 Vol.3, No.4, 343-353 (2013) Open Journal of Animal Sciences http://dx.doi.org/10.4236/ojas.2013.34051 Evaluation of the toxic effect of insecticide chlorantraniliprole on the silkworm Bombyx mori (Lepidoptera: Bombycidae) Roxelle Ethienne Ferreira Munhoz1, Thaís Souto Bignotto1, Naiara Climas Pereira1, Cláudia Regina das Neves Saez1, Rafaela Bespalhuk1, Verônica Aureliana Fassina1, Graziele Milani Pessini1, Mayarha Patrícia Dequigiovanni Baggio2, Lucinéia de Fátima Chasko Ribeiro2, Rose Meire Costa Brancalhão2, Shunsuke Mizuno3, Willian Shigeaki Aita3, Maria Aparecida Fernandez1* 1Departamento de Biotecnologia, Genética e Biologia Celular, Universidade Estadual de Maringá, Maringá, Brasil; *Corresponding Author: aparecidafernandez@gmail.com 2Universidade Estadual do Oeste do Paraná, Cascavel, Brasil 3Fiação de Seda Bratac S. A. Bastos, São Paulo, Brasil Received 10 September 2013; revised 15 October 2013; accepted 21 October 2013 Copyright © 2013 Roxelle Ethienne Ferreira Munhoz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The silkworm Bombyx mori feeds exclusiv ely on mulberry leaves and is highly sensitive to pesti- cides in general. Although mulberry plantations are free of agrochemicals, pesticide drift can occur. Chlorantraniliprole, a novel insecticide of the anthranilic diamides class, has been used to control pests in field crops. In this study, we investigated the biological effects of different concentrations of chlorantraniliprole on B. mori silkworm commercial Brazilian hybrids. To eva- luate the toxicity of chlorantraniliprole, bio- as- says were carried out and data on the lethal concentrations, symptomatology, morphology and variables of silk production were collected. Results indicated that B. mori is extremely sen- sitive to chlorantraniliprole, even in low con- centrations. The highest silkworm mortality rates were observed in the two highest chloran- traniliprole concentrations, 0.2 and 0.1 ppm. Al- though lower chlorantraniliprole concentrations did not cause death of all the silkworm larvae, various symptoms of toxicity were observed: feeding cessation, regurgitation, late develop- ment and incomplete ecdysis. Such symptoms reflect the morphological changes we observed in the midgut epithelium, which affected nutrient uptake and metabolism, and even the produc- tion of cocoons. Exposed larvae also produced thin-shelled cocoons, which constitutes a seri- ous economic problem because this type of cocoon is not useful for the silk industry. The results provided herein confirm the toxicity of chlorantraniliprole in silkworm larvae. There- fore, we strongly suggest that, competent au- thorities of the National Health Surveillance Agency, in pesticide management should take measures to reduce or eliminate the use of chlo- rantraniliprole in areas nearest to silkworm cul- tivation. Keywords: Silkworm, Pesticide Drift; Mortality; Symptomatology; Chlorantraniliprole 1. INTRODUCTION The silkworm Bombyx mori L. (Lepidoptera: Bomby- cidae), an insect originally from China, was domesticated 5000 years ago for silk production [1]. Silkworms are no longer found in nature and are totally dependent on man to survive. Bombyx mori most likely evolved from B. mandarina, a wild silkworm species with similar charac- teristics and reproduction to B. mori [2]. Silkworms are herbivorous and feed exclusively on fresh mulberry leaves during the larval phase [3]. The practice of sericulture includes mulberry cultiva- tion, egg production, larvae creation and maintenance until cocoon production and, finally, silk yard production by the industrial sector. Brazil’s weather is favorable for Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 344 cultivating both mulberry and silkworms, which makes sericulture a good alternative for this country, especially for small farmers. Bombyx mori is highly susceptible to insecticides, and the application of insecticides near sericulture projects is not advisable because the silkworms can be harmed by chemicals on the leaves, either through consumption of contaminated leaves or through other contact with the insecticides [4]. Hence, mulberry plantations intended for silkworms are free of pesticides. However, the com- plete elimination of pesticide drift is impossible and in- secticides that are employed in other crops, especially those that are applied through aerial spraying, can dam- age sericulture activity. Chlorantraniliprole is a novel insecticide belonging to a potent class of anthranilic diamides that is usually safe for mammals [5] and is commonly used in rice, coffee, sugar cane, apple and peach crops. Chlorantraniliprole eliminates Lepidoptera insects [6], as well as other pest orders such as Coleoptera, Diptera, Isoptera and Hemip- tera [7]. This insecticide, once ingested, acts as a selec- tive agonist for ryanodine receptors, a class of intracel- lular calcium channels. Ryanodine receptors play an im- portant role in muscle function because the contrac- tion of muscle cells requires exact liberation of calcium from intracellular deposits in the cytoplasm. Chloran- traniliprole causes unregulated Ca2+ release