 International Journal of Organic Chemistry, 2013, 3, 65-72 Published Online November 2013 (http://www.scirp.org/journal/ijoc) http://dx.doi.org/10.4236/ijoc.2013.33A007 Open Access IJOC Synthesis, Characterization, Anti-Bacterial and Anti-Fungal Activities of New Quinoxaline 1,4-di-N-Oxide Derivatives Dalia Hussein Soliman Pharmaceutical Chemistry Department, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt Email: odihss@yahoo.com Received September 4, 2013; revised October 8, 2013; accepted October 24, 2013 Copyright © 2013 Dalia Hussein Soliman. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A new series of quinoxaline 1,4-di-N-oxides were synthesized and evaluated for their antibacterial and antifungal ac- tivities. The best result was demonstrated by 3-amino-N-(4-methoxyphenyl)-2-quinoxalinecarboxamide 1,4-di-N-oxide 4e, MIC (0.24 µg/ml) against Aspergillus fumigatus, and (0.12 µg/ml) against Streptococcus pneumonia. Keywords: Quinoxaline 1,4-di-N-Oxide; Beirut Reaction; Benzofuroxanes; β-Ketoamide; β-Cyanoamide; Antibacterial; Antifungal 1. Introduction There is a growing need to develop new antibacterial agents in order to overcome the emergence of bacterial resistance to antibiotic therapy. Quinoxaline 1,4-di-N- oxide is a nucleus that displays a wide range of activities. Quinoxaline 1,4-di-N-oxides and their derivatives demon- strated excellent activities as antibacterial [1-5], antiplas- modial [2,6,7], antifungal [8] and as antimycobacterial [9-13]. Encouraged by these reported activities and with the aim of searching for new, broad spectrum and more potent antimicrobial compounds which can improve the current chemotherapeutic treatments, 20 new quinoxaline 1,4-di-N- oxide derivatives were synthesized and evaluated. In most of the quinoxaline 1,4-di-N-oxides reported as antibacte- rial and/or antimycobacterial, the carboxamide in position 2 was a common feature [11,13]. Therefore, the newly syn- thesized compounds 3a-n possessed a carboxamide in position 2 that was substituted with various aryl moieties bearing both electron-withrawing and electron donating groups. This series of quinoxaline carboxamide was pre- pared from unsubstituted benzofuroxane, 3a-g to be com- pared to their 7-chloro counterparts 3h-n in order to study the effect of substitution of chlorine atom in position 7. 2. Results and Discussion 2.1. Chemistry The synthetic route for the preparation of the quinoxaline 1,4-di-N-oxide were obtained by the Beirut reaction[14, 15]. This reaction, which has been referred to as the Bei- rut reaction, is an excellent method for preparing these heterocyclic compounds. Benzofuroxanes 1a,b were ob- tained by previously described methods [13,16]. Thus, the appropriate BFO reacted with the corresponding β-ketoamide (2a,b) or β-cyanoamide (3a-n) in the pres- ence anhydrous potassium carbonate as a catalyst (Scheme 1). The use of inorganic catalyst for this reac- tion provided better means of purification with consid- erably good yields, whereas the reaction of BFO with cyanoacetamide was better carried out using triethyl- amine as a catalyst and dimethylfomamide as the solvent. These two intermediates, β-ketoamide and β-cyanoamide, were prepared by condensation of either ethylacetoace- tate or ethylcyanoacetae with different amines [11,12,17]. When the target compounds were prepared from mono- substituted-BFO, the formation of isomeric quinoxaline 1,4-di-N-oxides was observed. In most cases, the 7-sub- stituted isomer prevailed over 6-substituted isomer, and when the methoxy substituted quinoxalines were pre- pared, only the 7-isomer was obtained, as previously de- scribed [18,19]. Moreover, in order to investigate the antimicrobial ac- tivity of fused tricyclic and tetracyclic di-N-oxides de- rived from quinoxaline 1,4-di-N-oxide the phenazine derivative 5 and the indenoquinoxaline 6 were synthe- sized. The Beirut reaction was similarly followed using 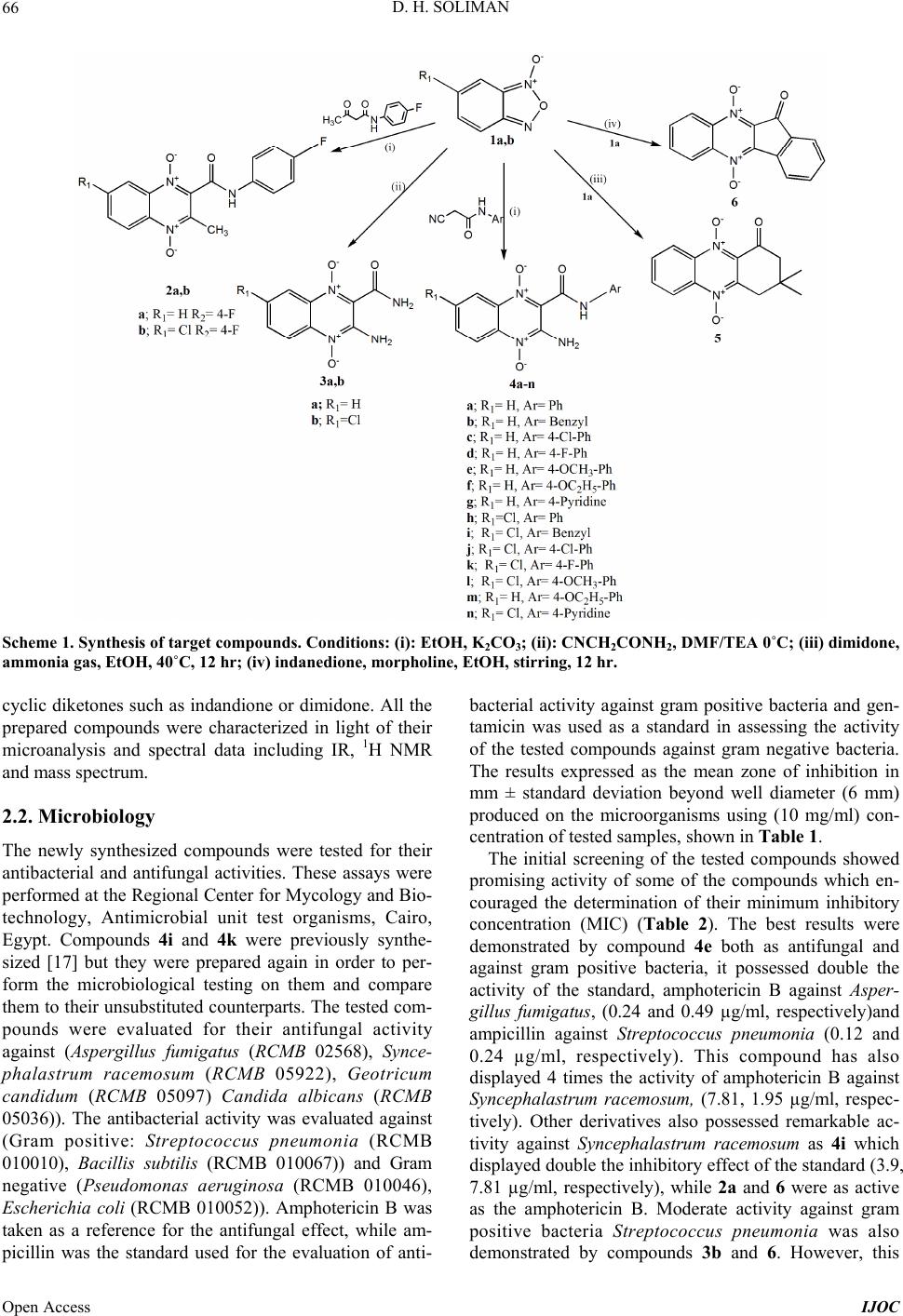 D. H. SOLIMAN 66 Scheme 1. Synthe sis of target compounds. Conditions: (i): EtOH, K2CO3; (ii): CNCH2CONH2, DMF/TEA 0˚C; (iii) dimidone, ammonia gas, EtOH, 40˚C, 12 hr; (iv) indanedione, morpholine, EtOH, stirring, 12 hr. cyclic diketones such as indandione or dimidone. All the prepared compounds were characterized in light of their microanalysis and spectral data including IR, 1H NMR and mass spectrum. 