 Green and Sustainable Chemistry, 2011, 1, 12-18 doi:10.4236/gsc.2011.11003 Published Online February 2011 (http://www.SciRP.org/journal/gsc) Copyright © 2011 SciRes. GSC The Synergy of Combined Use of DMSO and Bronsted Acid (Ionic Liquid) as a Catalyst ——Part I: Efficient Niementowski synthesis of modified quinazolinones Muthu Kumaradoss Kathiravan1, Rajeshwar Rajendra Jalnapurkar1, Aparna Surendra Chothe1, Trupti Sameer Chitre1, Riyaj Shaukat Tamboli2, Kumar V. Srinivasan*3 1Department of Pharmaceutical Chemistry, AISSMS College of Pharmacy, Near RTO Office, Pune, India 2Pharmacy Department, The Maharaja Sayajirao University of Baroda, India 3Division of Orga ni c C hemi st ry, National Chemical Laboratory, Pune, India E-mail: drmkkathir@gmail.com Received January 19, 2011; revised February 20, 2011; accepted February 24, 2011 Abstract A new rapid and versatile approach using Ionic Liquid/DMSO as a chemical reagent for the synthesis of fused heterocyclic compounds in a highly efficient way is described. This method offers the advantages of proceeding in neutral conditions, giving high to excellent isolated yields (83-92%) for Niementowski synthe- sis with easy workup procedure. The inherent Bronsted acidity of ionic liquid and high polarity of both IL and DMSO resulted in a significant enhancement in the reaction rate. Keywords: Ionic Liquids, DMSO, Niementowski 1. Introduction Rutaecarpine type alkaloids constitute an important class of indolopyridoquinazolinone heterocycles, which be- long to the subgroup of quinazoline type alkaloid, be- longing to Rutaceae family [1]. Rutaecarpine (Figure 1) shows a variety of pharmacological activities including antithrombotic [2], vasorelaxant [3], cyclooxygenase (CoX-2) inhibitor [4,5], thermoregulatory, anti-obesity [6], as well as effects on the cardiovascular and endocrine systems [7]. The progress in the studies of rutaecarpine has been reviewed [8]. Recently numerous thiophene analogues of rutaecarpine [9] as well as quinazoline have shown good anticancer activity [10], analgesic [11], anti-inflammatory [12-14] as well as antiparkinsonims activity [15]. Bioisosterism is a strategy of medicinal chemistry for the rational design of new drug, applied with a lead compound as a