from the sarcoplasmic reticulum of muscle cells [8], which im- pedes the insect’s ability to regulate muscle function and leads to permanent muscle contraction. Poisoning symp- toms include rapid cessation of feeding, lethargy, regur- gitation, muscle paralysis and, ultimately, insect death [9-11]. The effects of chlorantraniliprole in different insects have been well-studied in many taxa: Choristoneura ro- saceana [12], termites Reticulitermes flavipes [13], ho- ney bees Apis mellifera and bumble bees Bombus ter- restris [8] and Leptinotarsa decemlineata [14]. How- ever, little information is described about the effects of chlorantraniliprole on B. mori [11]. Although this insec- ticide is not used in mulberry fields, it can cause loss of cocoon production in Brazilian silkworm farms near ar- eas where chlorantraniliprole is used. Therefore, in this study, we investigated the effects of different concentra- tions of chlorantraniliprole on commercial Brazilian hy- brids of the B. mori silkworm. Recently, big crops such as sugarcane are using new kinds of insecticide to do the control of Diatrea sac- charalis larvae in Paraná State, Brazil. The areas of mulberry plantation, which are close to the sugarcane crops, are affected by aerial spraying of the insecticide, which results in B. mori larvae death. Studies made by the agriculture secretary of Paraná State show a loss of cocoons equal to 8,626 kilograms. This State is the big- gest producer of cocoons from Brazil, responsible for 92% of the total production in this country and due to this recent problem with cloranthraliniprole insecticide. This work was developed with the intention of describing the symptomatology of the intoxication in B. mori larvae that were exposed to this insecticide. The description of this symptom induced in laboratory can help the com- prehension of the toxic effect caused by cloranthralini- prole in the larvae, which has a commercial interest for several countries. Those data can further help the Brazil- ian sericulture, as the results can assist either the farmer who works with sericulture or the company behind all the production. 2. MATERIAL AND METHODS 2.1. Bombyx mori Culture The bioassays were carried out at the Fiação de Seda BRATAC laboratory at Bastos city, São Paulo, Brazil with the commercial hybrids of B. mori from this com- pany. Silkworm larvae were reared under hygienic conp- ditions and in controlled environments. Rearing rooms were adjusted to 28˚C ± 0.5˚C and 90% ± 5 % of relative humidity (RH) for the creation of 1st instar silkworm larvae, while 2nd instar larvae were reared at 27˚C ± 0.5˚C and with 85% ± 5 % of RH. Larvae of the 3rd, 4th and 5th instars were reared at 25 to 26˚C ± 0.5˚C and with RH of 70% ± 5 %. Artificial white lighting was employed in the rearing rooms to submit silkworm larvae to four- teen-hour daily of photoperiod with 10 hours of dark. During the experiments, larval mortality and symptoms of exposure were recorded. 2.2. Lethal Concentration Treatments consisted of three replicates, with 20 silk- worm larvae each. The following chlorantraniliprole concentrations, in ppm (parts per million), were used: 0.2, 0.1, 0.05, 0.025, 0.0125, 6.25 × 10−3, 3.13 ×10−3 and 1.57 × 10−3. Control groups consisted of silkworm larvae that were fed exclusively on mulberry leaves free of chloran- traniliprole. Silkworm larvae were exposed once to mulberry leaves containing chlorantraniliprole at different days and different instars, depending on the treatment, as fol- lows: 1st day of the 1st instar, 2nd day of the 1st instar, 1st day of the 2nd instar, 3rd day of the 2nd instar, 1st day of the 3rd instar, 3rd day of the 3rd instar, 1st day of the 4th instar, 3rd day of the 4th instar, 1st day of the 5th instar and 5th day of the 5th instar. Besides, bioassays also included 5th instar larvae that were exposed twice to chloran- tra- niliprole: on the 1st and 2nd days of the 5th instar and on the 5th and 6th days of the 5th instar. For the lethal concentration bioassays, mulberry leaves were immersed in aqueous solutions containing dif- Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 345 ferent concentrations of chlorantraniliprole, as mentioned above. Leaves were dried at room temperature and then offered to B. mori larvae. Each replicate included 20 silkworm larvae that were fed once a day in petri-dishes with different amounts of mulberry leaves containing chlorantraniliprole, as follows: larvae exposed to the insecticide in the 1st or 2nd days of the 1st instar were fed with 7 cm2 of mulberry leaves, while larvae exposed in the 1st or 3rd days of the 2nd instar were fed with 16 cm2; larvae in the 1st or 3rd days of the 3rd instar were fed with 10 g of mulberry leaves and those in the 1st or 3rd days of the 4th instar and in the 1st or 5th days of the 5th instar were fed with 30 g of mulberry leaves. Treatments in- volving silkworm larvae exposed twice to chloran- trani- liprole were fed with 30 g of mulberry leaves when ex- posure occurred in the 1st and 2nd days of the 5th instar and with 100 g of mulberry leaves when exposure oc- curred in the 5th and 6th days of the 5th instar. Before and after chlorantraniliprole exposure, larvae were fed in abundance with mulberry leaves free of chlorantrani- liprole. Mulberry trees used in the bioassays have been grown in the experimental field of the company Fiação de Seda BRATAC, Bastos city in São Paulo state, Brazil. Mul- berry leaves used for silkworm feeding were taken from 75-days mulberry trees. Silkworm larvae of the 1st, 2nd and 3rd instars were fed with young mulberry leaves tak- en from the top part of the principal branche, while old leaves were offered for 4th and 5th silkworm larvae. 