 Vol.4, No.11, 614-619 (2013) Agricultural Sciences http://dx.doi.org/10.4236/as.2013.411082 Extraction and characterization of gelling pectin from the peel of Poncirus trifoliata fruit Kouassi L. Koffi1, Beda M. Yapo2*, V. Besson3 1Food Research Unit, Polytechnique National Institute of Felix Houphouet Boigny, Abidjan, Côte d’Ivoire 2Subunit of Pedagogy in Biochemistry and Microbiology, University of Jean-Lourougnon Guédé, Daloa, Côte d’Ivoire; *Corresponding Author: bedamarcel@yahoo.fr 3Food Research and Technology Division, Cargill West Africa, Abidjan, Côte d’Ivoire Received 6 August 2013; revised 26 September 2013; accepted 18 October 2013 Copyright © 2013 Kouassi L. Koffi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT In the framework of searching for new pectin sources to partially compensate for domestic and regional demands, the peel (albedo) of the “non-comestible” fruit of Poncirus trifoliata was investigated using a relatively simple experi- ment al design for optimization, in which only the variable was the extraction pH (1.0, 1.5, and 2.0) on the basis of our previous studies on diverse pectin sources. The results showed that the yield of pectin (7.4% - 19.8%) was strongly in- fluenced by the extraction pH when the other parameters, namely the solid to liquid extractant (S/L) ratio, temperature (T ˚C), and time (t) were fixed to 1:25 ( w/v), 75˚C, a nd 90 mi n, re spec tiv ely. Likewise, the galacturonic acid content (GalA: 61.4% - 79.2%), total neutral sugar content (TNS: 9.1% - 22.5%), degree of branching (3.5% - 13.9%), homogalacturonan (HG) to rhamnoga- lacturonan-I (RG-I) ratio (2.2 - 5.6), degree of me- thyleste rificatio n (DM: 54 - 77), v i scosity av erage molecular weight (Mν: 57 - 82 ), and ge lling capac- ity (GC: 12 4 - 158) were all affe cted by the extrac- tion pH. The optimum pH for producing pectin with good yield, quality characteristics (GalA > 65%, DM > 60, Mν > 80 kDa), and gelling capacity (GC > 150), from the peel of P. trifoliata fruit, was found to be pH 1.5. Keywords: Ponciru s trifoli ata; Pectin; Block Copolymers; Macromolecular Characte ristics; Gelling Strength 1. INTRODUCTION Pectins are natural polysaccharides from all higher plant cell walls. They are generally composed of -D- galactopyranosyluronic acid ( -D-GalpA), and three neutral sugars, namely -L-rhamnopyranose ( -L-Rhap), -D-galactopyranose ( -D-Galp), and -L-arabinofu- ranose ( -L-Araf). The different sugars are linked to one another in the way that gives rise to pectin structure with two-to-three block copolymers, viz. homogalacturonan (HG), type one rhamnogalacturonan (RG-I), and type two rhamnogalacturonan (RG-II) to a lesser extent. HG is an 1,4- -D-GalpA polymer esterified with methyl al- cohol at C-6 position and sometimes with acetic acid at O-2 and/or O-3 positions. RG-I is an [1,4)- -D-GalpA- 1,2- -L-Rhap-(1,4] polymer, commonly branched with 1,5- -L-arabinan, 1,4- -D-galactan, and type one arabi- nogalactan. RG-II is a rhamnose-containing polymer, which has an oligogalacturonan core branched with four well-defined side chains [1. However, it remains a mat- ter of debate if all the block copolymers are covalently linked to one another, and how they are likely intercon- nected to one another within pectin structure, due to in- sufficiency in the availability of compelling and irrefuta- ble body of structural data. Nevertheless, it is worth un- derlying that three basic models have