2.2. Microbiology The newly synthesized compounds were tested for their antibacterial and antifungal activities. These assays were performed at the Regional Center for Mycology and Bio- technology, Antimicrobial unit test organisms, Cairo, Egypt. Compounds 4i and 4k were previously synthe- sized [17] but they were prepared again in order to per- form the microbiological testing on them and compare them to their unsubstituted counterparts. The tested com- pounds were evaluated for their antifungal activity against (Aspergillus fumigatus (RCMB 02568), Synce- phalastrum racemosum (RCMB 05922), Geotricum candidum (RCMB 05097) Candida albicans (RCMB 05036)). The antibacterial activity was evaluated against (Gram positive: Streptococcus pneumonia (RCMB 010010), Bacillis subtilis (RCMB 010067)) and Gram negative (Pseudomonas aeruginosa (RCMB 010046), Escherichia coli (RCMB 010052)). Amphotericin B was taken as a reference for the antifungal effect, while am- picillin was the standard used for the evaluation of anti- bacterial activity against gram positive bacteria and gen- tamicin was used as a standard in assessing the activity of the tested compounds against gram negative bacteria. The results expressed as the mean zone of inhibition in mm ± standard deviation beyond well diameter (6 mm) produced on the microorganisms using (10 mg/ml) con- centration of tested samples, shown in Table 1. The initial screening of the tested compounds showed promising activity of some of the compounds which en- couraged the determination of their minimum inhibitory concentration (MIC) (Table 2). The best results were demonstrated by compound 4e both as antifungal and against gram positive bacteria, it possessed double the activity of the standard, amphotericin B against Asper- gillus fumigatus, (0.24 and 0.49 µg/ml, respectively)and ampicillin against Streptococcus pneumonia (0.12 and 0.24 µg/ml, respectively). This compound has also displayed 4 times the activity of amphotericin B against Syncephalastrum racemosum, (7.81, 1.95 µg/ml, respec- tively). Other derivatives also possessed remarkable ac- tivity against Syncephalastrum racemosum as 4i which displayed double the inhibitory effect of the standard (3.9, 7.81 µg/ml, respectively), while 2a and 6 were as active as the amphotericin B. Moderate activity against gram positive bacteria Streptococcus pneumonia was also demonstrated by compounds 3b and 6. However, this Open Access IJOC 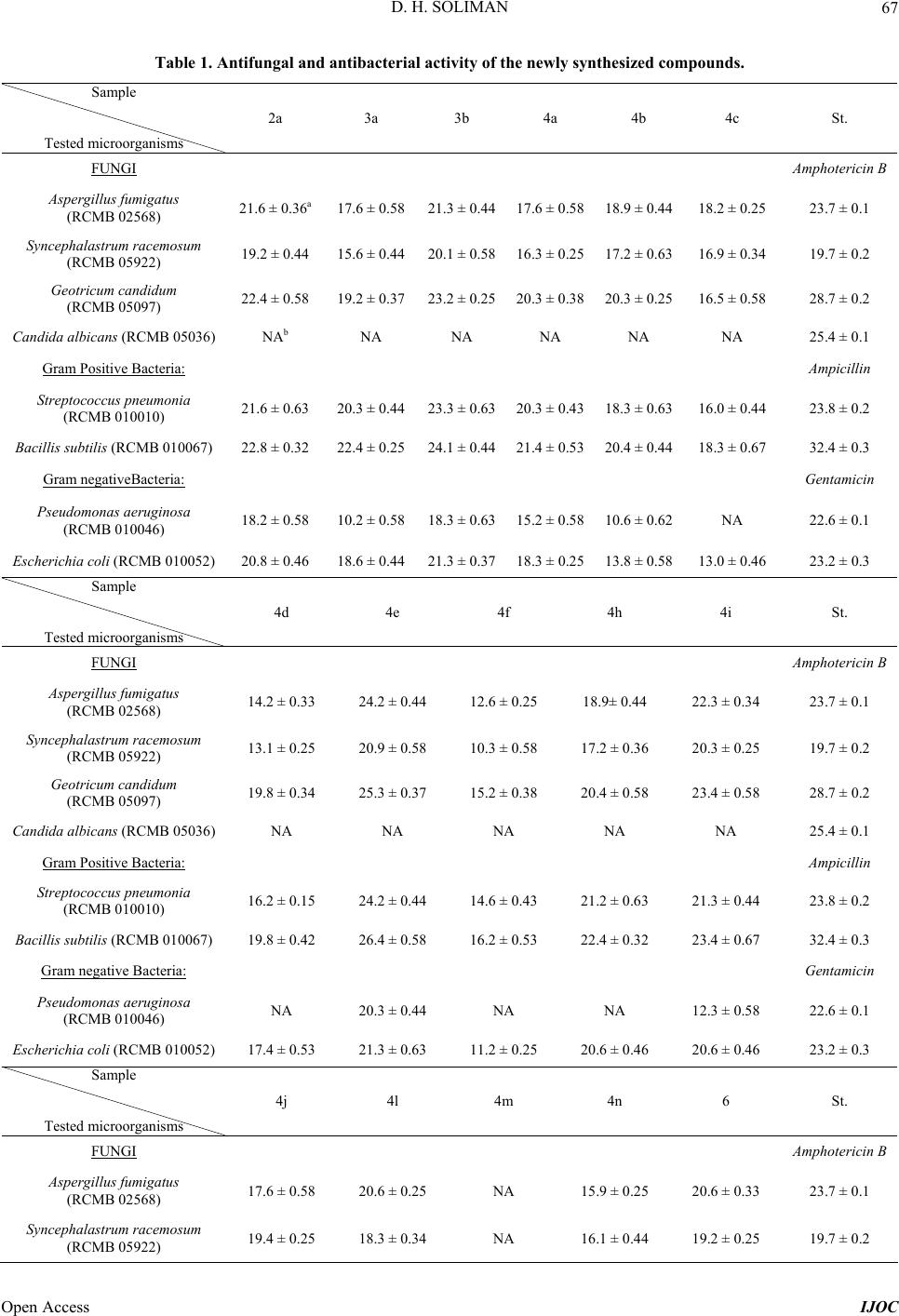 D. H. SOLIMAN 67 Table 1. Antifungal and antibacterial activity of the new ly synthe sized compounds. Sample Tested microorganisms 2a 3a 3b 4a 4b 4c St. FUNGI Amphotericin B Aspergillus fumigatus (RCMB 02568) 21.6 ± 0.36a 17.6 ± 0.5821.3 ± 0.4417.6 ± 0.5818.9 ± 0.4418.2 ± 0.25 23.7 ± 0.1 Syncephalastrum racemosum (RCMB 05922) 19.2 ± 0.44 15.6 ± 0.4420.1 ± 0.5816.3 ± 0.2517.2 ± 0.6316.9 ± 0.34 19.7 ± 0.2 Geotricum candidum (RCMB 05097) 22.4 ± 0.58 19.2 ± 0.3723.2 ± 0.2520.3 ± 0.3820.3 ± 0.2516.5 ± 0.58 28.7 ± 0.2 Candida albicans (RCMB 05036) NAb NA NA NA NA NA 25.4 ± 0.1 Gram Positive Bacteria: Ampicillin Streptococcus pneumonia (RCMB 010010) 21.6 ± 0.63 20.3 ± 0.4423.3 ± 0.6320.3 ± 0.4318.3 ± 0.6316.0 ± 0.44 23.8 ± 0.2 Bacillis subtilis (RCMB 010067) 22.8 ± 0.32 22.4 ± 0.2524.1 ± 0.4421.4 ± 0.5320.4 ± 0.4418.3 ± 0.67 32.4 ± 0.3 Gram negativeBacteria: Gentamicin Pseudomonas aeruginosa (RCMB 010046) 18.2 ± 0.58 10.2 ± 0.5818.3 ± 0.6315.2 ± 0.5810.6 ± 0.62NA 22.6 ± 0.1 Escherichia coli (RCMB 010052) 20.8 ± 0.46 18.6 ± 0.4421.3 ± 0.3718.3 ± 0.2513.8 ± 0.5813.0 ± 0.46 23.2 ± 0.3 Sample Tested microorganisms 4d 4e 4f 4h 4i St. FUNGI Amphotericin B Aspergillus fumigatus (RCMB 02568) 14.2 ± 0.33 24.2 ± 0.44 12.6 ± 0.25 18.9± 0.44 22.3 ± 0.34 23.7 ± 0.1 Syncephalastrum racemosum (RCMB 05922) 13.1 ± 0.25 20.9 ± 0.58 10.3 ± 0.58 17.2 ± 0.36 20.3 ± 0.25 19.7 ± 0.2 Geotricum candidum (RCMB 05097) 19.8 ± 0.34 25.3 ± 0.37 15.2 ± 0.38 20.4 ± 0.58 23.4 ± 0.58 28.7 ± 0.2 Candida albicans (RCMB 05036) NA NA NA NA NA 25.4 ± 0.1 Gram Positive Bacteria: Ampicillin Streptococcus pneumonia (RCMB 010010) 16.2 ± 0.15 24.2 ± 0.44 14.6 ± 0.43 21.2 ± 0.63 21.3 ± 0.44 23.8 ± 0.2 Bacillis subtilis (RCMB 010067) 19.8 ± 0.42 26.4 ± 0.58 16.2 ± 0.53 22.4 ± 0.32 23.4 ± 0.67 32.4 ± 0.3 Gram negative Bacteria: Gentamicin Pseudomonas aeruginosa (RCMB 010046) NA 20.3 ± 0.44 NA NA 12.3 ± 0.58 22.6 ± 0.1 Escherichia coli (RCMB 010052) 17.4 ± 0.53 21.3 ± 0.63 11.2 ± 0.25 20.6 ± 0.46 20.6 ± 0.46 23.2 ± 0.3 Sample Tested microorganisms 4j 4l 4m 4n 6 St. FUNGI Amphotericin B Aspergillus fumigatus (RCMB 02568) 17.6 ± 0.58 20.6 ± 0.25 NA 15.9 ± 0.25 20.6 ± 0.33 23.7 ± 0.1 Syncephalastrum racemosum (RCMB 05922) 19.4 ± 0.25 18.3 ± 0.34 NA 16.1 ± 0.44 19.2 ± 0.25 19.7 ± 0.2 Open Access IJOC 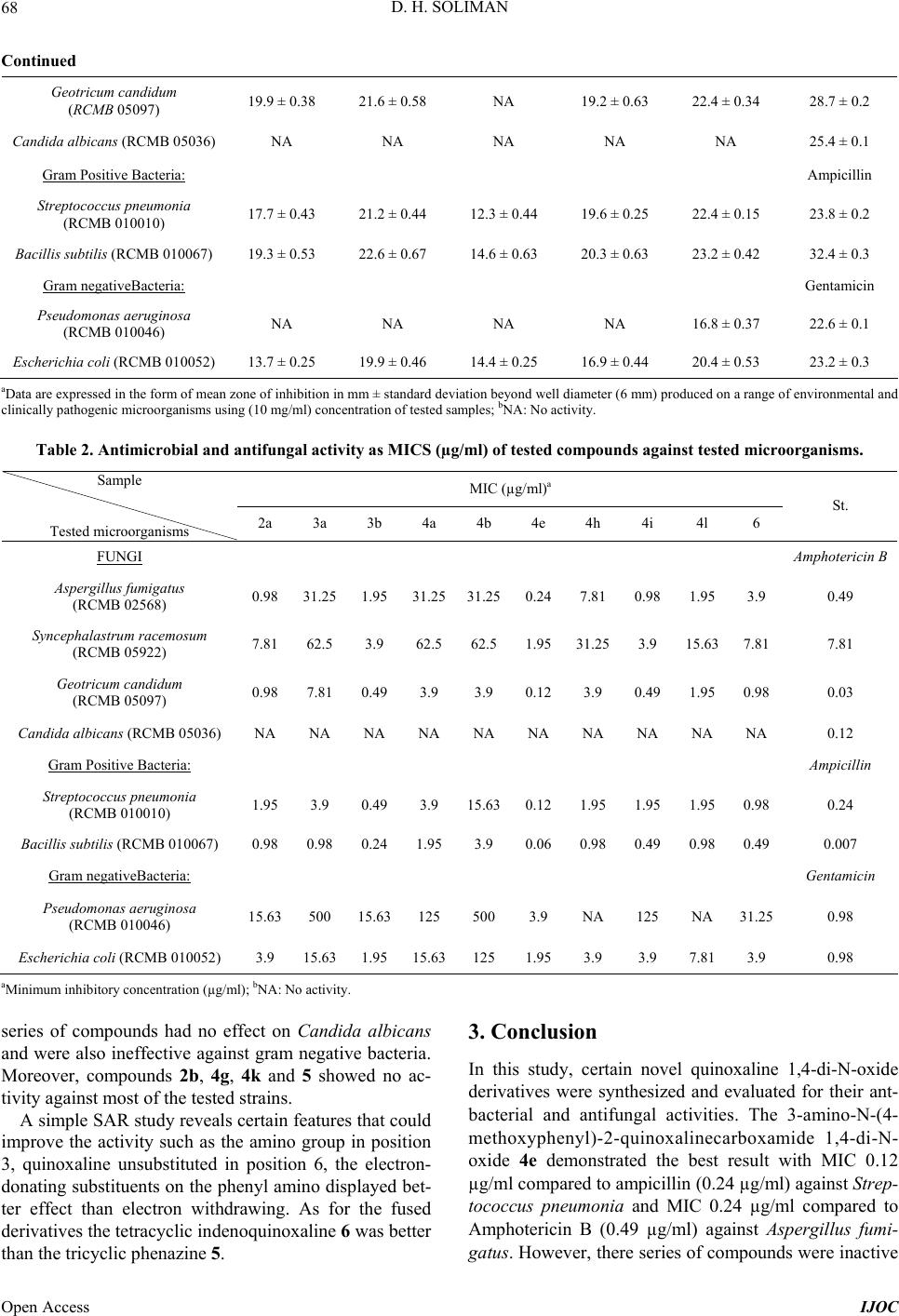 D. H. SOLIMAN 68 Continued Geotricum candidum (RCMB 05097) 19.9 ± 0.38 21.6 ± 0.58 NA 19.2 ± 0.63 22.4 ± 0.34 28.7 ± 0.2 Candida albicans (RCMB 05036) NA NA NA NA NA 25.4 ± 0.1 Gram Positive Bacteria: Ampicillin Streptococcus pneumonia (RCMB 010010) 17.7 ± 0.43 21.2 ± 0.44 12.3 ± 0.44 19.6 ± 0.25 22.4 ± 0.15 23.8 ± 0.2 Bacillis subtilis (RCMB 010067) 19.3 ± 0.53 22.6 ± 0.67 14.6 ± 0.63 20.3 ± 0.63 23.2 ± 0.42 32.4 ± 0.3 Gram negativeBacteria: Gentamicin Pseudomonas aeruginosa (RCMB 010046) NA NA NA NA 16.8 ± 0.37 22.6 ± 0.1 Escherichia coli (RCMB 010052) 13.7 ± 0.25 19.9 ± 0.46 14.4 ± 0.25 16.9 ± 0.44 20.4 ± 0.53 23.2 ± 0.3 aData are expressed in the form of mean zone of inhibition in mm ± standard deviation beyond well diameter (6 mm) produced on a range of environmental and clinically pathogenic microorganisms using (10 mg/ml) concentration of tested samples; bNA: No activity. Table 2. Antimicrobial and antifungal activity as MICS (µg/ml) of tested compounds against tested microorganisms. MIC (µg/ml)a Sample Tested microorganisms 2a 3a 3b 4a 4b 4e 4h 4i 4l 6 St. FUNGI Amphotericin B Aspergillus fumigatus (RCMB 02568) 0.98 31.25 1.9531.2531.250.247.810.981.95 3.9 0.49 Syncephalastrum racemosum (RCMB 05922) 7.81 62.5 3.9 62.562.51.9531.253.9 15.63 7.81 7.81 Geotricum candidum (RCMB 05097) 0.98 7.81 0.493.9 3.9 0.123.9 0.491.95 0.98 0.03 Candida albicans (RCMB 05036) NA NA NA NA NA NA NA NA NA NA 0.12 Gram Positive Bacteria: Ampicillin Streptococcus pneumonia (RCMB 010010) 1.95 3.9 0.493.9 15.630.121.951.951.95 0.98 0.24 Bacillis subtilis (RCMB 010067) 0.98 0.98 0.241.953.9 0.060.980.490.98 0.49 0.007 Gram negativeBacteria: Gentamicin Pseudomonas aeruginosa (RCMB 010046) 15.63 500 15.63125 500 3.9 NA 125 NA 31.25 0.98 Escherichia coli (RCMB 010052) 3.9 15.63 1.9515.63125 1.953.9 3.9 7.81 3.9 0.98 aMinimum inhibitory concentration (µg/ml); bNA: No activity. series of compounds had no effect on Candida albicans and were also ineffective against gram negative bacteria. Moreover, compounds 2b, 4g, 4k and 5 showed no ac- tivity against most of the tested strains. A simple SAR study reveals certain features that could improve the activity such as the amino group in position 3, quinoxaline unsubstituted in position 6, the electron- donating substituents on the phenyl amino displayed bet- ter effect than electron withdrawing. As for the fused derivatives the tetracyclic indenoquinoxaline 6 was better than the tricyclic phenazine 5. 3. Conclusion In this study, certain novel quinoxaline 1,4-di-N-oxide derivatives were synthesized and evaluated for their ant- bacterial and antifungal activities. The 3-amino-N-(4- methoxyphenyl)-2-quinoxalinecarboxamide 1,4-di-N- oxide 4e demonstrated the best result with MIC 0.12 µg/ml compared to ampicillin (0.24 µg/ml) against Strep- tococcus pneumonia and MIC 0.24 µg/ml compared to Amphotericin B (0.49 µg/ml) against Aspergillus fumi- gatus. However, there series of compounds were inactive Open Access IJOC  D. H. SOLIMAN 69 against gram negative bacteria as well as candida albi- cans 4. Experimental 4.1. Chemistry 4.1.1. Chemical Met hods All the solvents used were commercially available and distilled before use. Reactions were monitored by thin- layer chromatography (TLC) on silica gel plates (60 F254), visualizing with ultraviolet. Infra red spectra (KBr) were recorded on FT-IR 5300 spectrophotometer and Perkin Elmer spectrum RXIFT-IR system (ν, cm−1). 