special process of molecular modification [16]. Purine bases and their bioisoteric analogs were widely used as building block in combina- torial chemistry. The bioisoteres of purine bases such as thienopyrimidine and pyridopyrimidine possess wide range of biological activity such as PDE inhibitory activ- ity, antiparkinsonism, CNS depressant, analgesic, anti- inflammatory [17-19] etc. Since Niementowski’s preparation of 4(3H)-quina- zolinone by fusing anthranilic acid with formamide [20], several methods aimed toward the synthesis of modified quinazolinones have been pursued [21,22]. Recently a series of 1,3,10,12-tetrasubstituted-8H-pyrido-[2',3':4,5]- pyrimido[6,1-b]quinazolin-8-ones [23] and tetracyclic 1,2,9,11-tetrasubstituted-7H-thieno-[2',3':4,5]pyrimido- [6,1-b]-quinazolin-7-ones [24] has been reported by Laddha et al. However the reaction did not go to comple- tion in our hands as reported by the authors. Each of these methods has one or more of the following drawbacks. For instance, the use of expensive and toxic reagents, harsh reaction conditions, refluxing for a prolonged period of time, tedious work-ups etc. In addition, the known meth- ods made use of volatile organic solvents, leading to com- plex isolation and recovery procedures. Therefore, we sought to develop a more efficient and convenient method that avoids these drawbacks and could be used on both laboratory as well as industrial scale. Ionic Liquid (ILs) have been widely recognized as an efficient synthetic tool and its benefit has been well documented. Ionic liquids have been touted as replace- ments for traditional molecular solvents in synthesis be- cause of most of them are nonvolatility, nonflammability, their stability and ease of recyclability [25]. An extensive  M. K. KATHIRAVAN ET AL. 13 literature describing the diversity of ionic liquids are available [26]. Recently ionic liquid has been designed specifically for nucleophilic aromatic substitutions [27]. Very recently we have investigated the synergy of the combined use of ionic liquid and DMSO in the pro- portion 0.1:1 to synthesize a variety of esters in re- markably short reaction times from acyl or alkyl halides by their reaction with sodium carboxylates in the above -mentioned mixed