been proposed in the literature [2-4. The main functional property so far known to pectins is their ability to form gelling systems under specified conditions [5,6. It is now well known that the pectin HG domain is responsible for its gelling capability, while the pectin RG-I region chiefly plays a gel-stabilising role [6,7. Two different gelling mechanisms have been de- scribed, depending on the methylesterification degree (DM) of pectins. Thus, high methylesterified pectins (DM > 50%) are commonly used to prepare sugar-acid- mediated gels (HMP-SAG) and low methyl-esterified pectins (DM 50%) are utilized for the preparation of calcium-mediated (low calorie) gels. To date, commer- cial pectins, used for the preparation of different confec- Copyright © 2013 SciRes. OPEN ACCES S  K. L. Koffi et al. / Agricultural Sciences 4 (2013) 614-619 615 tions and gelling products (such as marmalades, jams, preserves, and low calorie gels), are principally produced, in Europe and in the United States of America, from two pectin-rich sources (lime albedo and apple pomace) un- der specified industrial extraction conditions: dry raw material to solvent ratio 1:35 - 1:15 (w/v), water acidi- fied with HNO3 (or HCl) to pH 1.0 - 3.0, temperature 60˚C - 100˚C, and time 30 - 180 min. Thus, commercial pectin import in emerging and especially in developing countries to satisfy their demand represents a high cost operation with low added values to domestically manu- factured gelling products. As a result, local food distribu- tion firms in developing countries such as Côte d’Ivoire are thus compelled to import ready-to-eat gelling prod- ucts. To partially remedy this problem, local agricul- tural byproducts, able to yield pectins with good gelling properties, are currently being searched for to compen- sate for the high cost linked with import of commercial lime and/or apple pectins. In this connection, the present study reports on the isolation and characterization of pec- tin with good yield, quality characteristics, and gelling capacity from the peel (albedo) of the underutilized Poncirus trifoliata fruit, a locally abundant and hitherto unexploited pectin source. The Poncirus fruit is a “non- comestible” fruit, because of its intense bitterness caused by the presence of poncirin and is so far locally proc- essed by naturotherapeutists who commonly use the fruit seeds, juice and essential oil (from the flavedo) as tradi- tional remedies against malaria and insect (mosquito and ant) bites. 2. MATERIAL AND METHODS 2.1. Pectin Extraction Fresh peels (albedo and flavedo) of the fruit of Poncirus trifoliata were separately collected from a me- dium-size plant of local producers of Poncirus juice, seeds and essential oil, in a city named Bacon, located in the southeastern region of Côte d’Ivoire. The peels were immediately blanched to prevent enzymatic degradation of the cell wall pectins, and oven-dried to about 7% final moisture content. The dried peels were ground in a hammer mill (Model 912, Winona Attrition Mill Co., Winona, MN) to pass through a 12 mm mean diameter size sieve and kept under moisture-free conditions until utilisation. Pectins were extracted only from the albedo using a relatively simple experimental design for optimization. On the basis of our previous work on other pectin sources, the solid to liquid (extractant) ratio (S/L), tem- perature (T ˚C), and time (t) were invariably fixed to 1:25 (w/v), 75˚C, and 