1HNMR spectra were recorded on Varian Gemini spec- trophotometer (300 MHz) in DMSO-d6 or CDCl3 as sol- vent. Proton chemical shifts (d) are relative to tetrame- thylsilane (TMS, d = 0.00) as internal standard and ex- pressed in ppm. Spin multiplicities are given as s (singlet), d (doublet), t (triplet), and m (multiplet) as well as b (broad). Coupling constants (J) are given in hertz. Melting points were determined by using melting point apparatus and are uncorrected. MS spectra were obtained on a GC Ms-QP 1000 EX mass spectrometer at 70 eV. Microanalyses were performed using a C H N S/O ana- lyzer. Elemental data are within ±0.4% of the theoretical values. All yields reported areunoptimized. The chemical reagents used in synthesis were purchased from Fluka, Sigma and Aldrich. 4.1.2. General Proce dure fo r Prepara ti on of Compounds 2a,b A warm solution of 5(6)-benzofuroxane 1a,b and the appropriate acetoacetanilide (0.01 mol) in ethanol (50 mL) was stirred at room temperature in the presence of catalytic amount of potassium carbonate. The yellow products precipitated over a period of 2 - 4 h. The resid- ual product was triturated with water, extracted with ethylacetate then the organic solvent was dried over an- hydrous sodium sulphate and removed in vacuo to give yellow crystals that were recrystallized out from ethanol. 1) 2-N-(4-Fluorophenyl)-3-methyl-2-quinoxaline car- boxamide 1,4-di-N-oxide 2a. Yield (78%); mp 221˚C. IR (KBr, cm−1): 1651 (C=O), 1575 (C=N), 3290 (NH), 1215, 1295 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 2.63 (3H, s, CH3), 11.04 (1H, s, NH-D2O exchangable), 7.23 (2H, d, Ar-H, J = 8.7 Hz), 7.6 (2H, d, Ar-H, J = 8.7 Hz), 7.99 (2H, d, H5, H8), 8.52 (m, 2H, H6, H7). MS (m/z): 313 (M+), 281 (M+-O2), 202 (M+-4-F-Ph-NH), 171 (M+-O2, -4-F-Ph-NH), 129 (quinoxaline+). Found, %: C; H; N. C 61.47; H 3.66; N, 13.12. C16H12FN3O3. Calc., %: C, 61.34; H, 3.86; N, 13.41. 2) 7-chloro-2-N-(4-fluorophenyl)-3-methyl-2-quinox- aline carboxamide 1,4-dioxide 2b. Yield (85%); mp 243˚C. IR (KBr, cm−1): 1670 (C=O), 1561 (C=N), 3211 (NH), 1334 (NO). MS (m/z): 349 (M+), 205 (M+-NH- C6H5-4-F, -O2). Found, %: C; H; N. C 54.47; H 3.66; N, 13.01. C16H13ClFN3O3. Calc., %: C, 54.95; H, 3.75; N, 12.01. 4.1.3. General Proce dure fo r Prepara ti on of Compounds 3a,b A mixture of 5(6)-substituted benzofuroxane 1a,b and cyanoacetamide (0.01 mol) was stirred for 10 min at 0˚C. Over the cooled suspension, a solution of triethylamine (5 drops) in dimethylformamide was added. The mixture was allowed to stand at room temperature over 24 h and then filtered off. The red solid product was filtered and recrystallized from ethanol. 1) 3-Amino-2-quinoxalinecarboxamide 1,4-dioxide 3a. Yield 71%; mp 260˚C - 265˚C. IR (KBr, cm−1): 1660 (C=O), 1640 (C=N), 3388, 3224 (2NH2), 1254 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 5.6 (s, 2H, NH2), 8.53 (s, 2H, CO-NH2), 7.26 - 8.2 (m, 4H, quinox-H). Found, %: C; H; N. C 49.47; H 2.96; N, 25.40. C9H8N4O3 C, 49.09; H, 3.66; N, 25.45. 2) 3-Amino-7-chloro-2-quinoxaline carboxamide 1,4- dioxide 3b. Yield 92%; mp. 270˚C - 271˚C. IR (KBr, cm−1): 1715 (C=O), 1640 (C=N), 1319, 1340 (NO), 3356, 3454, 3512 (2NH2). 1H NMR spectrum (DMSO-d6), δ, ppm 5.76 (s, 2H, NH2), 8.60 (s, 2H, CO-NH2), 7.36-8.32 (m, 4H, qui- nox-H). Found, %: C; H; N. C 42.47; H 2.66; N, 22.12. C9H7ClN4O3 C, 42.45; H, 2.77; N, 22.00. 4.1.4. General Proce dure fo r Prepara ti on of Compounds 4a-n A warm solution of 5(6)-benzofuroxane 1a,b and the appropriate cyanoacetanilide (0.01 mol) in ethanol (50 mL) was stirred at room temperature in the presence of catalytic amount of potassium carbonate. The solutions turned red and then they were stirred at room temperature for 1 - 5 hr depending on the BFO and the cyanoacetani- lides. The residue obtained was triturated with waterand then extracted with dichloromethane. The organic phase was dried with anhydrous sodium sulphate and filtered. The solvent was removed in vacuo and the red-brown precipitate was recrystallized out from ethanol. 