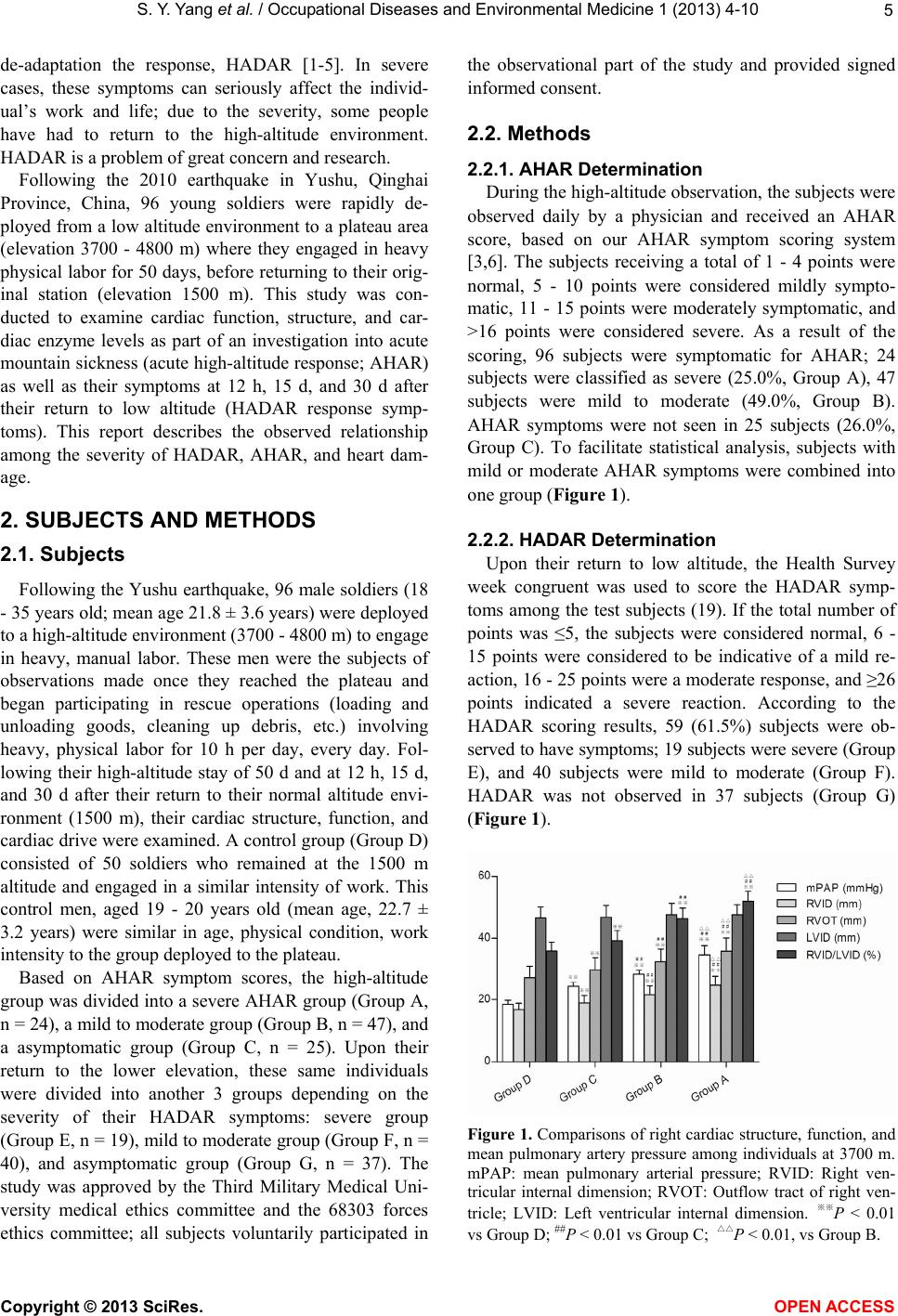 S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 5 de-adaptation the response, HADAR [1-5]. In severe cases, these symptoms can seriously affect the individ- ual’s work and life; due to the severity, some people have had to return to the high-altitude environment. HADAR is a problem of great concern and research. Following the 2010 earthquake in Yushu, Qinghai Province, China, 96 young soldiers were rapidly de- ployed from a low altitude environment to a plateau area (elevation 3700 - 4800 m) where they engaged in heavy physical labor for 50 days, before returning to their orig- inal station (elevation 1500 m). This study was con- ducted to examine cardiac function, structure, and car- diac enzyme levels as part of an investigation into acute mountain sickness (acute high-altitude response; AHAR) as well as their symptoms at 12 h, 15 d, and 30 d after their return to low altitude (HADAR response symp- toms). This report describes the observed relationship among the severity of HADAR, AHAR, and heart dam- age. 2. SUBJECTS AND METHODS 2.1. Subjects Following the Yushu earthquake, 96 male soldiers (18 - 35 years old; mean age 21.8 ± 3.6 years) were deployed to a high-altitude environment (3700 - 4800 m) to engage in heavy, manual labor. These men were the subjects of observations made once they reached the plateau and began participating in rescue operations (loading and unloading goods, cleaning up debris, etc.) involving heavy, physical labor for 10 h per day, every day. Fol- lowing their high-altitude stay of 50 d and at 12 h, 15 d, and 30 d after their return to their normal altitude envi- ronment (1500 m), their cardiac structure, function, and cardiac drive were examined. A control group (Group D) consisted of 50 soldiers who remained at the 1500 m altitude and engaged in a similar intensity of work. This control men, aged 19 - 20 years old (mean age, 22.7 ± 3.2 years) were similar in age, physical condition, work intensity to the group deployed to the plateau. Based on AHAR symptom scores, the high-altitude group was divided into a severe AHAR group (Group A, n = 24), a mild to moderate group (Group B, n = 47), and a asymptomatic group (Group C, n = 25). Upon their return to the lower elevation, these same individuals were divided into another 3 groups depending on the severity of their HADAR symptoms: severe group (Group E, n = 19), mild to moderate group (Group F, n = 40), and asymptomatic group (Group G, n = 37). The study was approved by the Third Military Medical Uni- versity medical ethics committee and the 68303 forces ethics committee; all subjects voluntarily participated in the observational part of the study and provided signed informed consent. 2.2. Methods 2.2.1. AHAR Determination During the high-altitude observation, the subjects were observed daily by a physician and received an AHAR score, based on our AHAR symptom scoring system [3,6]. The subjects receiving a total of 1 - 4 points were normal, 5 - 10 points were considered mildly sympto- matic, 11 - 15 points were moderately symptomatic, and >16 points were considered severe. As a result of the scoring, 96 subjects were symptomatic for AHAR; 24 subjects were classified as severe (25.0%, Group A), 47 subjects were mild to moderate (49.0%, Group B). AHAR symptoms were not seen in 25 subjects (26.0%, Group C). To facilitate statistical analysis, subjects with mild or moderate AHAR symptoms were combined into one group (Figure 1). 