90 min, respectively, and only the extraction pH was varied from 1.0 to 2.0 per 0.5 unit interval (1.0, 1.5 and 2.0). The peel was extracted twice before discarding insoluble fraction. At the end of each extraction, the slurry was filtered and pectin extract was rapidly brought to pH 4 for stability. The first and second extracts were combined, concentrated to desired volume, and then precipitated in 3 volumes of 95% ethanol at 5˚C for 2 h. Pectin precipitates were washed (twice) with 70% ethanol, followed by 95% ethanol and dry acetone, and kept for a while under a fume extractor (for residual acetone evaporation) and finally oven-dried at 45˚C overnight and weighed. Extraction of pectins was per- formed in three independent runs for each pH value. Dried pectin flakes were finely ground to pass through 60 mesh (0.25 mm) size sifters. Pectin flours were canned in plastic containers and kept at room tempera- ture under airless and moisture-free conditions until use. 2.2. Pectin Characterization 2.2.1. Pectin Purification Prior to characterization, the pectin samples were treated with a mixture of 1% (v/v) HCl/60% (v/v) etha- nol (three times), and the remaining pectin fractions were exhaustively washed with 60% (v/v) ethanol until the filtrate gave a negative response for chloride ions with silver nitrate, indicating that no free sugars were present within the so purified samples. This treatment indeed aimed at removing free sugars and salts and converting all the non-methylestrified carboxyl groups of pectin macromolecules to the free acid (-COOH) form for a correct titration with 1 N NaOH. Commercial citrus high methoxy pectin (CCHMP: 95% DM, Sigma-Aldrich Co., St. Louis, MO) was used for comparison, especially with regard to functional (gelling) properties, after enzymatic- controlled partial desertification to 74% DM (with or- ange peel PME (P5400) Sigma-Aldrich Co. St. Louis, MO), which did not significantly affect its sugar compo- sition and molecular characteristics [7. Pectins were characterized for the sugar composition, esterification degree, molecular weight, and gelling capacity. 2.2.2. Pectin Analysis The GalA content of pectin samples was colorimetri- cally quantified at 525 nm by a modified sulfamate- meta-hydroxydiphenyl (MHDP) assay using monoGalA standard [8. To assess the neutral sugar (NS) content, pectins were first hydrolyzed with 1 mol·L−1 H 2SO4 (100˚C, 3 h) and freed NS, notably galactose/arabinose (Megazyme procedure) and rhamnose [9 were quanti- fied spectrophotometrically at 340 nm using Megazyme assay kits (Megazyme International Ireland Ldt., Bray, Co. Wicklow, Ireland). The two NS assays were based on the quantitative oxidation of galactose/arabinose and rhamnose to corresponding lactonic derivatives (D-ga- lactono-(1,4)-lactone for α-L-Arabinose and β-D-Galac- tose and L-rhamno-(1,4)-lactone for α-L-rhamnose) in Copyright © 2013 SciRes. OPEN ACCES S  K. L. Koffi et al. / Agricultural Sciences 4 (2013) 614-619 616 the presence of corresponding dehydrogenases (β-ga- lactose dehydrogenase (β-GalDH) plus galactose mutaro- tase (GalM) for α-L-arabinose and β-D-galactose, and L- rhamnose dehydrogenase for α-L-rhamnose) and the co- enzyme NAD+, which is stoichiometrically reduced to NADH with maximum absorbance measured at 340 nm. D-galactose was quantitatively differentiated from L- arabinose by reading absorbance at different reaction times, viz. after 6 min- and 12 