2.3. Symptomatology After chlorantraniliprole applications, symptoms re- lated to insecticide exposure were registered immediately after the first day of exposure: feeding cessation, regur- gitation, late development and irregular ecdysis. These symptoms were classified according to the intensity, where 0 represents the absence of symptoms and 5 re- presents the highest level of the symptom. In addition, the following productive variables were collected: mean number of normal cocoons (NC), mean weight of normal cocoon (NCW), mean number of thin-shelled cocoons (TSC), mean weight of thin-shelled cocoons (TSCW) and mean number of live pupae (LP). Although no meas- urements were taken to distinguish thin-shelled cocoons from normal cocoons, thin-shelled cocoons can be visu- ally and unambiguously differentiated from normal co- coons since their shells are thinner and translucent. 2.4. Morphological Analysis The effects of chlorantraniliprole in B. mori midgut tissue were evaluated. Larvae of 5th instar were exposed to two different concentrations of this insecticide: 0.2 ppm and 0.1 ppm, in addition to the control group. On the 4th day after exposure to chlorantraniliprole, larvae were anesthetized and dissected. Segments of the midgut wall were removed and fixed in DuBosq Brasil, dehy- drated in ethanol and preserved in paraffin. Sequential cuts were made on an Olympus CUT4055 microtome at thicknesses ranging between 5 and 7 µm. Sections were stained with hematoxylin-eosin for general tissue analy- sis. Images were obtained with on Olympus BX 60 pho- tomicroscope. 2.5. Statistical Analysis The experimental delineation was completely ran- domized with three replicates per treatment (total 108 treatments). Twenty silkworm larvae were used per rep- licate. The data were compared with an analysis of vari- ance, followed by the Scott-Knott means test (0.05%) using the statistical software SISVAR 5.3 [15]. 3. RESULTS AND DISCUSSION This insecticide is frequently applied by spray in sug- arcane plantations next at silkworm producers in south of Brazil, causing damaging and lost of cocoon production. In the last years, Brazilian silkworm producers have been reporting great losses in cocoon production. Apparently, these losses are related to the period of time when insec- ticides are aerial sprayed in crops near silkworm farms. Recently, chlorantraniliprole has been employed in crops to eliminate Lepidoptera insects, especially sugarcane crops. In this study, we performed bioassays to verify if the symptoms caused by chlorantraniliprole in silkworm larvae are similar to those observed by producers in silkworm farms. To evaluate the toxicity and symptoma- tology of chlorantraniliprole in Brazilian hybrids of B. mori, we exposed groups of silkworm larvae to different concentrations of chlorantraniliprole. 3.1. Lethal Chlorantraniliprole Concentration We analyzed the mortality of silkworm larvae after they were exposed to eight different concentrations of chlorantraniliprole (Table 1). The two highest chloran- traniliprole concentrations, 0.2 ppm and 0.1 ppm, re- sulted in 100% larval mortality in most silkworm larvae instars, though not when exposure occurred on the 1st and 3rd day of the 2nd instar. Complete larval mortality was also observed in the groups exposed to 0.05 ppm in the 5th day of the 5th instar and in the 5th and 6th days of the 5th instar. Silkworm larvae that were exposed to a con- centration of 0.025 ppm on the 5th and 6th days of the 5th instar also had a high mortality rate of 98.33%. Overall, exposures of silkworm larvae on the 5th and 6th days of the 5th instar to the four highest chlorantraniliprole con- centrations resulted in a 100% rate of larval mortality, Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 Copyright © 2013 SciRes. OPEN ACCESS 346 Table 1. Bombyx mori larvae mortality considering instar, day of exposure and concentration of the insecticide chlorantraniliprole. Instar Day of exposure Chlorantraniliprole concentration (ppm) Mortality (%) 1st instar 1st day 0.2 100.00 a 1st instar 1st day 0.1 100.00 a 1st instar 1st day 0.05 80.00 b 1st instar 1st day 0.025 0.00 f 1st instar 1st day 0.0125 0.00 f 1st instar 1st day 6.25 × 10−3 0.00 f 1st instar 1st day 3.13 × 10−3 0.00 f 1st instar 1st day 1.57 × 10−3 0.00 f 1st instar 1st day Control group 0.00 f 1st instar 2nd day 0.2 100.00 a 1st instar 2nd day 0.1 100.00 a 1st instar 2nd day 0.05 96.66 a 1st instar 2nd day 0.025 0.00 f 1st instar 2nd day 0.0125 0.00 f 1st instar 2nd day 6.25 × 10−3 0.00 f 1st instar 