solvent medium in the absence of any added catalyst under ambient conditions [28,29]. Till now there is no reported method for Niemen- towski’s synthesis using ionic liquids to the best of our knowledge. We aimed to modify the procedure reported till now using ionic liquids which can be applicable not only for fused quinazoline but to a wide range of fused heterocycles such as substituted thienopyrimidine, pyri- dothienopyrimidines, triazinoquinazolines as well as quinazolines. In continuation of our ongoing work on condensed thienopyrimidines and quinazolines [30,31], we herein report the fused heterocyclic derivatives of thieno- pyrimidine and quinazoline as divalent and ring equivalent isosteres of Rutaecarpine. Hence a series of novel heterocyclic compounds belonging to thieno- pyrimidine fused with quinazoline, substituted thio- phene, pyridothiophene and quinazoline fused with substituted thiophene, pyridothiophene, quinazoline were synthesized using ionic liquid and DMSO. We initiated this study with the goal of expanding the effi- cacy and efficiency of the methodologies developed by us using ionic liquids for the synthesis of fused het- erocycles [32]. 2. Result and Discussion The starting material employed thiophene o-aminoesters, their subsequent cyclization with formamide and chlori- nation were done as per our earlier reported method [32]. The latter reaction is either carried out in microwave or by classical heating using polyphosphoric acid. The next step involves the reaction of anthranilic acid and condensed 4-chloro-pyrimidine to give the desired products. It is assumed that formation of products requires an acyl sub- stitution between the pyrimidine nitrogen and the car- boxylic acid group. Figure 1. Structure of rutaecarpine. All the present synthetic methodology for condensa- tion reaction have one or the other draw back of lengthy reaction time, high temperature and in many synthesis the use of Volatile Organic Compounds (VOC’s) such as tetrahydrofuran (Scheme 1) which are detrimental to the environment and solvents that need to be recovered and recycled completely adding to the economics of the process. Our research work devoted to the combined use of ionic liquid and DMSO in 0.1:1 proportion, we extended