2.2.2. HADAR De t er mination Upon their return to low altitude, the Health Survey week congruent was used to score the HADAR symp- toms among the test subjects (19). If the total number of points was ≤5, the subjects were considered normal, 6 - 15 points were considered to be indicative of a mild re- action, 16 - 25 points were a moderate response, and ≥26 points indicated a severe reaction. According to the HADAR scoring results, 59 (61.5%) subjects were ob- served to have symptoms; 19 subjects were severe (Group E), and 40 subjects were mild to moderate (Group F). HADAR was not observed in 37 subjects (Group G) (Figure 1). Figure 1. Comparisons of right cardiac structure, function, and mean pulmonary artery pressure among individuals at 3700 m. mPAP: mean pulmonary arterial pressure; RVID: Right ven- tricular internal dimension; RVOT: Outflow tract of right ven- tricle; LVID: Left ventricular internal dimension. ※※P < 0.01 vs Group D; ##P < 0.01 vs Group C; △△P < 0.01, vs Group B. Copyright © 2013 SciRes. OPEN ACCESS  S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 6 2.2.3. Cardiac Structure and Function Determination Color Doppler ultrasound (LOGIQ-type, GE Health- care, Little Chalfont, UK) was used to detect the average pulmonary artery pressure (mPAP), right ventricular di- ameter (RVID), right ventricular outflow tract (RVOT), left ventricular diameter (LVID), left ventricular ejection fraction (LVEF), and myocardial performance index (Tei index). Inspections were made with the subject in the left lateral position, with measurement taken over a 1-h pe- riod with the subject in a resting state. The pulsed Dop- pler sample volume involved the measurement of the steady-state heart rate, the spectrum of the mitral and aortic blood flow, and the measurement of the peak A mitral valve flow between the end of the next cardiac cycle E peak time period (a line), and the aortic ejection time (b line). The Tei index is calculated according to the formula: Tei index = (ab/b). Simpson’s method was used to calculate each subject’s LVEF [7]. mPAP measure- ments involved the determination of right ventricular ejection early time (RVPEP) and pulmonary blood flow acceleration time (AT), and was calculated according to the equation: mPAP (mmHg) = 42.1 (RVPEP/AT) - 15.7 [8,9]. RVID, RVOT, and LVID were measured accord- ing to established methods. 2.2.4. Determination of Cardiac Enzyme Levels A 3 ml, early morning, fasting blood sample was ob- tained, allowed to clot, and centrifuged at 4˚C for 10 min (3500 rpm, centrifugal radius = 15 cm). The resulting serum was collected and frozen at −20˚C, until analyzed. The rate method was used to determine the concentra- tions of serum creatine kinase isoenzyme-MB (CK-MB) and lactate dehydrogenase isoenzyme-1 (LDH-1). Meas- urements were made using a kit purchased from Lanzhou Biochem Bio (lanzhou, China), in strict accordance with the manufacturer’s instructions. An automatic biochemi- cal analyzer (BS-400 type; Mindray Medical Instrumen- tation, Shenzhen, China) was used to take the measure- ments. 2.3. Statistical Analysis SPSS 17.0 software (IBM, Armonk, NY, USA) was used for statistical analyses. Homogeneity of variance among the groups was determined by single factor anal- ysis of variance and Tamhane variance analysis when heterogeneity of variance was observed. Differ- ences between 2 groups were compared using Student’s t-test, and the results obtained at different times, within each group, were compared using a paired t-test. Corre- lation analysis was performed using the Pearson’s linear corre- lation analysis. P < 0.05 was considered statistically sig- nificant. 