min-reaction at room tem- perature, respectively. L-rhamnose was quantitatively determined after 1 h-reaction at room temperature. Total neutral sugar (TNS) was estimated either by the tri-re- agent (anthrone, orcinol, and MHDP) colorimetric- H2SO4 assay as reported previously [7 or by calculating the sum of the amounts of the three NS determined. The molar ratio of HG to RG-I block copolymers was roughly estimated using relation 1, reequated from pre- viously published work [1,10. GalA%Rha % HG RG-I%=1002Rha %Ara %Cal% (1) The degree of branching (DBr) of pectins rhamnosyl residues with arabinose- and galactose-containing side chains was roughly estimated, by Equation (2), as previ- ously reported [11. DBr100 Rha%Ara%Gal% (2) It expressed the minimum number of rhamnosyl resi- dues per 100 arabinosyl and galactosyl residues of pectin, which should be distinguished from the minimum length of arabinose- and galactose-containing side chains. The lower the DBr, the higher the level of branching of the pectin rhamnosyl residues. The overall esterification degree of pectins was poten- tiometrically determined as previously described [12. The acetylesterification degree (DAc) was colorimetri- cally measured at 510 nm by the hydroxamic acid assay using glucose pentaacetate standard [13, and the me- thylesterification degree (DM) was differentially evalu- ated. All the measurements were performed in triplicates. The molecular weight of pectin samples was analysed by GFC on a high resolution Superdex-200 HR 10/30 column (Amersham Biosciences Corp., NJ). A molecular weight kit of pullulan standards (Mw 6.0, 10.0, 21.7, 48.8, 113.0, 210.0, 393.0, and 805.0 kDa; Mw wn M 1.0-1.2) from American Polymer Standards Corp. (Mentor, OH) and homogenous HG standards (Mw 60 and 100 kDa, wn M 1.0-1.2) [14 with known in- trinsic viscosity ([ ]) and Mw values were used for cali- bration. To better evaluate the pectin Mν, the so-called universal calibration technique (UCT) was used by plot- ting log ([ ] Mw) versus the elution volume (Ve) of standards. Analyses were performed in triplicates. 2.3. Gelling Properties The gelling capacity (or power) of pectins was evalu- ated by the determination of the strength of gels prepared under the following conditions: 65.0% soluble solids (sucrose), 0.70 wt% pectin, pH 2.3, as previously de- scribed [12. 2.4. Statistical Analysis The data were statistically evaluated by the global test of a single-factor analysis of variance (ANOVA), fol- lowed by the Bonferroni’s posthoc test for multiple com- parisons, whenever applicable, using a GraphPad Prism V.3 software (GraphPad software Inc., San Diego, CA). Means of different treatments were considered signifi- cantly different at p < 0.05. 3. RESULTS AND DISCUSSION 3.1. Extraction Yield of Pectins The yield of extracted pectins from the peel P. trifoliata fruit is shown in Table 1. The yield varied from 7.4% to 19.8% as the pH was varied from 1.0 to 2.0, in- dicating that the yield of pectins was strongly pH-de- pendent when the other extraction parameters (solid to liquid ratio (S/L), temperature (T ˚C), and time (t)) were kept constant. The different pectin yields were signifi- cantly different from one another (p < 0.05). The yield (12.6%) obtained at pH 2.0 was lower than the yield (19.8%) obtained at pH 1.5, suggesting that the yield tended to increase with increase in the strength of (acid) extracting agent. However, the yield of pectin