the investigation of this system towards the present syn- thesis. We have exploited this type of reaction by em- ploying cyclic iminochlorides in the synthesis of fused heterocyclic in an attempt to increase the rate of reaction using ionic liquid. The reaction was initially carried out in DMSO and Ionic liquids [bbim]+ Br- independently. However, in DMSO there was no reaction but in ionic liquid the reac- tion went to completion in 10 hrs. Using the above sol- vent mixture conditions DMSO/IL (1:0.1 proportion), the reaction was completed in just 45-60 min (Scheme 2). The bronsted acidic IL was responsible for promotion of the reaction. We have successfully done first cyclization step using DMSO/IL in 45-60 minutes (Table 1) with better yields and purity. However the conventional heat- ing method of cyclization required 8-24 hr for each reac- tion. It was observed that under similar conditions, the substrate containing o-amino acids underwent condensa- tion with cyclic iminochlorides at a faster rate when compared to o-amino esters. Solvents with hydrogen- bond accepting properties can interact with the protons of the acid, increasing the electron density on the nitro- gen atom and therefore its nucleophilic character. This increase has been shown when DMSO is used as the solvent. Ionic liquids as bronsted acid can bond with sulphonyl oxygen of DMSO and give rise to dimsyl ion. Dimsyl ion can then interact with amine hydrogen of anthranilic acid as well as thiophenes resulting in naked -NH ions. The naked ion is highly reactive giving rise to the observed reaction. Thus the use of ionic liquid in synergy with DMSO was found to accelerate and significantly improved the yield and reduced the time of the reaction. The synergy of combined use of IL and DMSO is evident from the observation that the reaction did not proceed at all either in DMSO or in [bbim]+Br- individually under similar Scheme 1 Copyright © 2011 SciRes. GSC 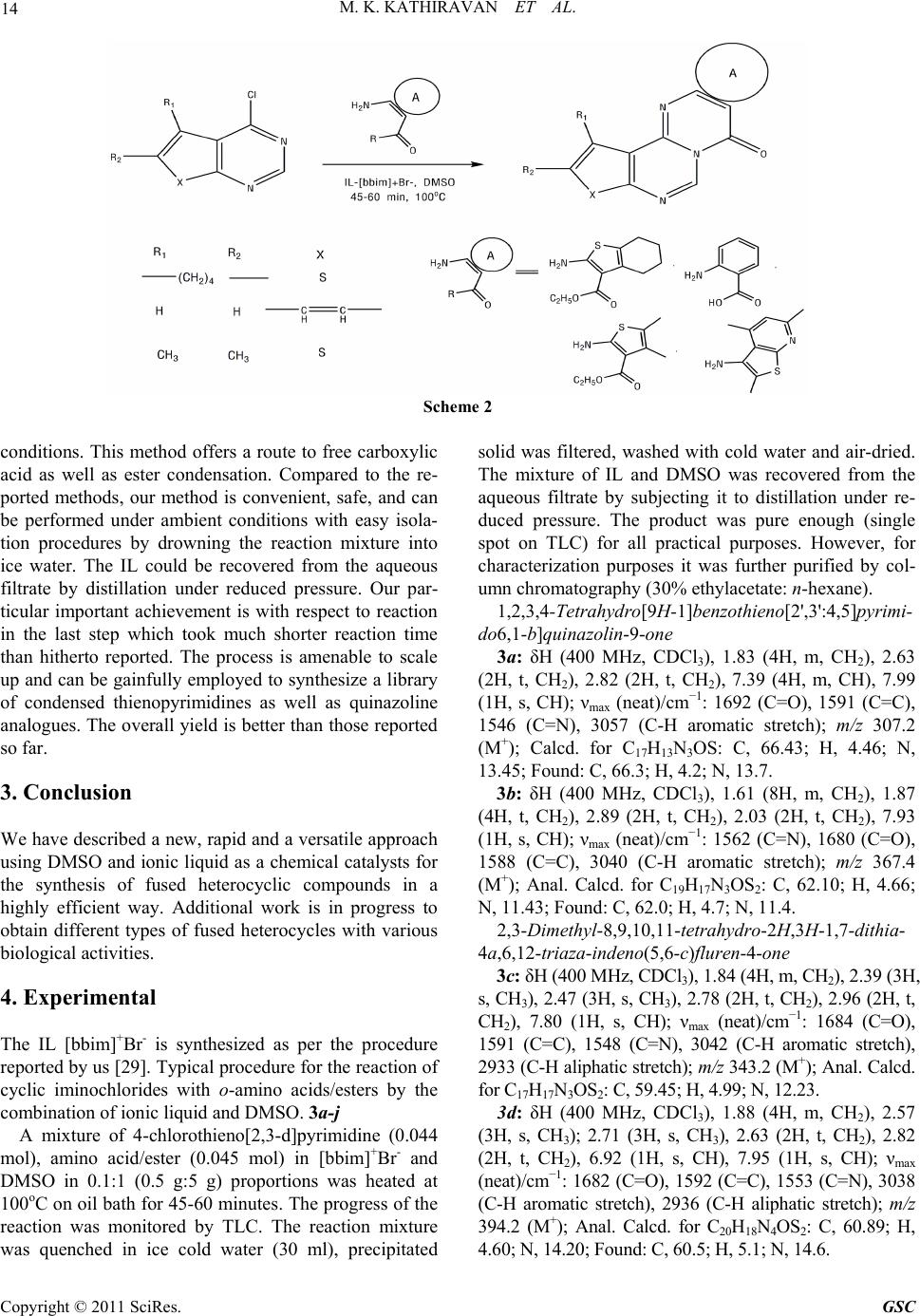 M. K. KATHIRAVAN ET AL. Copyright © 2011 SciRes. GSC 14 Scheme 2 conditions. This method offers a route to free carboxylic acid as well as ester condensation. Compared to the re- ported methods, our method is convenient, safe, and can be performed under ambient