3. RESULTS 3.1. Cardiac Structure, Function, and Myocardial Enzymes in Each Group The mPAP, RVID, RVOT, RVID/LVID ratio, Tei in- dex, CK-MB level, and LDH-1 level were significantly higher in severe AHAR group than in mild to moderate AHAR group, asymptomatic group or control group (Figure 2). No significant differences were evident in the LVID values (Figure 1). LVEF was significantly lower among severe AHAR group individuals than among those in mild to moderate AHAR group, asymptomatic group or control group. There was also a significant difference between mild to moderate AHAR group and asymptomatic group and control group; asymptomatic group was also signifi- cantly different from control group (P < 0.01) (Figure 2). 3.2. Relationship between AHAR with HADAR Among the 96 subjects observed, 59 demonstrated HADAR symptoms upon returning to the lower altitude. Among the 24 subjects with severe AHAR, 18 experi- enced severe HADAR (75.0%), 5 (20.8%) experienced light to moderate HADAR, and 1 (4.2%) did not experi- ence HADAR symptoms. Of the 47 subjects who suffer- ing from mild to moderate AHAR, 1 (2.1%) experienced severe HADAR symptoms, 32 (68.1%) experienced mild to moderate HADAR, and 14 (29.8%) did not experience HADAR. Of the 25 subjects not suffering from AHAR, the HADAR was mild to moderate in 3 individuals and did not occur in 22 (88.0%) subjects. A linear correlation analysis revealed that the total AHAR points and the Figure 2. Comparisons of Tei index, left cardiac function, and cardiac muscle enzyme levels among groups at 3700 m. LVEF: Left ventricular ejection fraction; Tei index: Cardiac muscle work index; CK-MB: Creatine kinase isoenzyme-MB; LDH- 1:Lactic dehydrogenase isoenzyme-1; Group A scores ≥ 16; Group B scores 5 - 15; Group C scores ≤ 4; Group D, normal controls at an altitude of 1500 m. ※※P < 0.01 vs Group D; ##P < 0.01 vs Group C; △△P < 0.01, vs Group B. Copyright © 2013 SciRes. OPEN ACCESS  S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 7 total HADAR points exhibited a significant positive cor- relation (r = 0.863, P < 0.001). 3.3. Relationship between HADAR Severity and Cardiac Structure, Function, and Cardiac Enzyme Levels after Returning to a Lower Altitude Upon returning to a lower altitude for 12 h, Group E individuals demonstrated mPAP, RVID, RVOT, RVID/ LVID ratio, Tei index, CK-MB levels, and LDH-1 levels that were significantly higher than those for subjects in mild to moderate Group, asymptomatic group, and con- trol group. LVEF was significantly lower in severe HADAR Group individuals than in individuals from mild to moderate HADAR Group, asymptomatic group, and control group; mild to moderate Group, asymptomatic group, and control group also demonstrated significant differences (P < 0.01). There were no significant differ- ences in LVID between the groups (Figure 3). After 15 d at low altitude, severe HADAR Group mPAP, RVID, RVOT, and RVID/LVID ratios were sig- nificantly higher than in mild to moderate HADAR Group, asymp- tomatic group, and control group (P < 0.05); there was also a significant difference between mild to moderate HADAR Group individuals and those in asymptomatic group and control group (P < 0.05), but not between as- ymptomatic group and control group. The LVEF, Tei index, CK-MB levels, and LDH-1 levels in each group did not demonstrate a significant differ- ence (P > 0.05). All data are shown in Figure 4. After returning to a lower altitude for 30 days, there were no significant differences among the indicators for Figure 3. Relationship between HADAR, cardiac structure, function, and mean pulmonary artery pressures 12 h after re- turning to 1500 m. mPAP: Mean pulmonary arterial pressure; RVID: Right ventricular internal dimension; RVOT: Outflow tract of right ventricle; LVID: Left ventricular internal dimen- sion; LVEF: Left ventricular ejection fraction; Tei index: Car- diac muscle work index; CK-MB: Creatine kinase isoenzyme- MB; LDH-1: Lactic dehydrogenase isoenzyme-1; Group E scores ≥ 26; Group F scores 6 - 25; Group G scores ≤ 5; Group D, normal controls at an