2nd day 3.13 × 10−3 0.00 f 1st instar 2nd day 1.57 × 10−3 0.00 f 1st instar 2nd day Control group 0.00 f 2nd instar 1st day 0.2 100.00 a 2nd instar 1st day 0.1 85.00 b 2nd instar 1st day 0.05 0.00 f 2nd instar 1st day 0.025 0.00 f 2nd instar 1st day 0.0125 0.00 f 2nd instar 1st day 6.25 × 10−3 0.00 f 2nd instar 1st day 3.13 × 10−3 0.00 f 2nd instar 1st day 1.57 × 10−3 0.00 f 2nd instar 1st day Control group 0.00 f 2nd instar 3rd day 0.2 88.33 b 2nd instar 3rd day 0.1 55.00 c 2nd instar 3rd day 0.05 0.00 f 2nd instar 3rd day 0.025 0.00 f 2nd instar 3rd day 0.0125 0.00 f 2nd instar 3rd day 6.25 × 10−3 0.00 f 2nd instar 3rd day 3.13 × 10−3 0.00 f 2nd instar 3rd day 1.57 × 10−3 0.00 f 2nd instar 3rd day Control group 0.00 f 3rd instar 1st day 0.2 100.00 a 3rd instar 1st day 0.1 100.00 a 3rd instar 1st day 0.05 5.00 f 3rd instar 1st day 0.025 0.00 f 3rd instar 1st day 0.0125 0.00 f 3rd instar 1st day 6.25 × 10−3 0.00 f 3rd instar 1st day 3.13 × 10−3 0.00 f  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 347 Continued 3rd instar 1st day 1.57 × 10−3 0.00 f 3rd instar 1st day Control group 0.00 f 3rd instar 3rd day 0.2 100.00 a 3rd instar 3rd day 0.1 98.33 a 3rd instar 3rd day 0.05 33.33 d 3rd instar 3rd day 0.025 13.33 e 3rd instar 3rd day 0.0125 0.00 f 3rd instar 3rd day 6.25 × 10−3 0.00 f 3rd instar 3rd day 3.13 × 10−3 0.00 f 3rd instar 3rd day 1.57 × 10−3 0.00 f 3rd instar 3rd day Control group 0.00 f 4th instar 1st day 0.2 100.00 a 4th instar 1st day 0.1 100.00 a 4th instar 1st day 0.05 3.33 f 4th instar 1st day 0.025 1.66 f 4th instar 1st day 0.0125 3.33 f 4th instar 1st day 6.25 × 10−3 6.66 f 4th instar 1st day 3.13 × 10−3 1.66 f 4th instar 1st day 1.57 × 10−3 3.33 f 4th instar 1st day Control group 0.00 f 4th instar 3rd day 0.2 100.00 a 4th instar 3rd day 0.1 100.00 a 4th instar 3rd day 0.05 15.00 e 4th instar 3rd day 0.025 5.00 f 4th instar 3rd day 0.0125 0.00 f 4th instar 3rd day 6.25 × 10−3 5.00 f 4th instar 3rd day 3.13 × 10−3 1.66 f 4th instar 3rd day 1.57 × 10−3 3.33 f 4th instar 3rd day Control group 1.66 f 5th instar 1st day 0.2 100.00 a 5th instar 1st day 0.1 100.00 a 5th instar 1st day 0.05 13.33 e 5th instar 1st day 0.025 3.33 f 5th instar 1st day 0.0125 3.33 f 5th instar 1st day 6.25 × 10−3 1.66 f 5th instar 1st day 3.13 × 10−3 3.33 f 5th instar 1st day 1.57 × 10−3 1.66 f 5th instar 1st day Control group 0.00 f 5th instar 5th day 0.2 100.00 a 5th instar 5th day 0.1 100.00 a 5th instar 5th day 0.05 100.00 a 5th instar 5th day 0.025 56.66 c 5th instar 5th day 0.0125 1.66 f 5th instar 5th day 6.25 × 10−3 0.00 f Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 Copyright © 2013 SciRes. OPEN ACCESS 348 Continued 5th instar 5th day 3.13 × 10−3 1.66 f 5th instar 5th day 1.57 × 10−3 0.00 f 5th instar 5th day Control group 0.00 f 5th instar 1st - 2nd days 0.2 100.00 a 5th instar 1st - 2nd days 0.1 100.00 a 5th instar 1st - 2nd days 0.05 56.66 c 5th instar 1st - 2nd days 0.025 3.33 f 5th instar 1st - 2nd days 0.0125 1.66 f 5th instar 1st - 2nd days 6.25 × 10−3 0.00 f 5th instar 1st - 2nd days 3.13 × 10−3 0.00 f 5th instar 1st - 2nd days 1.57 × 10−3 0.00 f 5th instar 1st - 2nd days Control group 0.00 f 5th instar 5th - 6th days 0.2 100.00 a 5th instar 5th - 6th days 0.1 100.00 a 5th instar 5th - 6th days 0.05 100.00 a 5th instar 5th - 6th days 0.025 98.33 a 5th instar 5th - 6th days 0.0125 0.00 f 5th instar 5th - 6th days 6.25 × 10−3 5.00 f 5th instar 5th - 6th days 3.13 × 10−3 0.00 f 5th instar 5th - 6th days 1.57 × 10−3 3.33 f 5th instar 5th - 6th days Control group 0.00 f Overall mean 28.34 VC (%) 23.78 (a-f) in the columns are different by the Scott-Knott test (P < 0.05). which indicates that larvae are more susceptible to the detrimental effects of this insecticide under such condi- tions. Exposure to chlorantraniliprole at a concentration of 0.05 ppm resulted in variable rates of mortality, as seen in Table 1. The highest silkworm larvae mortality (96.66%) occurred when the insecticide was applied once on the 2nd day of the 1st instar, on the 5th day of the 5th instar or twice on the 5th and 6th days of the 5th instar. High mortality (80%) was also observed when exposure occurred on the 1st day of the 1st instar. On the other hand, the lowest rates of larval mortality were observed follow- ing exposure on the 1st or 3rd days of the 2nd instar, the 1st day of the 3rd instar or on the 1st day of the 4th instar. Few larvae died when they were exposed to a concen- tration of 0.05 ppm of chlorantraniliprole in the early days of the instar. This finding can be explained by the fact that, once ecdysis occurs, silkworm larvae are le- thargic and eat less than in the last days of each instar. When chlorantraniliprole exposure occurred in the last days of an instar, the larvae likely consumed more con- taminated leaves and therefore suffered a higher mortal- ity rate. The other chlorantraniliprole concentrations (0.025, 0.0125, 6.25 × 10−3, 3.13 × 10−3 and 1.57 × 10−3 ppm) resulted in lower or no silkworm larval mortality. An exception occurred when larvae were exposed to chlor- antraniliprole at a concentration of 0.025 ppm on the 5th day of the 5th