1) 3-Amino-N-phenyl-2-quinoxaline carboxamide 1,4- di-N-oxide 4a. Yield (67%); mp 180˚C. IR (KBr, cm−1): 1668 (C=O), 1563 (C=N), 3210 (NH), 3256, 3390 (NH2),1301 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 4.2 (s,2H,NH2), 7.1 (t, 1H, H4’), 7.26 (t, 2H, H3’ + H5’), 7.49 (d, 2H, H2’ + H6’), 7.85 - 7.97 (m, 2H, H6 + H7), 8.1 (d, 2H, H5 + H8), 10.79 (s, 1H, NH-D2O exchangeable) ppm. Found, %: C; H; N. C 60.47; H 4.66; N, 18.12. C15H12N4O3. Calc., %: C, 60.81; H, 4.08; N, 18.91. 2) 3-Amino-N-benzyl-2-quinoxaline carboxamide 1,4- Open Access IJOC  D. H. SOLIMAN 70 di-N-oxide 4b. Yield (62%); mp 192˚C. IR (KBr, cm−1): 1659 (C=O), 1547 (C=N), 3230 - 33313 (NH, NH2), 1298 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 4.32, 4.64 (2d, 2H, N-CH2), 7.26 - 7.46 (m, 5H, phenyl-H), 7.52 - 7.55 (m, 2H, H6 + H7), 8.48 (s, 2H, NH2-D2O exchangeable), 8.61 (t, 1H, NH-D2O exchangeable) ppm. Found, %: C; H; N. C 61.47; H 4.66; N, 18.12. C16H14N4O3. Calc., %: C, 61.93; H, 4.55; N, 18.06. 3) 3-Amino-N-(4-chlorophenyl)-2-quinoxaline carbox- amide 1,4-di-N-oxide 4c. Yield (83%); mp 209˚C. IR (KBr, cm−1): 1673 (C=O), 1631 (C=N), 3269 (NH), 3347, 3401(NH2), 1245 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.05 (t, H6 + H7), 7.2 (d, 2H, H5 + H8), 7.74 (d, 2H, H3’ + H5’), 7.79 (d, 2H, H2’ + H4’), 10.12 (s, 1H, NH-D2O exchangeable), 8.0 (s, 2H, NH2-D2O exchangeable) ppm. Found, %: C; H; N. C 54.37; H 3.66; N, 16.12. C15H11ClN4O3. Calc., %: C, 54.47; H, 3.35; N, 16.94. 4) 3-Amino-N-(4-fluorophen yl)-2-quinoxaline carbox- amide 1,4-di-N-oxide 4d. Yield (81%); mp 210˚C. IR (KBr, cm−1): 1670 (C=O), 1621 (C=N), 3256 (NH), 3327, 3398 (NH2),1305 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.08 (t, 2H, H6 + H7), 7.62 (d, 2H, H5 + H8), 7.74 (d, 2H, H3’ + H5’, 7.99 (d, 2H, H2’ + H4’), 10.10(s, 1H, NH-D2O exchangeable), 10.85 (br. s, 2H, NH2-D2O exchangeable) ppm. MS (m/z): 314 (M+), 282 (M+-O2), 172 (M+-O2-NH-C6H5-4-F,). Found, %: C; H; N. C 57.47; H 3.66; N, 17.12. C15H11FN4O3. Calc., %: C, 57.33; H, 3.53; N, 17.83. 5) 3-Amino-N-(4-methoxyphenyl)-2-quinoxaline car- boxamide 1,4-di-N-oxide 4e. Yield (76%); mp 195˚C. IR (KBr, cm−1): 1662 (C=O), 1611 (C=N), 3250 - 3378 (NH, NH2), 1325 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 3.77(s, 3H, OCH3), 3.89 (br. s, 2H, NH2-D2O exchangeable), 6.92 - 7.278 (m, 8H, Ar-H + quinox-H), 78.06(d, 2H, H2’ + H4’), 10.32 (s, 1H, NH-D2O exchangeable), ppm. Found, %: C; H; N. C 58.47; H 4.66; N, 17.12. C16H14N4O4. Calc., %: C, 58.89; H, 4.32; N, 17.17. 6) 3-Amino-N-(4-ethoxyphenyl)-2-quinoxaline carbox- amide 1,4-di-N-oxide 4f. Yield (79%); mp 198˚C. IR (KBr, cm−1): 1660 (C=O), 1598 (C=N), 3259 - 3421 (NH, NH2), 1355 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 1.29 (t, 3H, OCH2CH3), 4.02 (q, 2H, OCH2CH3), 6.97 (m, 2H, H6 + H7), 7.28 (d, 2H, H3’ + H5’), 7.73 (d, 2H, H5 + H8), 8.04 (d, 2H, H2’ + H4’), 10.67(s, 1H, NH-D2O exchangeable), 12.12 (br. s, 2H, NH2-D2O exchangeable), ppm. Found, %: C; H; N. C 59.47; H 4.66; N, 16.12. C17H16N4O4. Calc., %: C, 59.99; H, 4.74; N, 16.46. 7) 3-Amino-N-(pyridine-4-yl)-2-quinoxaline carbox- amide 1,4-di-N-oxide 4g. Yield (59%); mp 211˚C - 214˚C. IR (KBr, cm−1): 1663 (C=O), 1604 (C=N), 3278 - 3435 (NH, NH2), 1345 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.41 (d, 2H, H5 + H8), 7.71(m, 2H, H6 + H7), 8.22(d, 2H, H3’ + H5’), 8.37 (d, 2H, H2’ + H4’), 10.18 (s, 1H, NH-D2O exchangeable), 12.46 (br. s, 2H, NH2-D2O exchangeable), ppm. Found, %: C; H; N. C 56.47; H 3.66; N, 23.12. C14H11N5O3. Calc., %: C, 56.56; H, 3.73; N, 23.56. 8) 3-Amino-7-Chloro -N-phenyl-2-quinoxaline carbox- amide 1,4-di-N-oxide 4h. Yield (83%); mp 223˚C - 224˚C. IR (KBr, cm−1): 1670 (C=O), 1615 (C=N), 3221 (NH), 3266, 3421 (NH2), 1353 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.43 (m, 1H, H4’), 7.66 (m, 2H, H3’ + H5’), 7.89(d, 2H, H2’ + H6’), 7.92 (d, 1H, H5), 8.02 (s, 1H, H8), 8.29 (d, 1H, H6), 10.70 (s, 1H, NH-D2O exchangeable), 13.6 (s, 2H, NH2-D2O exchangeable), ppm. Found, %: C; H; N. C 54.47; H 3.66; N, 16.12. C15H11ClN4O3 C, 54.47; H, 3.35; N, 16.94. 