altitude of 1500 m. ※※P < 0.01 vs Group D; ##P < 0.01 vs Group G; △△P < 0.01, vs Group F. any of the groups (Figure 5). The diameter of pulmonary artery and the right ven- tricular outflow tract in the plateau at fiftieth days was significantly larger than the diameter of pulmonary artery and the right ventricular outflow tract in 30 days after returned to lower altitude, furthermore, compared with 50 days the exposure to high altitude, the diameter of right ventricular outflow tract was significantly larger than the diameter of pulmonary artery in 30 days after returned to lower altitude, suggesting that the right ven- tricular recovery than pulmonary artery slower recovery (Figure 6). 4. DISCUSSION The results of this study showed that the right ven- tricular function, structure, myocardial enzyme level were significantly increased in the subjects with severe AHAR. In contrast, the left ventricular function was sig- nificantly lower. The responses of the human body to the high-altitude hypoxic environment and heavy physical work included more severe AHAR, pulmonary hyperten- sion, changes in the right ventricle, reduced cardiac func- tion and myocardial damage; but the changes in the left ventricular structure were less obvious. The reason may be that people engaged in heavy labor, in high-altitude, hypoxic environments, require a substantial increase in oxygen consumption. Therefore, when the body is ex- posed to a hypoxic environment, the hypoxia causes pulmonary vasoconstriction, pulmonary hypertension, and right ventricular load increase, leading to right ven- tricular enlargement. The AHAR severity, mPAP level, Figure 4. Relationship between HADAR, cardiac structure, function, and mean pulmonary artery pressure 15 d after re- turning to 1500 m. mPAP: Mean pulmonary arterial pressure; RVID: Right ventricular internal dimension; RVOT: Outflow tract of right ventricle; LVID: Left ventricular internal dimen- sion. LVEF: Left ventricular ejection fraction; Tei index: Car- diac muscle work index; CK-MB: Creatine kinase isoenzyme- MB; LDH-1: Lactic dehydrogenase isoenzyme-1; Group E scores ≥ 26; Group F scores 6 - 25; Group G scores ≤ 5; Group D, normal controls at an altitude of 1500 m. ※※P < 0.01 vs Group D; ##P < 0.01 vs Group G; △△P < 0.01, vs Group F. Copyright © 2013 SciRes. OPEN ACCESS  S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 8 Figure 5. Relationship between HADAR and right cardiac structure, function, and mean pulmonary artery pressure 30 d after returning to 1500 m. mPAP: Mean pulmonary arterial pressure; RVID: Right ventricular internal dimension; RVOT: Outflow tract of right ventricle; LVID: Left ventricular internal di- mension; Group E scores ≥ 26; Group F scores 6 - 25; Group G scores ≤ 5; Group D, normal controls at an altitude of 1500 m. and right ventricular size may be related to differences in individual tolerances and responses to hypoxia. In people with poor hypoxia tolerance, hypoxic mitochondrial ATP synthesis impairment in myocardial cells may result in a reduced myocardial energy supply, leading to obvious, severe symptoms. When hypoxia tolerance is relatively good, the inhibition of mitochondrial ATP synthesis is relatively mild, resulting in relatively mild heart damage and other symptoms [10-13]. For individuals who do not demonstrate AHAR, only sub-clinical effects on cardiac structure and function may occur. The results of this study also show that the total AHAR and HADAR points showed a significant positive correlation. Upon returning to the lower altitude, subjects in the severe HADAR group demonstrated mPAP, RVID, RVOT, RVID/LVID ratio, Tei index, CK-MB level, and LDH-1 level results that were significantly higher than among those in the other groups; LVEF