substan- tially decreased as the strength of extractant was further increased up to pH 1.0. This unexpected drastic drop in the yield of pectin extracted at pH 1.0 could only be ex- plained by considerable degradation of pectin polymers, during solubilization from the cell wall, into smaller oli- gomers, which were ethanol-soluble, and therefore were not precipitated in the final sample. This observation is consistent with various previous studies in which notable degradation of pectins in hot acid media was reported [15-17. In the light of the differences observed in yields, the extraction conditions of S/L (1:25 w/v), T (75˚C), t (90 min), and pH 1.5 appeared to be the optimum condi- tions for isolating pectin with good yield from the peel (albedo) of P. trifoliata fruit. 3.2. Characteristics of Extracted Pectins 3.2.1. Sugar Composition and Block Copoly mers The sugar composition of the extracted pectins is also shown in Ta bl e 1 . The galacturonic acid (GalA) content of pectins ranged from 61.4% to 79.2% as the extraction pH was varied from pH 1.0 to pH 2.0. The pectin GalA amounts were significantly different from one another (p Copyright © 2013 SciRes. OPEN ACCES S 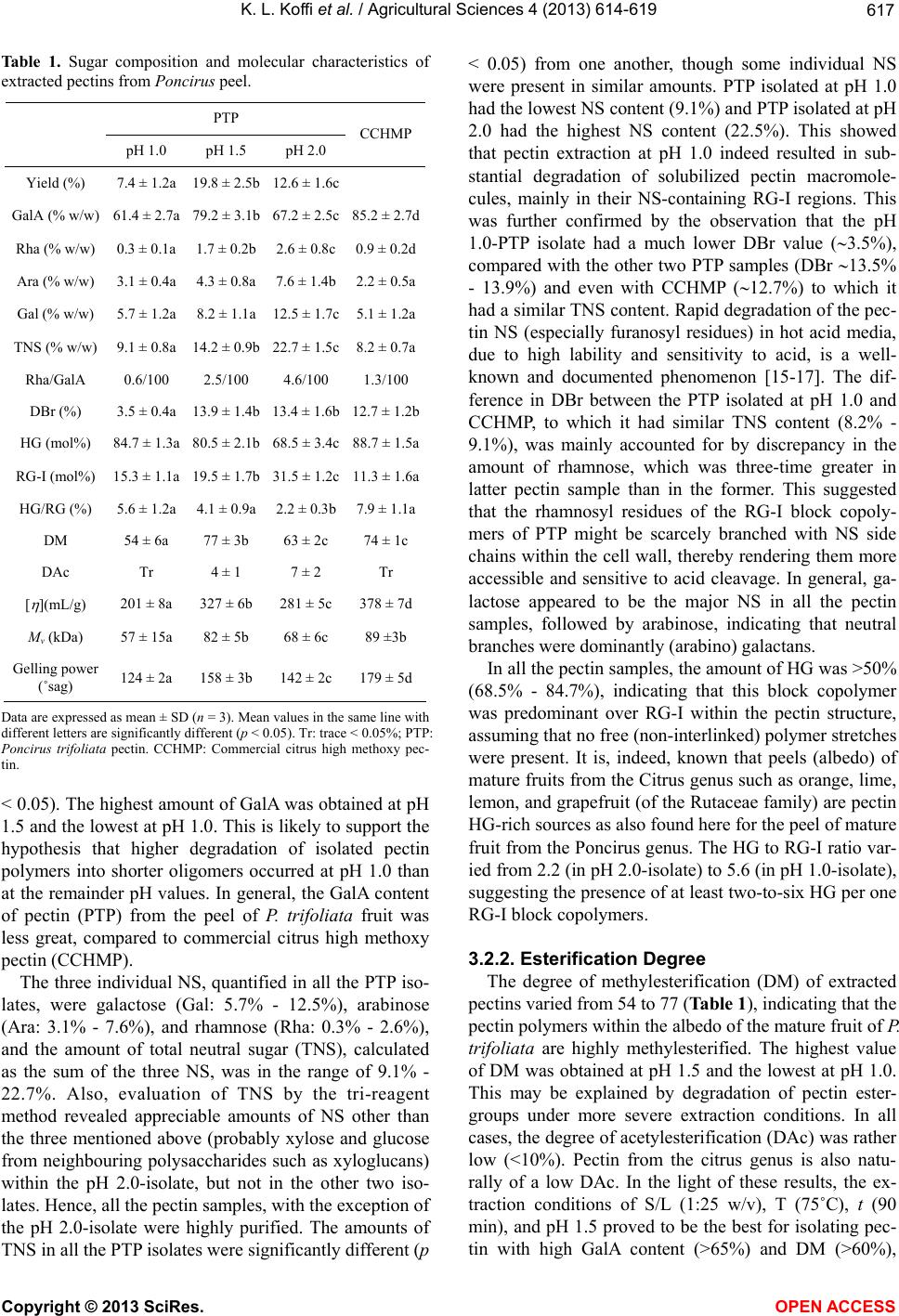 K. L. Koffi et al. / Agricultural Sciences 4 (2013) 614-619 617 Table 1. Sugar composition and molecular characteristics of extracted pectins from Poncirus peel. PTP pH 1.0 pH 1.5 pH 2.0 CCHMP Yield (%) 7.4 ± 1.2a 19.8 ± 2.5b 12.6 ± 1.6c GalA (% w/w) 61.4 ± 2.7a 79.2 ± 3.1b 67.2 ± 2.5c 85.2 ± 2.7d Rha (% w/w) 0.3 ± 0.1a 1.7 ± 0.2b 2.6 ± 0.8c 0.9 ± 0.2d Ara (% w/w) 3.1 ± 0.4a 4.3 ± 0.8a 7.6 ± 1.4b 2.2 ± 0.5a Gal (% w/w) 5.7 ± 1.2a 8.2 ± 1.1a 12.5 ± 1.7c 5.1 ± 1.2a TNS (% w/w) 9.1 ± 0.8a 14.2 ± 0.9b 22.7 ± 1.5c 8.2 ± 0.7a Rha/GalA 0.6/100 2.5/100 4.6/100 1.3/100 DBr (%) 3.5 ± 0.4a 13.9 ± 1.4b 13.4 ± 1.6b 12.7 ± 1.2b HG (mol%) 84.7 ± 1.3a 80.5 ± 2.1b 68.5 ± 3.4c 88.7 ± 1.5a RG-I (mol%) 15.3 ± 1.1a 19.5 ± 1.7b 31.5 ± 1.2c 11.3 ± 1.6a HG/RG (%) 5.6 ± 1.2a 4.1 ± 0.9a 2.2 ± 0.3b 7.9 ± 1.1a DM 54 ± 6a 77 ± 3b 63 ± 2c 74 ± 1c DAc Tr 4 ± 1 7 ± 2 Tr [ ](mL/g) 201 ± 8a 327 ± 6b 281 ± 5c 378 ± 7d Mv (kDa) 57 ± 15a 82 ± 5b 68 ± 6c 89 ±3b Gelling power (˚sag) 124 ± 2a 158 ± 3b 142 ± 2c 179 ± 5d Data are expressed as mean ± SD (n = 3). Mean values in the same line with different letters are significantly different (p < 0.05). Tr: trace < 0.05%; PTP: Poncirus trifoliata pectin. CCHMP: Commercial citrus high methoxy pec- tin. < 0.05). The highest amount of GalA was obtained at pH 1.5 and the lowest at pH 1.0. This is likely to support the hypothesis that higher degradation of isolated pectin polymers into shorter oligomers occurred at pH 1.0 than at the remainder pH values. In general, the GalA content of pectin (PTP) from the peel of P. trifoliata fruit was less great, compared to commercial citrus high methoxy pectin (CCHMP). The three individual NS, quantified in all the PTP iso- lates, were galactose (Gal: 5.7% - 12.5%), arabinose (Ara: 3.1% - 7.6%), and rhamnose (Rha: 0.3% - 2.6%), and the amount of total neutral sugar (TNS), calculated as the sum of the three NS, was in the range of 9.1% - 22.7%. Also, evaluation of TNS by the tri-reagent method revealed appreciable amounts of NS other than the three mentioned above (probably xylose and glucose from neighbouring polysaccharides such as xyloglucans) within the pH 2.0-isolate, but not in the other two iso- lates. Hence, all the pectin samples, with the exception