conditions with easy isola- tion procedures by drowning the reaction mixture into ice water. The IL could be recovered from the aqueous filtrate by distillation under reduced pressure. Our par- ticular important achievement is with respect to reaction in the last step which took much shorter reaction time than hitherto reported. The process is amenable to scale up and can be gainfully employed to synthesize a library of condensed thienopyrimidines as well as quinazoline analogues. The overall yield is better than those reported so far. 3. Conclusion We have described a new, rapid and a versatile approach using DMSO and ionic liquid as a chemical catalysts for the synthesis of fused heterocyclic compounds in a highly efficient way. Additional work is in progress to obtain different types of fused heterocycles with various biological activities. 4. Experimental The IL [bbim]+Br- is synthesized as per the procedure reported by us [29]. Typical procedure for the reaction of cyclic iminochlorides with o-amino acids/esters by the combination of ionic liquid and DMSO. 3a-j A mixture of 4-chlorothieno[2,3-d]pyrimidine (0.044 mol), amino acid/ester (0.045 mol) in [bbim]+Br - and DMSO in 0.1:1 (0.5 g:5 g) proportions was heated at 100oC on oil bath for 45-60 minutes. The progress of the reaction was monitored by TLC. The reaction mixture was quenched in ice cold water (30 ml), precipitated solid was filtered, washed with cold water and air-dried. The mixture of IL and DMSO was recovered from the aqueous filtrate by subjecting it to distillation under re- duced pressure. The product was pure enough (single spot on TLC) for all practical purposes. However, for characterization purposes it was further purified by col- umn chromatography (30% ethylacetate: n-hexane). 1,2,3,4-Tetrahydro[9H-1]benzothieno[2',3':4,5]pyrimi- do6,1-b]quinazolin-9 -one 3a: δH (400 MHz, CDCl3), 1.83 (4H, m, CH2), 2.63 (2H, t, CH2), 2.82 (2H, t, CH2), 7.39 (4H, m, CH), 7.99 (1H, s, CH); νmax (neat)/cm−1: 1692 (C=O), 1591 (C=C), 1546 (C=N), 3057 (C-H aromatic stretch); m/z 307.2 (M+); Calcd. for C17H13N3OS: C, 66.43; H, 4.46; N, 13.45; Found: C, 66.3; H, 4.2; N, 13.7. 