was significantly lower than in the other groups. Among subjects in the mild to moderate HADAR group there were also similar differences between these individuals and those in the asymptomatic and control groups. After returning to a lower elevation for 15 d, individuals in the severe HA- DAR group continued to demonstrate mPAP, RVID, RVOT, and RVID/LVID ratio results that were signifi- cantly higher than those in the mild to moderate HADAR group, the asymptomatic HADAR group, or the control group. Subjects in the mild to moderate HADAR group also demonstrated significant differences in these pa- rameters as compared with subjects in the asymptomatic HADAR group or in the control group. However, there were no differences between the HADAR group and the control group. The LVEF, Tei index, CK-MB level, and LDH-1 level values were restored to the values observed (a) (b) (c) (d) Figure 6. Pulmonary artery diameter and right ventricular out- flow tract diameter return to lower altitude 30 days. (a) Pulmonary artery diameter up to 34 mm expsure 50 days under high altitude expansion environment; (b) Pulmonary artery diameter is 19 mm return to lower altitude 30 days; (c) Right ventricular outflow tract diameter up to 40 mm exposure 50 days under high altitude environment; (d) Right ventricular outflow tract diameter is 40 mm return to lower altitude 30 days. in the control group level after being at the low altitude for 30 days, The HADAR and AHAR severities were correlated with the degree of heart damage in these subjects. Those experiencing more severe AHAR while at the elevated elevation and having more significant HADAR symptoms upon their return to the low altitude experienced more severe structural damage to the right side of their hearts and a slower recovery time. After a period of adaptation to high-altitude, hypoxic environments, the return to low- altitude environments results in hypoxia-reoxygenation injuries. The mechanism may involve the following components. 1) Energy metabolism: enhanced myocar- dial tissue hypoxia results in anaerobic glycolysis and decreased ATP generation, resulting in a decrease in cellular energy supply. Hypoxic damage to the mito- chondria may result in the mitochondria not being effec- tive when aerobic conditions are restored to the myocar- dial cells [9]. 2) Reactive oxygen species generation: oxygen free radicals (OFR) involved in clearing tissue hypoxia may result in the generation of reactive oxygen species; restoration of oxygen may result in the genera- tion of a large number of OFR in the membranes of myocardial cells, resulting in the formation of lipid per- oxides that react with intracellular proteins and nucleic acids. These peroxides may cause structural and func- Copyright © 2013 SciRes. OPEN A CCESS  S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 9 tional changes to cells, leading to myocardial cell dam- age [14]. Zhang K, et al. [15] study confirmed, with en- hanced of myocardial injuries, the increased levels of Malondialdehyde (MDA), lactate dehydrogenase (LDH) and interleukin 6 (IL-6), the LOB (Chrysoeriol7-O- [-D-glucuronopyran-osyl-(1 → 2)-O–D-glucuronopy- ranoside]) can decreased plasma levels of MDA, LDH, IL-6, suggesting that the LOB could be a potential the- rapeutic agent for myocardial ischemia/reperfusion (I/R) injury and hypoxia/reoxygenation (H/R) injury. 