of the pH 2.0-isolate were highly purified. The amounts of TNS in all the PTP isolates were significantly different (p < 0.05) from one another, though some individual NS were present in similar amounts. PTP isolated at pH 1.0 had the lowest NS content (9.1%) and PTP isolated at pH 2.0 had the highest NS content (22.5%). This showed that pectin extraction at pH 1.0 indeed resulted in sub- stantial degradation of solubilized pectin macromole- cules, mainly in their NS-containing RG-I regions. This was further confirmed by the observation that the pH 1.0-PTP isolate had a much lower DBr value (3.5%), compared with the other two PTP samples (DBr 13.5% - 13.9%) and even with CCHMP (12.7%) to which it had a similar TNS content. Rapid degradation of the pec- tin NS (especially furanosyl residues) in hot acid media, due to high lability and sensitivity to acid, is a well- known and documented phenomenon [15-17. The dif- ference in DBr between the PTP isolated at pH 1.0 and CCHMP, to which it had similar TNS content (8.2% - 9.1%), was mainly accounted for by discrepancy in the amount of rhamnose, which was three-time greater in latter pectin sample than in the former. This suggested that the rhamnosyl residues of the RG-I block copoly- mers of PTP might be scarcely branched with NS side chains within the cell wall, thereby rendering them more accessible and sensitive to acid cleavage. In general, ga- lactose appeared to be the major NS in all the pectin samples, followed by arabinose, indicating that neutral branches were dominantly (arabino) galactans. In all the pectin samples, the amount of HG was >50% (68.5% - 84.7%), indicating that this block copolymer was predominant over RG-I within the pectin structure, assuming that no free (non-interlinked) polymer stretches were present. It is, indeed, known that peels (albedo) of mature fruits from the Citrus genus such as orange, lime, lemon, and grapefruit (of the Rutaceae family) are pectin HG-rich sources as also found here for the peel of mature fruit from the Poncirus genus. The HG to RG-I ratio var- ied from 2.2 (in pH 2.0-isolate) to 5.6 (in pH 1.0-isolate), suggesting the presence of at least two-to-six HG per one RG-I block copolymers. 3.2.2. Esterification Degree The degree of methylesterification (DM) of extracted pectins varied from 54 to 77 (Table 1), indicating that the pectin polymers within the albedo of the mature fruit of P. trifoliata are highly methylesterified. The highest value of DM was obtained at pH 1.5 and the lowest at pH 1.0. This may be explained by degradation of pectin ester- groups under more severe extraction conditions. In all cases, the degree of acetylesterification (DAc) was rather low (<10%). Pectin from the citrus genus is also natu- rally of a low DAc. In the light of these results, the ex- traction conditions of S/L (1:25 w/v), T (75˚C), t (90 min), and pH 1.5 proved to be the best for isolating pec- tin with high GalA content (>65%) and DM (>60%), Copyright © 2013 SciRes. OPEN ACCES S  K. L. Koffi et al. / Agricultural Sciences 4 (2013) 614-619 618 from the peel of P. trifoliata fruit, two of the quality characteristics that need to be fulfilled for possible mass production and marketing. 