9) 3-Amino-7-Chloro-N-benzyl-2-quinoxaline carbox- amide 1,4-di-N-oxide 4i. Yield (84%); mp 214˚C - 216˚C. IR (KBr, cm−1): 1664 (C=O), 1572 (C=N), 3223 - 3345 (NH, NH2), 1331 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 4.63 (d, 2H, N-CH2), 7.26 - 7.46, 7.79 (2m, 7H, phenyl-H, H5 + H6), 7.99 (s, 1H, H8), 8.48 (s, 2H, NH2-D2O exchangeable), 8.81 (t, 1H, NH-D2O exchangeable) ppm. Found, %: C, 55.34; H, 3.70; N, 16.12.C16H13ClN4O3. Calc., %: C, 55.74; H, 3.80; N, 16.25. 10) 3-Amino-7-Chloro-N-(4-chlorophenyl)-2-quinoxa- line carboxamide 1,4-di-N-oxide 4j. Yield (89%); mp 240˚C. IR (KBr, cm−1): 1674 (C=O), 1587 (C=N), 3224 (NH), 3276, 3470 (NH2), 1335 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.46 (d, 1H, H2’ + H6’), 7.59(d, 1H, H6), 7.73 (d, 2H H3’ + H5’), 7.83 (s, 1H, H8), 7.88 (d, 1H, H5), 10.89 (s, 1H, NH-D2O exchangeable), 13.69(s, 2H, NH2-D2O exchangeable), ppm. MS (m/z): 364 (M+), 332(M+-O2), 191(M+-O2-NH- C6H5-4-Cl-NH). Found, %: C 49.47; H 2.66; N, 15.12. C15H10Cl2N4O3 C, 49.34; H, 2.76; N, 15.34. 11) 3-Amino-7-Chloro-N-(4-flourorophenyl)-2-quinoxa- line carboxamide 1,4-di-N-oxide 4k. Yield (91%); mp 244˚C. IR (KBr, cm−1): 1669 (C=O), 1602 (C=N), 3210 - 3380 (NH, NH2), 1301 (NO). MS (m/z): 348 (M+), 350 (M + 2), 332 (M+-O2), 221 (M+-O2- NH-C6H5-4-F). Found, %: C; H; N. C 51.47; H 2.66; N, 16.12. C15H10ClFN4O3 C, 51.66; H, 2.89; N, 16.07. 12) 3-Amino-7-Chloro-N-(4-methoxyphenyl)-2-quinoxa- linecarboxamide 1,4-di-N-oxide 4l. Yield (82%); mp 219˚C. IR (KBr, cm−1): 1664 (C=O), 1520 (C=N), 3298 (NH), 3620, 3589 (NH2), 1248 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 3.75 (s, 3H, OCH3), 6.97 (d, 2H, H3’ + H5’), 7.62 (d, 2H, H2’ + H6’), 7.81 (s. 1H, H8), 8.00 (d, 1H, H5), 8.22 (D, 1H, H6), 10.27 (s, 1H, NH-D2O exchangeable), 13.60 (s, 2H, NH2- D2O exchangeable), ppm. MS (m/z): 360 (M+), 362 (M + Open Access IJOC  D. H. SOLIMAN 71 2), 206 (M+-O2-NH-C6H5-4-OCH3). Found, %: C; H; N. C 53.47; H 3.66; N, 15.12. C16H13ClN4O4 C, 53.27; H, 3.63; N, 15.53. 13) 3-Amino-7-Chloro-N-(4-ethoxyphenyl)-2-quinoxa- line carboxamide 1,4-di-N-oxide 4m. Yield (84%); mp 219˚C - 220˚C. IR (KBr, cm−1): 1663 (C=O), 1613 (C=N), 3255, 3389 (NH, NH2), 1351 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 3.75 (s, 3H, OCH3), 6.97(d, 2H, H3’ + H5’), 7.62(d, 2H, H2’ + H6’), 7.81 (s. 1H, H8), 8.00 (d, 1H, H5), 8.22 (D, 1H, H6), 10.27 (s, 1H, NH-D2O exchangeable), 13.60 (s, 2H, NH2- D2O exchangeable), ppm. MS (m/z): 360 (M+), 362 (M + 2), 206 (M+-O2-NH-C6H5-4-OCH3). Found, %: C; H; N. C 53.07; H 3.26; N, 15.12. C16H13ClN4O4 C, 53.27; H, 3.63; N, 15.53. 14) 3-Amino-7-Chlo ro -N-(pyridin-4-yl)-2-quinoxaline carboxamide 1,4-di-N-oxide 4n. Yield (63%); mp 223˚C. IR (KBr, cm−1): 1658 (C=O), 1554 (C=N), 3202 - 3325(NH, NH2), 1251 (NO). 1H NMR spectrum (DMSO-d6), δ, ppm 7.03 (d, 1H, H5), 7.91 (d, 1H, H6), 8.14 (d, 2H, H2’ + H6’), 8.17 (s. 1H, H8), 8.34 (d, 2H, H3’ + H5’), 10.27 (s, 1H, NH-D2O ex- changeable), 13.60 (s, 2H, NH2-D 2O exchangeable), ppm. MS (m/z): 331 (M+), 333 (M + 2), 317 (M+-O), 302 (M+-O2), 208(M+-NH-pyridine), 192(M+-O2-NH-pyridine- NH2). Found, %: C; H; N. C 49.77; H 2.96; N, 21.15. C14H10ClN5O3C, 50.69; H, 3.04; N, 21.11. 4.1.5. 3,3-Dimethyl-1-Oxo-1,2,3,4-Tetrahydrophenazine 5,10-Dioxide 5 A mixture of 5(6)-substituted benzofuroxane 1a (0.01 mol) and dimidone (0.01 mol) in ethanol (50 mL) was stirred and heated at 60˚C, while ammonia gas was bub- bled into the solution for 1 hr. The solution was stirred at room temperature for another 11 hr. The solvent was then removed and finally a yellow precipitate was ob- tained which was recrystallized out from ethanol. Yield 75%; mp. 267˚C - 268˚C. IR (KBr, cm−1): 1670 (C=O), 1627 (C=N), 1319, 1340 (NO). 1H NMR spec- trum (DMSO-d6), δ, ppm 1.16, 1.22 (2s, 6H, 2CH3), 3.05 (s, 2H, H2), 3.34 (s, 2H, H4), 7.76 (m, 2H, H7), 7.86 (m, 2H, H8), 8.43 (d, 1H, H6), 8.61 (d, 1H, H9). Found, %: C; H; N. C 65.17; H 5.36; N, 11.12. C14H14N2O3, C, 65.11; H, 5.46; N, 10.85. 4.1.6. 