instar or on the 5th and 6th days of the 5th instar, which resulted in 56.66% and 98.33% larval mor- tality rates, respectively. These lower concentrations are not generally fatal to B. mori larvae, but they may cause damage to silkworms, in the end days of the last instar like discussed above. High concentrations of chlorantraniliprole, such as 0.2 ppm and 0.1 ppm, caused 100% larval mortality in most of the larval instars, except when the insecticide was applied in the 1st day of the 2nd instar, in this case only for 0.1 ppm, or in the 3rd day of the 2nd instar (Table 1 ). Although lower concentrations of this insecticide cause little or no larval mortality, they still result in damage such as cessation of feeding and production of a thin- shelled cocoon, which is economically not viable. Hence, our results demonstrated that Brazilian hybrids of the B. mori silkworm are extremely sensitive to chloran- trani- liprole. In experiments conducted in China was de- tected a high sensitivity to chlorantraniliprole in the B. mori silkworm and identified a threshold value of LC50 at 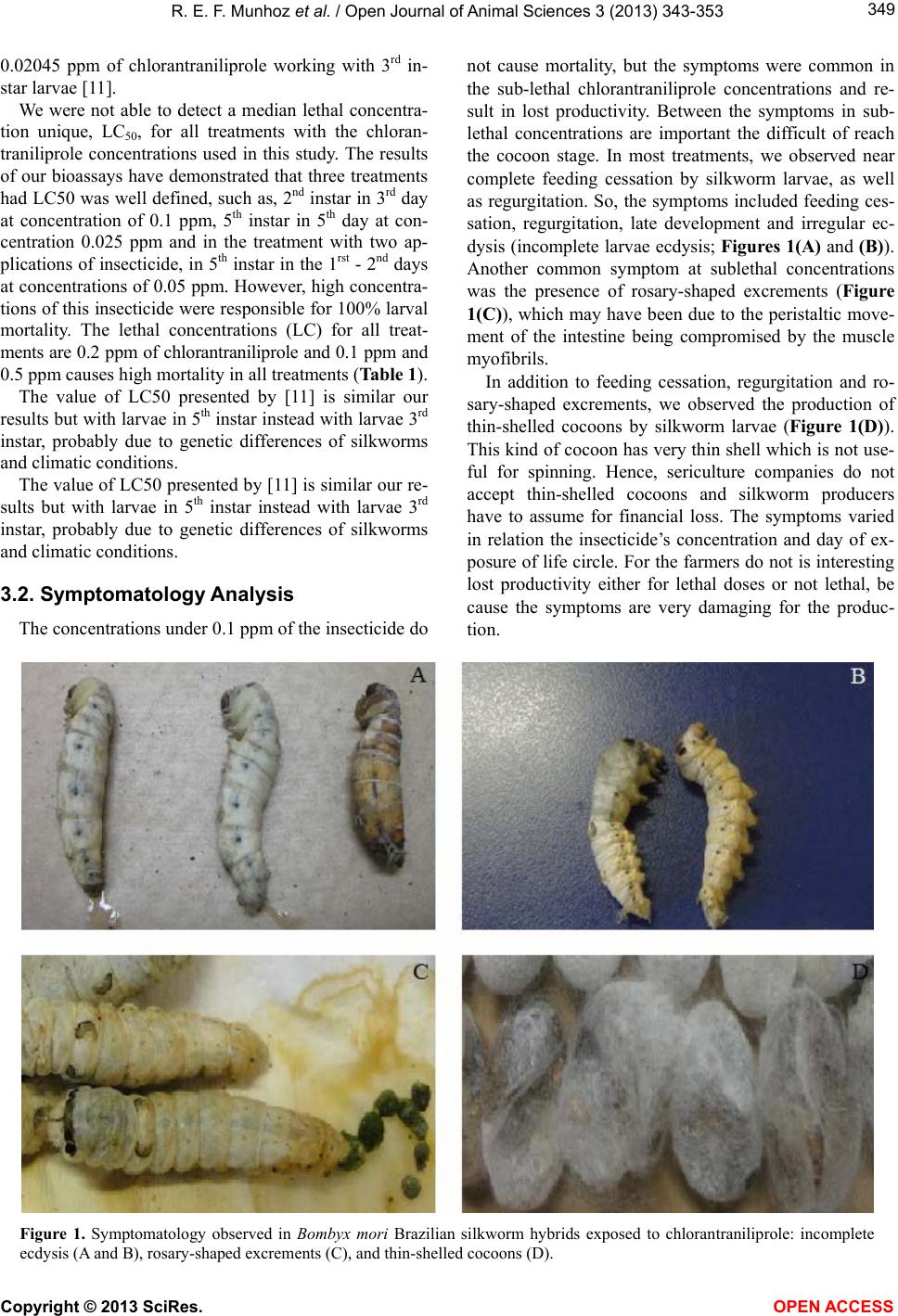 R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 349 0.02045 ppm of chlorantraniliprole working with 3rd in- star larvae [11]. We were not able to detect a median lethal concentra- tion unique, LC50, for all treatments with the chloran- traniliprole concentrations used in this study. The results of our bioassays have demonstrated that three treatments had LC50 was well defined, such as, 2nd instar in 3rd day at concentration of 0.1 ppm, 5th instar in 5th day at con- centration 0.025 ppm and in the treatment with two ap- plications of insecticide, in 5th instar in the 1rst - 2nd days at concentrations of 0.05 ppm. However, high concentra- tions of this insecticide were responsible for 100% larval mortality. The lethal concentrations (LC) for all treat- ments are 0.2 ppm of chlorantraniliprole and 0.1 ppm and 0.5 ppm causes high mortality in all treatments (Table 1). The value of LC50 presented by [11] is similar our results but with larvae in 5th instar instead with larvae 3rd instar, probably due to genetic differences of silkworms and climatic conditions. The value of LC50 presented by [11] is similar our re- sults but with larvae in 5th instar instead with larvae 3rd instar, probably due to genetic differences of silkworms and climatic conditions. 3.2. Symptomatology Analysis The concentrations under 0.1 ppm of the insecticide do not cause mortality, but the symptoms were common in the sub-lethal chlorantraniliprole concentrations and re- sult in lost productivity. Between the symptoms in sub- lethal concentrations are important the difficult of reach the cocoon stage. In most treatments, we observed near complete feeding cessation by silkworm larvae, as well as regurgitation. So, the symptoms included feeding ces- sation, regurgitation, late development and irregular ec- dysis (incomplete larvae ecdysis; Figures 1(A) and (B)). Another common symptom at sublethal concentrations was the presence of rosary-shaped excrements (Figure 1(C)), which may have been due to the peristaltic move- ment of the intestine being compromised by the muscle myofibrils. In addition to feeding cessation, regurgitation and ro- sary-shaped excrements, we observed the production of thin-shelled cocoons by silkworm larvae (Figure 1(D)). This kind of cocoon has very thin shell which is not use- ful for spinning. Hence, sericulture companies do not accept thin-shelled cocoons and silkworm producers have to assume for financial loss. The symptoms varied in relation the insecticide’s concentration and day of ex- posure of life circle. For the farmers do not is interesting lost productivity either for lethal doses or not lethal, be cause the symptoms are very damaging for the produc- tion. Figure 1. Symptomatology observed in Bombyx mori Brazilian silkworm hybrids exposed to chlorantraniliprole: incomplete ecdysis (A and B), rosary-shaped excrements (C), and thin-shelled cocoons (D). Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 350 We observed symptoms similar to those found by [11]. They also reported that symptoms emerged very quickly, in agreement with our observations. The ob- served symptoms are most likely related to the unregu- lated calcium release, which controls muscle myofibril activity [9], and alterations in feeding lead to changes in the conversion of ingested and digested food, which may eventually cause abnormalities in subsequent develop- ment [16]. We also evaluated productive variables of silkworm cocoons and pupae. Analyses were conducted only for treatments in which exposure to chlorantraniliprole oc- curred in 4th and 5th silkworm instars; treatments with concentrations equal to 0.2 ppm and 0.1 ppm were ex- cluded from the analysis because they were lethal, caus- ing 100% of larval mortality. Table 2 illustrates the data obtained for the following variables: mean number of normal cocoons (NC), mean weight of normal cocoon (NCW), mean number of thin-shelled cocoons (TSC), mean weight of thin-shelled cocoons (TSCW) and mean number of live pupae (LP). In general, there was little variation among treatments. There was no statistical variation among treatments in which chlorantraniliprole exposure occurred in the 4th larval. When the larvae were exposed during later stages of the 5th instar, there was greater statistical variation, mainly in the NC, NCW and LP variables. After expo- sure on the 1st day of the 5th instar, there were no signifi- cant differences and only modest variation in the variable NCW. Low values of NCW were observed at chloran- traniliprole concentrations of 0.025 ppm and 0.0125 ppm, with values equal to 2.23 and 2.26 g, respectively. When exposure to chlorantraniliprole occurred on the 5th day of the 5th instar, the response was variable in each variable except TSCW. A chlorantraniliprole concentra- tion of 0.05 ppm resulted in the production of only thin-shelled cocoons by the silkworms, so the variables NC, NCW and LP could not be assessed. The NC, NCW, TSC and LP for the groups exposed to 0.025 ppm were significantly different from the other chlorantraniliprole concentrations, and their values were 6.66 cocoons, 2.13 g, 7.66 cocoons and 8.66 pupae, respectively. Treatments with chlorantraniliprole also involved two exposures of this insecticide at different ages of the 5th instar. When chlorantraniliprole was applied twice in the 5th instar, on the 1st and 2nd days, there was no variation among NCW, TSC and, consequently, TSCW in the treatments. The NC and the LP, had the lowest values in the treatment with chlorantraniliprole at a concentration of 0.05 ppm, reaching values of 8.33 cocoons and 11.33 pupae, respectively. Finally, our experiment also evaluated exposure to chlorantraniliprole twice on the 5th and 6th days of the 5th instar. At a concentration of 0.05 ppm, there was total mortality, and no further data are available. NC, NCW and LP varied at the chlorantraniliprole concentration of 0.025 ppm and had the overall lowest values at this con- centration: 0.33 cocoons, 0.70 g and 0.33 pupae, respec- tively. It is important to note that the TSCW was less than half of the NCW for most of the treatments. More- over, the NC decreased significantly when high concen- trations of chlorantraniliprole were applied on the 5th day of the 5th instar. 3.3. Morphological Alterations The morphological features of the columnar epithelial cells in the midgut of B. mori larvae were altered by chlorantraniliprole exposure. The apical columnar cell surface exhibited protrusions in addition to the con- spi- cuous microvilli related to apocrine vesicles. Some of these vesicles contained cell nuclei (Figures 2(B) and (D); black arrows). Cell fragments enclosed by a membrane with mor- phology similar to apoptotic bodies were observed along the midgut epithelium. The term apoptotic body is often used during the process of death by classical apoptosis to define the membrane-bound portion of the cell contain- ing nuclear and cytoplasmic residue from fragmentation during apoptosis [17]. The presence of the nucleus in the structures observed in light microscopy was confirmed to be the result of the death of epithelial cells of the midgut. Columnar cells are most abundant in the midgut epithe- lium of insects and are responsible for processing food, secreting digestive enzymes and absorbing the final di- gestion products [18]. Their malfunction affects the entire metabolism, in- cluding silk production. The regenerative cells presented in the midgut epithe- lium underwent hypertrophy and hyperplasia (Figure 2(D)), changing their appearance. The regenerative cells are responsible for cell renewal [18]. It is assumed that these cells are the precursors of others cells types, in- cluding columnar cells [18,19]. However, the regenera- tion of columnar cells did not occur due to changes and hypertrophy resulting from exposure. We also observed the intestinal wall and its layers of longitudinal and circular muscles, which work in peri- stalsis. These muscles were disorganized, possibly due to the disarray of their myofibrils (Figures 2(B) and (D); MM). Figure 2(A) shows normal muscle control for comparison. The midgut is responsible for the food di- gestion and absorption of necessary nutrients for the de- velopment and maintenance of normal metabolic func- tion of the insect. Thus, the microscopic changes ob- served in response to chlorantraniliprole at concentra- tions of 0.2 ppm and 0.1 ppm harmed the development of their normal organic functions and was responsible for Copyright © 2013 SciRes. OPEN ACCESS 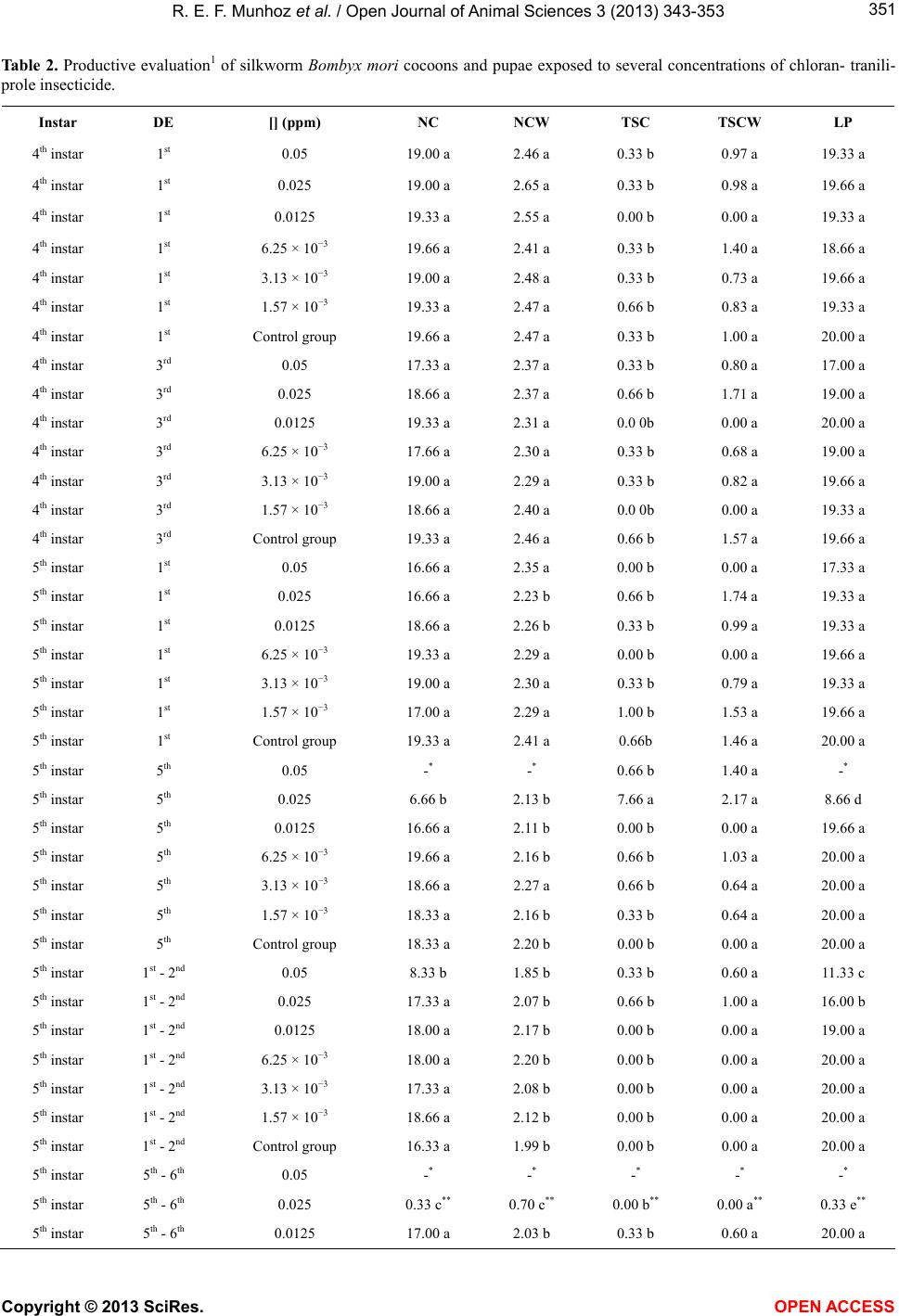 R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 351 Tab le 2. Productive evaluation1 of silkworm Bombyx mori cocoons and pupae exposed to several concentrations of chloran- tranili- prole insecticide. Instar DE [] (ppm) NC NCW TSC TSCW LP 4th instar 1st 0.05 19.00 a 2.46 a 0.33 b 0.97 a 19.33 a 4th instar 1st 0.025 19.00 a 2.65 a 0.33 b 0.98 a 19.66 a 4th instar 1st 0.0125 19.33 a 2.55 a 0.00 b 0.00 a 19.33 a 4th instar 1st 6.25 × 10−3 19.66 a 2.41 a 0.33 b 1.40 a 18.66 a 4th instar 1st 3.13 × 10−3 19.00 a 2.48 a 0.33 b 0.73 a 19.66 a 4th instar 1st 1.57 × 