3) Cal- cium overload: anaerobic glycolysis may enhance myo- cardial hypoxia, causing intracellular acidosis. As the extracellular pH gradually returns to normal after the restoration of normal oxygenation, intracellular and ex- tracellular formation of transmembrane pH gradients may result in enhanced sodium and hydrogen exchange, increasing the intracellular Na concentration. Since the cells generate less ATP after reoxygenation, the cell membrane and sarcoplasmic reticulum calcium and so- dium pump functions may be reduced, leading to intra- cellular calcium overload. The increase in the intracellu- lar calcium concentration can further activate endothelial cells, promoting OFR generation, and leading to further damage [16,17]. But Li Q [18] study shows that endocan- nabinoids can suppresses calcium overload through inhi- bition of INCX during perfusion with simulated ischemic solution; the effects may be mediated by CB2 receptor via PTX-sensitive Gi/o proteins. 4) High altitude hypoxia stress induced myocardial injury, restore oxygen after myocardial injury has not been fully restored, or restore later than other functions. Hu J, et al. [19] study show that simple plateau hypoxia exposure endothelin (ET)-1 α concentrations gradually increased whereas HIF-1 ex- pression in myocardial cells was significantly higher (P < 0.01). There was low pressure hypoxia exposure after myocardial mitochondria numbers were reduced during the initial phase of acute stress response to hypoxia and cellular injury but, later, mitochondrial numbers were restored to normal values. Plasma VEGF concentrations increased under exposure group hypoxia in low pressure hypoxia exposure, which were significantly higher than those of control group. Therefore Hu concluded that high-altitude hypoxia exposure: a) induced HIF-1 α ex- pression; b) prompted adaptation/acclimatization after initial stress and cellular injury; and c) enhanced VEGF expression. The mechanism of HADAR and hypoxia- reoxygenation injury on the body is extremely complex and requires further in-depth studies in order to more fully elucidate them. 5. ACKNOWLEDGEMENTS The authors thank the personnel from the 68303 Infantry brigade and 68303 Troop Hospital of the People’s Liberation Army for their assis- tance in this study. This work was funded by the National Science and Technology Ministry (Grant#2009BAI85B03) and Army Health Sub- ject (Grant# 2013BJZ032). 6. AUTHOR CONTRIBUTIONS Conceived and designed the experiments: Shengyue Yang, Qiquan Zhou; Performed the experiments: Enzhi Feng, Ziqiang Yan, Zhongxin Tian, He Yin, Zifu Shi; Analyzed the data: Shengyue Yang; Contributed reagents/ materials/analysis tools: Zifu Shi; Wrote the manuscript: Shengyue Yang, Qiquan Zhou; English translation: Yong Fan. REFERENCES [1] Fan, Y. and Zhou, Q. (2012) Research progress of de- adaptation to high altitude. Journal of Preventive Medi- cine of Chinese People’s Liberation Army, 30, 227-230. [2] He, B., Wang, J., Qian, G., Hu, M., Qu, X., Wei, Z., Li, J., Chen, Y., Chen, H., Zhou, Q. and Wang, G. (2013) Analy- sis of high-altitude de-acclimatization syndrome after ex- posure to high altitudes: A cluster-randomized controlled trial. PLoS One, 8, e62072. http://dx.doi.org/10.1371/journal.pone.0062072 [3] Zhou, Q.Q., Yang, S.Y., Luo, Y.J., Qi, Y.S., Yan, Z.Q., Shi, Z.F. and Fan, Y. (2012) A randomly-controlled study on the cardiac function at the early stage of return to the plains after short-term exposure to high altitude. PLoS One, 7, e31097. [4] Shi, Z., Zhou, Q., Xiang, L., Ma, S., Yan, C. and Luo, H. (2011) Three preparations of compound Chinese herbal medicines for de-adaptation to high altitude: A random- ized, placebo-controlled trial. Journal of Chinese Integra- tive Medicine, 9, 395-401. http://dx.doi.org/10.3736/jcim20110408 [5] Zhou, Q., Yang, S., Yuan, Z., Wang, Y., Zhang, X., Gao, W., Shi, Z., Yang, Y., Wu, Y., Fan, Y., Wang, G. and Gao, Y. (2012) A research in diagnostic criteria of high altitude de-adaptation for plateau migrants returning to the plains: a multicenter, randomized controlled trial. Medical Jour- nal of