3.2.3. Macromolecular Features The intrinsic viscosity ([ ]) of PTP varied from 201 to 327 mL/g (Table 1), with the pH 1.0-PTP and pH 1.5- PTP isolates possessing the lowest and highest intrinsic viscosities, respectively (p < 0.05). Nevertheless, these intrinsic viscosities were great enough, suggesting that PTP macromolecules probably had an overall extended rod-like conformation, as imposed by the dominance of HG over NS-ramified RG-I block copolymers, rather than a compact sphere-like conformation. Likewise, the viscosity-average molecular weight (Mν) of PTP varied from 57 to 82 kDa (Tabl e 1), with the pH 1.0-PTP and pH 1.5-PTP isolates exhibiting the lowest and highest values, respectively (p < 0.05). Both results definitively substantiated the fact that the extraction condition using pH 1.0 induced higher degradation of pectin polymers than the remainder, and that the extraction condition us- ing pH 1.5 was more suitable for producing PTP with a Mν comparable to that of CCHMP having a similar DM. 3.3. Gelling Properties of Extracted Pectins The gelling capacity of PTP varied from 124 to 158 (Ta bl e 1). The pH 1.0-PTP and pH 1.5-PTP isolates dis- played the lowest and highest values, respectively. This difference could be attributed to discrepancy in GalA content, DM, and Mν. The gelling capability of pectin is (one of) the most important factor(s) for labeling as a food additive and international marketing. To date, only citrus (lime) peel and apple pomace are used for the production of commercial pectins in Western countries, mainly because citrus and apple pectins pos- sess high gelling strengths [5,6 in addition for the two raw materials to being available in large quantities. How- ever, new sources such as mango and yellow passion fruit rinds are more and more domestically used in dif- ferent emerging and developing countries such as India, Brazil, and South Africa to a lesser extent to partially solve the problem of high cost pectin import-low added value to locally manufactured gelling products. Our re- sults showed that pectin with good yield, quality charac- teristics and gelling capacity (amply comparable to the benchmark citrus pectin) can be produced from the peel of underutilized Poncirus trifoliata fruit. 4. CONCLUSION This study shows that Poncirus peel is a pectin-rich source. About 20% pectin isolate, with a high galactu- ronic acid content (>65%), methylesterification degree (>60%), viscosity-average molecular weight (>80 kDa), and good gelling capability (>150) can be produced un- der the optimum extraction conditions of 1:25-solid to solvent ratio, 75˚C-temperature, 90 min-time, and pH 1.5. Our future investigation should mainly focus on the rheological (viscoelastic) properties of the pH 1.5-pectin isolate to find out the optimum conditions (temperature, time, and velocity) of gelation for possible small-size industrial production to satisfy the increasing indoors and outdoors demands at a reasonable cost. 5. ACKNOWLEDGEMENTS We are indebted to Cargill West Africa for some financial support. REFERENCES [1] Yapo, B.M. (2011) Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polymer Reviews, 51, 391-413. http://dx.doi.org/10.1080/15583724.2011.615962 [2] Schols, H.A. and Voragen, A.G.J. (1996) Complex pectins: Structure elucidation using enzymes. In: Visser, J. and Voragen A.G.J., Eds., Pectins and Pectinases, Progress in Biotechnology, Elsevier Science, Amsterdam, 3-19. http://dx.doi.org/10.1016/S0921-0423(96)80242-5 [3] Vincken, J.P., Schols, H.A., Oomen R.J.F.J., McCann, M.C., Ulvskov, P., Voragen, A.G.J. and Visser, R.G.F. (2003) If homogalacturonan were a side chain of rham- nogalaturonan I. Implications for cell wall architecture. Plant Physioliogy, 132, 1781-1789. http://dx.doi.org/10.1104/pp.103.022350 [4] Yapo, B.M. (2011) Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydrate Polymers, 86, 373-385. http://dx.doi.org/10.1016/j.carbpol.2011.05.065 [5] May, C.D. (1990) Industrial pectins: Sources, production and applications. Carbohydrate. Polymers, 12, 79-99. http://dx.doi.org/10.1016/0144-8617(90)90105-2 [6] Voragen, A.G.J., Pilnik, W., Thibault, J.