10−3 19.33 a 2.47 a 0.66 b 0.83 a 19.33 a 4th instar 1st Control group 19.66 a 2.47 a 0.33 b 1.00 a 20.00 a 4th instar 3rd 0.05 17.33 a 2.37 a 0.33 b 0.80 a 17.00 a 4th instar 3rd 0.025 18.66 a 2.37 a 0.66 b 1.71 a 19.00 a 4th instar 3rd 0.0125 19.33 a 2.31 a 0.0 0b 0.00 a 20.00 a 4th instar 3rd 6.25 × 10−3 17.66 a 2.30 a 0.33 b 0.68 a 19.00 a 4th instar 3rd 3.13 × 10−3 19.00 a 2.29 a 0.33 b 0.82 a 19.66 a 4th instar 3rd 1.57 × 10−3 18.66 a 2.40 a 0.0 0b 0.00 a 19.33 a 4th instar 3rd Control group 19.33 a 2.46 a 0.66 b 1.57 a 19.66 a 5th instar 1st 0.05 16.66 a 2.35 a 0.00 b 0.00 a 17.33 a 5th instar 1st 0.025 16.66 a 2.23 b 0.66 b 1.74 a 19.33 a 5th instar 1st 0.0125 18.66 a 2.26 b 0.33 b 0.99 a 19.33 a 5th instar 1st 6.25 × 10−3 19.33 a 2.29 a 0.00 b 0.00 a 19.66 a 5th instar 1st 3.13 × 10−3 19.00 a 2.30 a 0.33 b 0.79 a 19.33 a 5th instar 1st 1.57 × 10−3 17.00 a 2.29 a 1.00 b 1.53 a 19.66 a 5th instar 1st Control group 19.33 a 2.41 a 0.66b 1.46 a 20.00 a 5th instar 5th 0.05 -* - * 0.66 b 1.40 a -* 5th instar 5th 0.025 6.66 b 2.13 b 7.66 a 2.17 a 8.66 d 5th instar 5th 0.0125 16.66 a 2.11 b 0.00 b 0.00 a 19.66 a 5th instar 5th 6.25 × 10−3 19.66 a 2.16 b 0.66 b 1.03 a 20.00 a 5th instar 5th 3.13 × 10−3 18.66 a 2.27 a 0.66 b 0.64 a 20.00 a 5th instar 5th 1.57 × 10−3 18.33 a 2.16 b 0.33 b 0.64 a 20.00 a 5th instar 5th Control group 18.33 a 2.20 b 0.00 b 0.00 a 20.00 a 5th instar 1st - 2nd 0.05 8.33 b 1.85 b 0.33 b 0.60 a 11.33 c 5th instar 1st - 2nd 0.025 17.33 a 2.07 b 0.66 b 1.00 a 16.00 b 5th instar 1st - 2nd 0.0125 18.00 a 2.17 b 0.00 b 0.00 a 19.00 a 5th instar 1st - 2nd 6.25 × 10−3 18.00 a 2.20 b 0.00 b 0.00 a 20.00 a 5th instar 1st - 2nd 3.13 × 10−3 17.33 a 2.08 b 0.00 b 0.00 a 20.00 a 5th instar 1st - 2nd 1.57 × 10−3 18.66 a 2.12 b 0.00 b 0.00 a 20.00 a 5th instar 1st - 2nd Control group 16.33 a 1.99 b 0.00 b 0.00 a 20.00 a 5th instar 5th - 6th 0.05 -* - * - * - * - * 5th instar 5th - 6th 0.025 0.33 c** 0.70 c** 0.00 b** 0.00 a** 0.33 e** 5th instar 5th - 6th 0.0125 17.00 a 2.03 b 0.33 b 0.60 a 20.00 a Copyright © 2013 SciRes. OPEN ACCESS 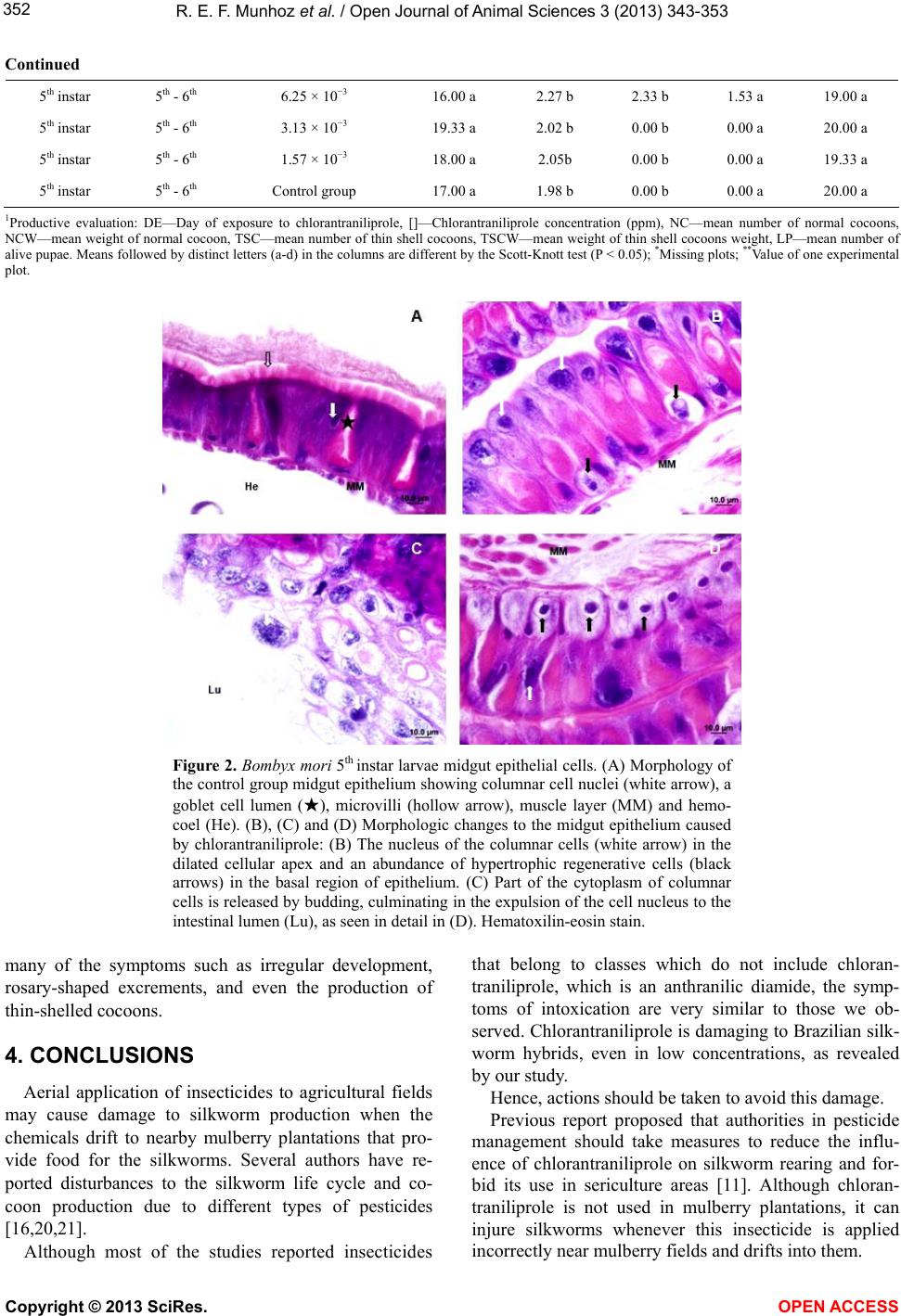 R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 352 Continued 5th instar 5th - 6th 6.25 × 10−3 16.00 a 2.27 b 2.33 b 1.53 a 19.00 a 5th instar 5th - 6th 3.13 × 10−3 19.33 a 2.02 b 0.00 b 0.00 a 20.00 a 5th instar 5th - 6th 1.57 × 10−3 18.00 a 2.05b 0.00 b 0.00 a 19.33 a 5th instar 5th - 6th Control group 17.00 a 1.98 b 0.00 b 0.00 a 20.00 a 1Productive evaluation: DE—Day of exposure to chlorantraniliprole, []—Chlorantraniliprole concentration (ppm), NC—mean number of normal cocoons, NCW—mean weight of normal cocoon, TSC—mean number of thin shell cocoons, TSCW—mean weight of thin shell cocoons weight, LP—mean number of alive pupae. Means followed by distinct letters (a-d) in the columns are different by the Scott-Knott test (P < 0.05); *Missing plots; **Value of one experimental plot. Figure 2. Bombyx mori 5th instar larvae midgut epithelial cells. (A) Morphology of the control group midgut epithelium showing columnar cell nuclei (white arrow), a goblet cell lumen (★), microvilli (hollow arrow), muscle layer (MM) and hemo- coel (He). (B), (C) and (D) Morphologic changes to the midgut epithelium caused by chlorantraniliprole: (B) The nucleus of the columnar cells (white arrow) in the dilated cellular apex and an abundance of hypertrophic regenerative cells (black arrows) in the basal region of epithelium. (C) Part of the cytoplasm of columnar cells is released by budding, culminating in the expulsion of the cell nucleus to the intestinal lumen (Lu), as seen in detail in (D). Hematoxilin-eosin stain. many of the symptoms such as irregular development, rosary-shaped excrements, and even the production of thin-shelled cocoons. 4. CONCLUSIONS Aerial application of insecticides to agricultural fields may cause damage to silkworm production when the chemicals drift to nearby mulberry plantations that pro- vide food for the silkworms. Several authors have re- ported disturbances to the silkworm life cycle and co- coon production due to different types of pesticides [16,20,21]. Although most of the studies reported insecticides that belong to classes which do not include chloran- traniliprole, which is an anthranilic diamide, the symp- toms of intoxication are very similar to those we ob- served. Chlorantraniliprole is damaging to Brazilian silk- worm hybrids, even in low concentrations, as revealed by our study. Hence, actions should be taken to avoid this damage. Previous report proposed that authorities in pesticide management should take measures to reduce the influ- ence of chlorantraniliprole on silkworm rearing and for- bid its use in sericulture areas [11]. Although chloran- traniliprole is not used in mulberry plantations, it can injure silkworms whenever this insecticide is applied incorrectly near mulberry fields and drifts into them. Copyright © 2013 SciRes. OPEN ACCESS  R. E. F. Munhoz et al. / Open Journal of Animal Sciences 3 (2013) 343-353 353 5. ACKNOWLEDGEMENTS This work was supported by CAPES, CNPq, FINEP/Fundação Arau- cária and Secretaria de Estado da Ciência, Tecnologia