11-Oxo-11H-inde no[1,2-b]Quinoxaline 5,10-Dioxide 6 A solution of 5(6)-substituted benzofuroxane 1a and in- danedione (0.01 mol) inethanol (50 Ml) was stirred for 12 hr at room temperature in the presence of morpholine as a catalyst. The red precipitate separated after standing at room temperature for an additional 12 hr. The product was finally filtered and recrystallized out from ethanol. Yield 61%; mp. 222˚C - 225˚C. IR (KBr, cm−1): 1715 (C=O), 1642 (C=N), 1321, 1345 (NO).1H NMR spectrum (DMSO-d6), δ, ppm 7.68 (m, 2H, H7 + H8), 7.73 (d, 2H, H6 + H9), 8.00 (m, 1H, H3), 8.03 (m, 1H, H2), 8.35 (d, 2H, H4), 8.85 (d, 1H, H1), MS (m/z): 264 (M+), 248 (M+-O), 232 (M+-O2), 204(M+-CO). Found, %: C; H; N. C 69.01; H 2.66; N, 11.12. C15H8N2O3C, 68.18; H, 3.05; N, 10.60. 4.2. Antimicrobial and Antifungal Assays Antimicrobial activity was determined using the agar well diffusion assay method as described by [20]. The tested organisms were subcultured on nutrient agar me- dium (Oxoid laboratories, UK) for bacteria and Saboroud dextrose agar (Oxoid laboratories, UK) for fungi. Am- picillin and Gentamycin were used as a positive control for bacterial strains. Amphotericin B was used as a posi- tive control for fungi. The plates were done in triplicate. Bacterial cultures were incubated at 37˚C for 24 h while the other fungal cultures were incubated at (25˚C - 30˚C) for 3 - 7 days. Antimicrobial activity was determined by measurement zone of inhibition [21]. Determination of MIC The minimum inhibitory concentration (MIC) of the samples was estimated for each of the tested organisms in triplicates. Varying concentrations of the samples (1000 - 0.007 µg/ml), nutrient broth were added and then a loopful of the test organism previously diluted to 0.5 McFarland turbidity standard was introduced to the tubes. A tube containing broth media only was seeded with the test organisms to serve as control. Tubes containing tested organisms cultures were then incubated at 37˚C for 24 h while the other fungal cultures were incubated at (25˚C - 30˚C) for 3 - 7 days. The tubes were then exam- ined for growth by observing for turbidity [22]. REFERENCES [1] Y. Deepika, P. Surendra, N. K. Sachin and S. Shewta, “Biological Activity of Quinoxaline Derivatives,” Inter- national Journal of Current Pharmaceutical Review and Research, Vol. 1, No. 3, 2011, pp. 33-46. [2] B. Ganley, G. Chowdhury, J. Bhansali, J. S. Daniels and K. S. Gates, “Redox-Activated, Hypoxia-Selective DNA Cleavage by Quinoxaline 1,4-di-N-oxide,” Bioorganic and Medicinal Chemistry, Vol. 9, No. 9, 2001, pp. 2395- 2401. http://dx.doi.org/10.1016/S0968-0896(01)00163-8 [3] R. G. Glushkov, T. I. Vozyakova, E. V. Adamskaya, S. A. Aleinikova, T. P. Radkevich, L. D. Shepilova, E. N. Pa- deiskaya and T. A. Gus’kova, “Synthesis and Antibacte- rial Activity of New Derivatives of 1,4-di-N-Oxides of Quinoxaline,” Pharmaceutical Chemistry Journal, Vol. 28, No. 1, 1994, pp. 17-20. http://www.springer.com/10.1007/BF02218945 [4] E. Vicente, R.Villar, A. Burguete, B. Solano, S. Pérez- Silanes, I. Aldana, J. A. Maddry, A. J. Lenaerts, S. G. Open Access IJOC  D. H. SOLIMAN Open Access IJOC 72 Franzblau, S. Cho, A. Monge and R. C. Goldman, “Effi- cacy of Quinoxaline-2-Carboxylate 1,4-Di-N-Oxide De- rivatives in Experimental Tuberculosis,” Antimicrobial Agents and Chemotherapy, Vol. 52, No. 9, 2008, pp. 3321-3326. http://dx.doi.org/10.1128/AAC.00379-08 [5] M. Loriga, A. Nuvole, G. Paglietti, G. Fadda and S. Za- netti, “2-Phenyl-6(7)-R Substituted Quinoxalines N-Oxi- des. Synthesis, Structure Elucidation and Antimicrobial Activity,” European Journal of Medicinal Chemistry, Vol. 25, No. 6, 1990, pp. 527-532. http://dx.doi.org/10.1016/0223-5234(90)90147-U [6] D. Benitez, M. Cabrera, P. Hernández, L. Boiani, M. L. Lavaggi, R. Di Maio, G. Yaluff, E. Serna, S. Torres, M. E. Ferreira, N. Vera de Bilbao, E. Torres, S. Pérez-Silanes, B. Solano, E. Moreno, I. Aldana, A. López de Ceráin, H. Cerecetto, M. González and A. Monge, “Trifluorome- thylquinoxaline N,N'-dioxides as Anti-Trypanosomatid Agents. Identification of Optimal Anti-T. Cruzi Agents and Mechanism of Action Studies,” Journal of Medici- nal Chemistry, Vol. 54, No. 10, 2011, pp. 3624-3636. http://dx.doi.org/10.1021/jm2002469 [7] G. Aguirre, H. Cerecetto, R. Di Maio, M. González, M. E. Alfaro, A. Jaso, B. Zarranz, M. A. Ortega, I. Aldana and A. Monge-Vega, “Quinoxaline N,N'-Dioxide Derivatives and Related Compounds as Growth Inhibitors of Try- panosoma Cruzi. Structure-Activity Relationships” Bio- organic and