3b: δH (400 MHz, CDCl3), 1.61 (8H, m, CH2), 1.87 (4H, t, CH2), 2.89 (2H, t, CH2), 2.03 (2H, t, CH2), 7.93 (1H, s, CH); νmax (neat)/cm−1: 1562 (C=N), 1680 (C=O), 1588 (C=C), 3040 (C-H aromatic stretch); m/z 367.4 (M+); Anal. Calcd. for C19H17N3OS2: C, 62.10; H, 4.66; N, 11.43; Found: C, 62.0; H, 4.7; N, 11.4. 2,3-Dimethyl-8,9,10,11-tetrahydro-2H,3H-1,7-dithia- 4a,6,12-triaza-indeno(5,6-c)fluren-4-one 3c: δH (400 MHz, CDCl3), 1.84 (4H, m, CH2), 2.39 (3H, s, CH3), 2.47 (3H, s, CH3), 2.78 (2H, t, CH2), 2.96 (2H, t, CH2), 7.80 (1H, s, CH); νmax (neat)/cm−1: 1684 (C=O), 1591 (C=C), 1548 (C=N), 3042 (C-H aromatic stretch), 2933 (C-H aliphatic stretch); m/z 343.2 (M+); Anal. Calcd. for C17H17N3OS2: C, 59.45; H, 4.99; N, 12.23. 3d: δH (400 MHz, CDCl3), 1.88 (4H, m, CH2), 2.57 (3H, s, CH3); 2.71 (3H, s, CH3), 2.63 (2H, t, CH2), 2.82 (2H, t, CH2), 6.92 (1H, s, CH), 7.95 (1H, s, CH); νmax (neat)/cm−1: 1682 (C=O), 1592 (C=C), 1553 (C=N), 3038 (C-H aromatic stretch), 2936 (C-H aliphatic stretch); m/z 394.2 (M+); Anal. Calcd. for C20H18N4OS2: C, 60.89; H, .60; N, 14.20; Found: C, 60.5; H, 5.1; N, 14.6. 4 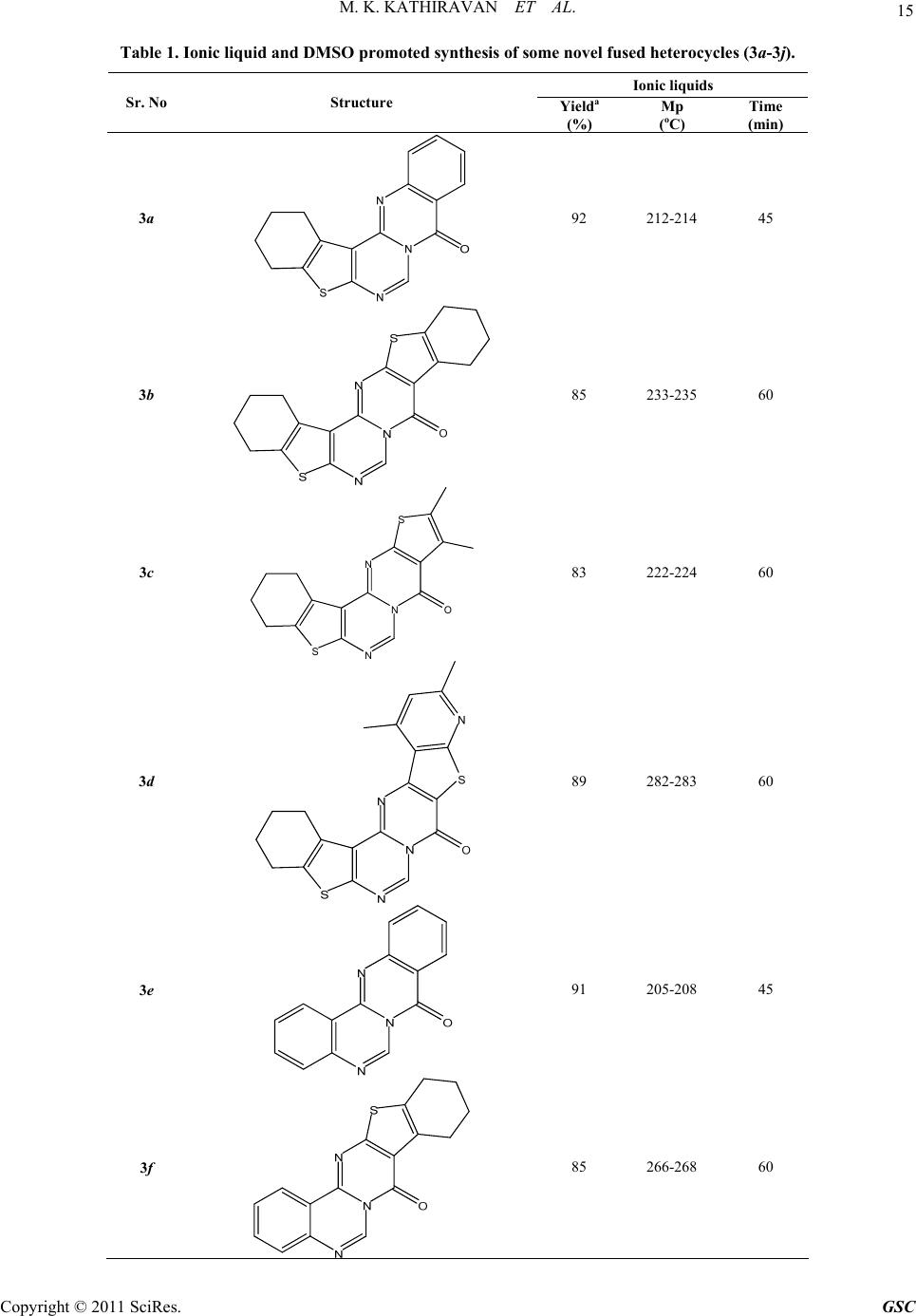 M. K. KATHIRAVAN ET AL. Copyright © 2011 SciRes. GSC 15 Table 1. Ionic liquid and DMSO promoted synthe sis of some novel fused he terocycles (3a-3j). Ionic liquids Sr. No Structure Yielda (%) Mp (oC) Time (min) 3a 92 212-214 45 3b 85 233-235 60 3c S N N O S 83 222-224 60 3d 89 282-283 60 3e 91 205-208 45 3f 85 266-268 60 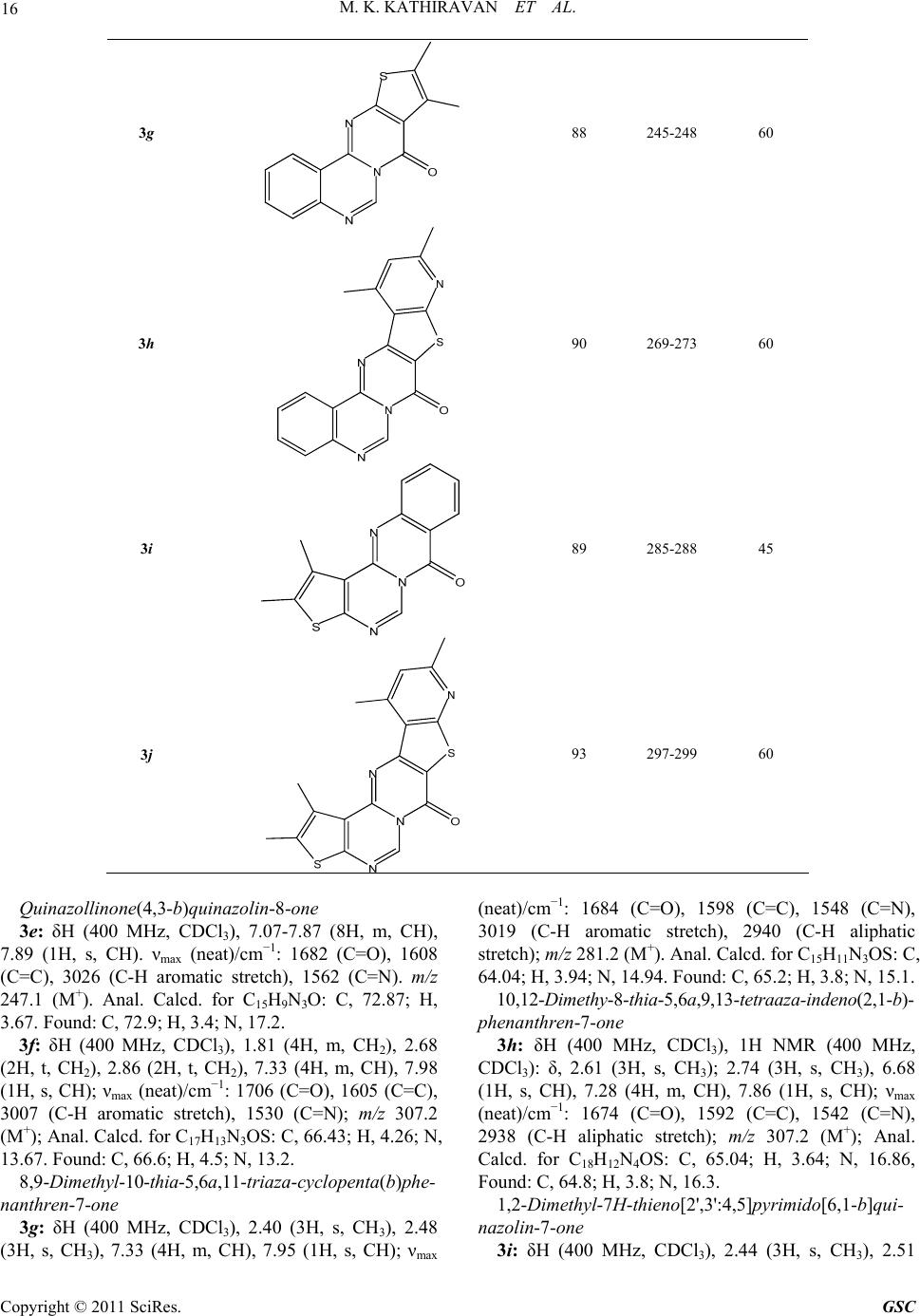 16 M. K. KATHIRAVAN ET AL. 3g 88 245-248 60 3h 90 269-273 60 3i 89 285-288 45 3j 93 297-299 60 Quinazollinone(4,3-b)quinazolin-8-one 3e: δH (400 MHz, CDCl3), 7.07-7.87 (8H, m, CH), 7.89 (1H, s, CH). νmax (neat)/cm−1: 1682 (C=O), 1608 (C=C), 3026 (C-H aromatic stretch), 1562 (C=N). m/z 247.1 (M+). Anal. Calcd. for C15H9N3O: C, 72.87; H, 3.67. Found: C, 72.9; H, 3.4; N, 17.2. 3f: δH (400 MHz, CDCl3), 1.81 (4H, m, CH2), 2.68 (2H, t, CH2), 2.86 (2H, t, CH2), 7.33 (4H, m, CH), 7.98 (1H, s, CH); νmax (neat)/cm−1: 1706 (C=O), 1605 (C=C), 3007 (C-H aromatic stretch), 1530 (C=N); m/z 307.2 (M+); Anal. Calcd. for C17H13N3OS: C, 66.43; H, 4.26; N, 13.67. Found: C, 66.6; H, 4.5; N, 13.2. 