Chinese People’s Liberation Army, 37, 146-155. [6] West, J.B. (2010) English translation of “Nomenclature, classification, and diagnostic criteria of high altitude dis- ease in China”. High Altitude Medicine & Biology, 11, 169-172. http://dx.doi.org/10.1089/ham.2010.1014 [7] Simpson, J., Miller, O., Bell, A., Bellsham-Revell, H., McGhie, J. and Meijboom, F. (2012) Image orientation for three-dimensional echocardiography of congenital heart disease. The International Journal of Cardiovascu- lar Imaging, 28, 743-753. http://dx.doi.org/10.1007/s10554-011-9893-3 [8] Fakhri, A.A., Hughes-Doichev, R.A., Biederman, R.W. and Murali, S. (2012) Imaging in the evaluation of pul- monary artery hemodynamics and right ventricular struc- ture and function. Heart Failure Clinics, 8, 353-372. http://dx.doi.org/10.1016/j.hfc.2012.04.004 [9] Zhao, S., Deng, Y.B., Chen, X.L. and Liu, R. (2012) As- Copyright © 2013 SciRes. OPEN A CCESS  S. Y. Yang et al. / Occupational Diseases and Environmental Medicine 1 (2013) 4- 10 Copyright © 2013 SciRes. OPEN A CCESS 10 sessment of right ventricular function in recipient twin of twin to twin transfusion syndrome with speckle tracking echocardiography. Ultrasound in Medicine and Biology, 38, 1502-1507. http://dx.doi.org/10.1016/j.ultrasmedbio.2012.05.009 [10] Li, B., Liu, J. and Chen, L. (2005) Changes of adenylate content and distribution in myocardium and mitochondria of rats after hypoxic exposure. Medical Journal of Na- tional Defending Forces In Northwest China, 26, 90-92. [11] Li, J. and Xing, L. (2012) The effects of simulated 3500 m different hypoxic training on free radical metabolism and respiratory chain function of mitochondrial in myo- cardium after exhaustive running in rat. Journal of Shanghai Physical Education Institute, 36, 51-55. [12] Rozova, K.V. (2008) Effect of normo-and hypobaric hy- poxia on ultrastructure of the lung and myocardial tissue. Fiziolohichnyi Zhurnal, 54, 63-68. [13] Zhao, Y. and Ao, H. (2011) Research progress of myocar- dial ischemia reperfusion injury. Chinese Circulation Journal, 26, 396-398. [14] Kin, J.K., Pedram, A., Razandi, M. and Levin, E.R. (2006) Estrogen prevents cardiomyocyte apoptosis through inhi- bition of reactive oxygen species and differential regula- tion of p38 kinase isoforms. Journal of Biological Chem- istry, 281, 6760-6767. http://dx.doi.org/10.1074/jbc.M511024200 [15] Zhang, K., Bai, Y., Song, T. and Zhang, G. (2013) In vivo and in vitro evidence of protective effects of a natural flavone on rat myocardial ischemia-reperfusion and hy- poxia-reoxygenation injuries. Journal of Cardiovascular Pharmacology and Therapeutics, 18, 31-36. http://dx.doi.org/10.1177/1074248412461713 [16] Feygin, J., Hu, Q., Swingen, C. and Zhang, J. (2008) Relationships between regional myocardial wall stress and bioenergetics in hearts with left ventricular hypertro- phy. American Journal of Physiology: Heart and Circu- latory Physiology, 294, H2313-H2321. http://dx.doi.org/10.1152/ajpheart.01288.2007 [17] Zhang, D.W., Bian, Z.P., Xu, J.D., Wu, H.F., Gu, C.R., Zhou, B., Chen, X.J. and Yang, D. (2012) Astragaloside IV alleviates hypoxia/reoxygenation-induced neonatal rat cardiomyocyte injury via the protein kinase A pathway. Pharmacology, 90, 95-101. http://dx.doi.org/10.1159/000339476 [18] Li, Q., Cui, N., Du, Y., Ma, H. and Zhang, Y. (2013) An- andamide reduces intracellular Ca2+ concentration through suppression of Na+/Ca2+ exchanger current in rat cardiac myocytes. PLoS One, 8, e63386. http://dx.doi.org/10.1371/journal.pone.0063386 [19] Hu, J., Wang, Q.J., Hu, Y.H. and Li, Y.F. (2012) A study of high-altitude hypoxia-induced cell stress in murine model. Cell Biochemistry and Biophysics, 64, 85-88. http://dx.doi.org/10.1007/s12013-012-9374-x
|