Medicinal Chemistry Letters, Vol. 14, No. 14, 2004, pp. 3835-3839. http://dx.doi.org/10.1016/j.bmcl.2004.04.088 [8] A. Carta, M. Loriga, G. Paglietti, A. Mattana, P. L. Fiori, P. Mollicotti, L. Sechi and S. Zanetti, “Synthesis Anti- Mycobacterial, Anti-Trichomonas and Anti-Candida in Vitro Activities of 2-Substituted-6,7-Diflouro-3-Methyl- quinoxaline 1,4-dioxides,” European Journal of Medici- nal Chemistry, Vol. 39, No. 2, 2004, pp. 195-203. http://dx.doi.org/10.1016/j.ejmech.2003.11.008 [9] S. Ancizu, E. Moreno, E. Torres, A. Burguete, S. Pérez- Silanes, D. Benítez, R. Villar, B. Solano, A. Marín, I. Aldana, H. Cerecetto, M. González and A. Monge, “Het- erocyclic-2-Carboxylic Acid (3-Cyano-1,4-di-Noxide- quinoxalin-2-yl) Amide Derivatives as Hits for the De- velopment of Neglected Disease Drugs,” Molecules, Vol. 14, No. 6, 2009, pp. 2256-2272. [10] A. Jaso, B. Zarranz, I. Aldana and A. Monge, “Synthesis of New Quinoxaline-2-Carboxylate 1,4-Dioxide Deriva- tives as Anti-Mycobacterium Tuberculosis Agents,” Jour- nal of Medicinal Chemistry, Vol. 48, No. 6, 2005, pp. 2019-2025. http://dx.doi.org/10.1021/jm049952w [11] E. Moreno, S. Ancizu, S. Pérez-Silanes, E. Torres, I. Al- dana and A. Monge, “Synthesis and Antimycobacterial Activity of New Quinoxaline-2-Carboxamide 1,4-di-N- Oxide Derivatives,” European Journal of Medicinal Che- mistry, Vol. 45, No. 10, 2010, pp. 4418-4426. http://dx.doi.org/10.1016/j.ejmech.2010.06.036 [12] E. Vicente, S. Pérez-Silanes, L. M. Lima, S. Ancizu, A. Burguete, B. Solano, R. Villar, I. Aldana and A. Monge, “Selective Activity against Mycobacterium tuberculosis of New Quinoxaline 1,4-di-N-Oxides,” Bioorganic and Medicinal Chemistry, Vol. 17, No. 2, 2009, pp. 385-389. http://dx.doi.org/10.1016/j.bmc.2008.10.086 [13] B. Zarranz, A. Jaso, I. Aldana and A. Monge, “Synthesis and Antimycobacterial Activity of New Quinoxaline-2- Carboxamide 1,4-di-N-Oxide Derivatives,” Bioorganic and Medicinal Chemistry, Vol. 11, No. 10, 2003, pp. 2149-2156. http://dx.doi.org/10.1016/S0968-0896(03)00119-6 [14] A. Monge, J. A. Palop, M. Gonzaliz, F. J. Martinez Cre- spo, A. Lopez de Cerain, Y. Sainz and S. Narro, “New Hypoxia-Selective Cytotoxines Derived from Quinoxa- line 1,4-Dioxides,” Journal of Heterocyclic Chemistry, Vol. 32, No. 4, 1995, pp. 1213-1217. http://dx.doi.org/10.1002/jhet.5570320420 [15] M. J. Haddadin, G. A. gopian, C. H. Issidorides, “Synthe- sis and Photolysis of Some Substituted Quinoxaline di-N- Oxides,” Journal of Organic Chemistry, Vol. 36, No. 4, 1971, pp. 514-517. http://dx.doi.org/10.1021/jo00803a005 [16] M. A. Ortega, Y. Sainz, M. E. Montoya, A. Jaso, B. Zar- ranz, I. Aldana and A. Monge, “Anti-Mycobacterium tu- berculosis Agents Derived from Quinoxaline-2-Carbo- nitrile and Quinoxaline-2-Carbonitrile 1,4-di-N-oxide,” Arzneimittelforschung, Vol. 52, 2002, pp. 113-119. [17] K. M. Amin, M. M. F. Ismail, E. Noaman, D. H. Soliman and Y. A. Ammar, “New Quinoxaline 1,4-di-N-Oxides. Part 1: Hypoxia-Selective Cytotoxins and Anticancer Agents Derived from Quinoxaline 1,4-di-N-Oxides,” Bio- organic and Medicinal Chemistry, Vol. 14, 2006, pp. 6917-6923. http://dx.doi.org/10.1016/j.bmc.2006.06.038 [18] G. W. H. Cheeseman, “Condensed Pyrazines,” Wiley and Sons, New York, 1979, p. 35. [19] B. Zarranz, A. Jaso, I. Aldana and A. Monge, “Synthesis and Anticancer Activity Evaluation of New 2-Alkylcar- bonyl and 2-Benzoyl-3-Trifluoromethyl-Quinoxaline 1,4- di-N-oxide Derivatives,” Bioorganic and Medicinal Che- mistry, Vol. 12, No. 37, 2004, pp. 3711-3721. http://dx.doi.org/10.1016/j.bmc.2004.04.013 [20] I. A. Holder and S. T. Boyce, “Agar Well Diffusion As- say Testing of Bacterial Susceptibility to Various Antim- icrobials in Concentrations Non-Toxic for Human Cells in Culture,” Burns, Vol. 20, 1994, pp. 426-429. http://dx.doi.org/10.1016/0305-4179(94)90035-3 [21] H. Agwa, M. M. Aly and R. Bonaly, “Isolation and Char- acterization of Two Streptomyces Species Produced Non Polyenic Antifungal Agents,” Journal of Union Arab Bioogyl, Vol. 7, 2000, pp. 62-82. [22] J. H. Doughari, “Antimicrobial Activity of Tamarindus indica Linn. Trop,” Journal of Pharmaceutical Research, Vol. 5, No. 2, 2006, pp. 597-603.
|