-F., Axelos, M.A.V. and Renard, C.M.G.C. (1995) Pectins. In: Stephen, A.M., Ed., Food polysaccharides and Their Applications, Mar- cel Dekker, New York, 287-339. [7] Yapo B.M. and Koffi K.L. (2013) Utilisation of model pectins reveals the effect of demethylated block size fre- quency on calcium gel formation. Carbohydrate Poly- mers, 92, 1-10. http://dx.doi.org/10.1016/j.carbpol.2012.09.010 [8] Yapo, B.M. (2010) Improvement of the compositional quality of monocot pectin extracts contaminated with glucuronic acid-containing components using a step-wise purification procedure. Food and Bioproducts Processing, 88, 283-290. http://dx.doi.org/10.1016/j.fbp.2009.07.001 [9] Turecek, P.L., Buxbaum, E. and Pittner, F. (1989) Quan- titative determination of pectic substances as an example of a rhamnopolysaccharide assay. Journal of Biochemical and Biophysical Methods, 19, 215-222. http://dx.doi.org/10.1016/0165-022X(89)90028-6 Copyright © 2013 SciRes. OPEN ACCES S  K. L. Koffi et al. / Agricultural Sciences 4 (2013) 614-619 Copyright © 2013 SciRes. OPEN ACCES S 619 [10] M’sakni N.H., Majdoub, H., Roudesli, S., Picton, L., Le Cerf, D., Rihouey, C. and Morvan, C. (2006) Composi- tion, structure and solution properties of polysaccharides extracted from leaves of Mesembryanthenum crystal- linum. European Polymer Journal, 42, 786-795. http://dx.doi.org/10.1016/j.eurpolymj.2005.09.014 [11] Yapo, B.M. and Koffi, K. L. (2006) Yellow passion fruit rind—A potential source of low-methoxyl pectin. Journal of Agriculture and Food Chemistry, 54, 2738-2744. http://dx.doi.org/10.1021/jf052605q [12] Yapo, B.M. (2009) Biochemical characteristics and gel- ling capacity of pectin from yellow passion fruit rind as affected by acid extractant nature. Journal of Agriculture and Food Chemistry, 57, 1572-1578. http://dx.doi.org/10.1021/jf802969m [13] McComb, E.A. and McCready, R. M. (1957). Determina- tion of acetyl in pectin and in acetylated carbohydrate polymers. Analytical Chemistry, 29, 819-821. http://dx.doi.org/10.1021/ac60125a025 [14] Yapo, B.M. (2009) Pineapple and banana pectins com- prise fewer homogalacturonan building blocks with a smaller degree of polymerization as compared with yel- low passion fruit and lemon pectins: Implication for gel- ling properties. Biomacromolecules, 10, 717-721. http://dx.doi.org/10.1021/bm801490e [15] Canteri-Schemin, M.H., Fertonani, H.C.R., Waszczynskyj, N. and Wosiacki, G. (2005) Extraction of pectin from ap- ple pomace. Brazilian Archives of Biology and Technol- ogy, 48, 259-266. http://dx.doi.org/10.1590/S1516-89132005000200013 [16] Garna, H., Mabon, N., Nott, K., Wathelet, B. and Paquot, M. (2006) Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chemistry, 96, 477-484. http://dx.doi.org/10.1016/j.foodchem.2005.03.002 [17] Yapo, B.M., Robert, C., Etienne, I., Wathelet, B. and Paquot, M. (2007) Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chemistry, 100, 1356-1364. http://dx.doi.org/10.1016/j.foodchem.2005.12.012
|