e Ensino Superior— FUNDO PARANÁ. REFERENCES [1] Xiang, Z.H. (1995) Genetics and breeding of the silk- worm. China Agriculture Press, Beijing. [2] Goldsmith, M.R., Shimada, T. and Abe, H. (2002) The genetics and genomics of the silkworm, Bombyx mori. Annual Review of Entomology, 50, 71-100. http://dx.doi.org/10.1146/annurev.ento.50.071803.130456 [3] Nagaraju, J. and Goldsmith, M.R. (2002) Silkworm ge- nomics—Progress and prospects. Current Science, 83, 411-425. [4] Bora, D., Khanikor, B. and Gogoi, H. (2012) Plant based pesticides: Green environment with special reference to silk worms. In: Soundararajan, R.P., Eds., Pesticides— Advances in Chemical and Botanical Pesticides, InTech, Rijeka, 171-206. http://dx.doi.org/10.5772/47832 [5] Lahm G.P., et al. (2007) Rynaxypyr™: A new insecticide anthranilic diamide that acts as a potent and selective ry- anodine receptor activator. Bioorganic and Medicinal Chemistry Letters, 17, 6274-6279. http://dx.doi.org/10.1016/j.bmcl.2007.09.012 [6] Temple, J. H., Pommireddy, P.L., Cook, D.R., Marçon, P. and Leonard, B.R. (2009) Susceptibility of selected lepi- dopteran pests to Rynaxypyr®, a novel insecticide. Jour- nal of Cotton Science, 13, 23-31. [7] Hannig, G. T., Ziegler, M. and Marcon, P.G. (2009) Feed- ing cessation effects of chlorantraniliprole, a new anthra- nilic diamide insecticide, in comparison with several in- secticides in distinct chemical classes and mode-of-action groups. Pest Management Science, 65, 969-974. http://dx.doi.org/10.1002/ps.1781 [8] Dinter, A., Brugger, K.E., Frost, N.-M. and Woodward, M.D. (2009) Chlorantraniliprole (Rynaxypyr): A novel DuPont™ insecticide with low toxicity and low risk for honey bees (Apis mellifera) and bumble bees (Bombus terrestris) providing excellent tools for uses in integrated pest management. Proceedings, Hazards of pesticides to bees—10th International Symposium of the ICP-Bee Pro- tection Group, 8-10 October 2008, Bucharest, 984-996. [9] Cordova, D., Benner, E., Sacher, M., Rauh, J., Sopa, J., Lahm, G., Selby, T., Stevenson, T., Flexner, L. and Gut- teridge, S. (2006) Anthranilic diamides: a new class of in- secticides with a novel mode of action, ryanodine recep- tor activation. Pesticide Biochemistry and Physiology, 84, 196-214. http://dx.doi.org/10.1016/j.pestbp.2005.07.005 [10] Sattelle, D.B., Cordova, D. and Cheek, T.R. (2008) Insect ryanodine receptors: Molecular targets for novel pest con- trol chemicals. Invertebrate Neuroscience, 8, 107-119. http://dx.doi.org/10.1007/s10158-008-0076-4 [11] Chen, W.G., Dong, R.H., Sun, H.Y., Dai, J.Z., Zhu, H.L. and Wu, F.A. (2010) An investigation on toxicity of the agricultural pesticide chlorantraniliprole to the silkworm, Bombyx mori. Science of Sericulture, 1, 84-90. [12] Sial, A.A., Brunner, J.F. and Garczynski, S.F. (2011) Bi- ochemical characterization of chlorantraniliprole and spinetoram resistance in laboratory-selected obliqueban- ded leafroller, Choristoneura rosaceana (Harris) (Lepi- doptera: Tortricidae). Pesticide Biochemistry and Physi- ology, 99, 274-279. http://dx.doi.org/10.1016/j.pestbp.2011.01.006 [13] Spomer, N.A., Kamble, S.T. and Siegfried, B.D. (2009) Bioavailability of chlorantraniliprole and indoxacarb to eastern subterranean termites (Isoptera: Rhinotermitidae) in various soils. Journal of Economic Entomology, 102, 1922-1927. http://dx.doi.org/10.1603/029.102.0524 [14] Jiang, W.H.; Lu, W.P., Guo, W.C., Xia, Z.H., Fu, W.J. and Li, G.Q. (2012) Chlorantraniliprole susceptibility in Lep- tinotarsa decemlineata in the North Xinjiang Uygur auto- nomous region in China. Journal of Economic Entomol- ogy, 105, 549-554. http://dx.doi.org/10.1603/EC11194 [15] Ferreira, D.F. (1999) SISVAR user's manual, version 5.3. Universidade Federal de Lavras, Minas Gerais. http://www.dex.ufla.br/~danielff/softwares.htm [16] Nasr, H.M. (2011) Toxicological and biochemical effects of chlorpyrifos, chlorfluazuron and oxymatrine on larvae of Bombyx mori. Journal of Agricultural Research of Ka- fer El-Sheikh University, 37, 209-222. [17] Kerr, J.F., Wyllie, A.H. and Currie, A.R. (1972) Apoptosis: A basic biological phenomenon with wide-ranging impli- cations in tissue kinetics. British Journal of Cancer, 26, 239-257. http://dx.doi.org/10.1038/bjc.1972.33 [18] Lehane, M.J. and Billingsley, P.F. (1996) Biology of the insect midgut. Chapman & Hall, Cambridge. http://dx.doi.org/10.1007/978-94-009-1519-0 [19] Illa-Bochaca, I. and Montuenga, L.M. (2006) The regen- erative nidi of the locust midgut as a model to study epi- thelial cell differentiation from stem cells. The Journal of Experimental Biology, 209, 2215-2223. http://dx.doi.org/10.1242/jeb.02249 [20] Kuribayashi, S. (1988) Damage of silkworms caused by pesticides and preventive measures. Japan Agricultural Research Quaterly, 21, 274-283. [21] Nath, B.S., Suresh, A., Verma, B.M. and Kumar, R.P.S. (1997) Changes in protein metabolism in haemolymph and fat body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to OP insecticidal toxicity. Eco- toxicology and Environmetal Safety, 36, 169-173. http://dx.doi.org/10.1006/eesa.1996.1504 Copyright © 2013 SciRes. OPEN ACCESS
|