8,9-Dimethyl-10-thia-5,6a,11-triaza-cyclopenta(b)phe- nanthren-7-one 3g: δH (400 MHz, CDCl3), 2.40 (3H, s, CH3), 2.48 (3H, s, CH3), 7.33 (4H, m, CH), 7.95 (1H, s, CH); νmax (neat)/cm−1: 1684 (C=O), 1598 (C=C), 1548 (C=N), 3019 (C-H aromatic stretch), 2940 (C-H aliphatic stretch); m/z 281.2 (M+). Anal. Calcd. for C15H11N3OS: C, 64.04; H, 3.94; N, 14.94. Found: C, 65.2; H, 3.8; N, 15.1. 10,12-Dimethy-8-thia-5,6a,9,13- tetraaza-indeno(2,1-b)- phenanthren-7-one 3h: δH (400 MHz, CDCl3), 1H NMR (400 MHz, CDCl3): δ, 2.61 (3H, s, CH3); 2.74 (3H, s, CH3), 6.68 (1H, s, CH), 7.28 (4H, m, CH), 7.86 (1H, s, CH); νmax (neat)/cm−1: 1674 (C=O), 1592 (C=C), 1542 (C=N), 2938 (C-H aliphatic stretch); m/z 307.2 (M+); Anal. Calcd. for C18H12N4OS: C, 65.04; H, 3.64; N, 16.86, Found: C, 64.8; H, 3.8; N, 16.3. 1,2-Dimethyl-7H-thieno[2',3 ':4 ,5]pyrimido[6,1-b]qui- nazolin-7-one 3i: δH (400 MHz, CDCl3), 2.44 (3H, s, CH3), 2.51 Copyright © 2011 SciRes. GSC  M. K. KATHIRAVAN ET AL. 17 (3H, s, CH3), 7.26 (4H, m, CH), 7.88 (1H, s, CH); νmax (neat)/cm−1: 1695 (C=O), 1601 (C=C), 1542 (C=N), 3034 (C-H aromatic stretch), 2938 (C-H aliphatic stretch); m/z 281.2 (M+); Anal. Calcd. for C15H11N3OS: C, 64.04; H, 3.94; N, 14.94. Found: C, 64.1; H, 4.2; N, 14.7. 3j: δH (400 MHz, CDCl3) δ, 2.41 (3H, s, CH3), 2.48 (3H, s, CH3), 2.65 (3H, s, CH3); 2.72 (3H, s, CH3), 6.42 (1H, s, CH), 7.94 (1H, s, CH); νmax (neat)/cm−1: 1690 (C=O), 1610 (C=C), 1554 (C=N), 3018 (C-H aromatic stretch), 2938 (C-H aliphatic stretch); m/z 366.4 (M+). Anal. Calcd. for C18H14N4OS2: C, 58.99; H, 3.85; N, 15.29. Found: C, 59.3; H, 3.6; N, 15.4. 5. Acknowledgments We are grateful to Dr. Mrs. A. R. Madgulkar, Principal, AISSM’s College of Pharmacy, Pune, for providing us with the necessary financial support and infrastructure for carrying out this work. 6. References [1] Y. Asahina and K. Kashiwaki, “Chemical Constituents of the Fruits of Evodia Rutaecarpa,” Yakugaku Zasshi, Vol. 405, 1915, pp. 1273-1293. [2] L. R. Sheu, W. C. Hung, Y. M. Lee and M. H. Yen, “Mechanism of Inhibition of Platelet Aggregation by Ru- taecarpine, an Alkaloid Isolated from Evodia Rutae- carpa,” European Journal of Pharmacology, Vol. 318, 1996, pp. 469-475. [3] W. F. Chiou, J. F. Liao and C. F. Chen, “Comparative Study on Vasodilatory Effects of Three Quinazoline Al- kaloids Isolated from Evodia Rutaecarpa,” Journal of Natural Products, Vol. 59, 1996, pp. 374-378. doi:10.1021/np960161+ [4] S. Hibino and T. Choshi, “Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit,” Natural Product Reports, Vol. 18, 2001, pp. 66-87. doi:10.1039/b004055j [5] T. C. Moon, M. Murakami, I. Kudo, K. H. Son, H. P. Kim, S. S. Kang and H. W. Chang, “A New Class of COX-2 Inhibitor, Rutaecarpine from Evodia Rutaecarpa,” Inflammation Research, Vol. 48, 1999, pp. 621-625. doi:10.1007/s000110050512 [6] H. K. Raymond, “Sur la Ceto-yobyrine,” Comptes Rendus, Vol. 226, 1948, pp. 1379-1381. [7] J. Tames, G. Bujtas, K. Horvath-Dora and O. Clauder, “Alkaloids Containing the Indolo [2,3-c]-quinazolino- [3,2-a] Pyridine Skeleton, IV. The Mass Spectra of Rutaecarpine, Evodiamine and 3,14-Dihydrorutaecarpine,” Acta Chimica Academiae Scientiarum Hungaricae, Vol. 89, 1976, pp. 85-89. [8] S. H. Lee, J. K Son, B. S. Jeong, T. C. Jeong, W. C. Hyeon, E. S. Lee and Y. Jahng, “Progress in the Studies on Rutaecarpine,” Molecules, Vol. 13, No. 2, 2008, pp. 272-300. doi:10.3390/molecules13020272 [9] A. Hamid, A. Elomrib and A. Daich, “Expedious and Practical Synthesis of the Bioactive Alkaloids Rutaecarpine, Euxylophoricine A, Deoxyvasicinone and Their Heterocyclic Homologues,” Tetrahedron Letters, Vol. 47, 2006, pp. 1777-1781. [10] L. M. Yang, S. J Lin, L. C Lin and Y. H. Kuo, “Antitumor Agents. 2. Synthesis and Cytotoxic Evaluation of 10-bromorutaecarpine,” Chinese Pharmaceutical Journal, Vol. 51, 1999, pp. 219-225. [11] H. Miyamatsn, S. Ueno, M. Shimizu, J. Hosono, M. Tomari, K. Seida, T. Suzuki and J. Wada, “New Nonsteroidal Antiinflammatory Agent. 3. Analogs of 2-substituted 5-benzothiazoleacetic Acids and Their De- rivatives,” Journal of Medicinal Chemistry, Vol. 17, 1974, pp. 491-496. doi:10.1021/jm00251a004 [12] E. Bansal, T. Ram, S. Sharma, M. Tyagi, A. P. Rani, K. Bajaj, R. Tyagi, B. Goel, V. K. Srivastava, J. N. Gurtu and A. Kumar, “Thiazolidinyl-triazinoquinazolines as Potent Anti-inflammatory Agents,” Indian Journal of Chemistry, Vol. 40B, 2001, p. 307. [13] K. Okumura, T. Oine, Y. Yamada, G. Hayashi, M. Nakama and T. Nose, “4-Oxo-1,2,3,4-tetrahydroquinazolines. II. Synthesis of 1-Alkyl- and 1-[2,(Distributed amino) ethyl]- 2-methyl-3-aryl-4-oxo-1,2,3,4-tetrahydroquinazolines,” Journal of Med ic inal Chemistr y, Vol. 11, 1968, pp. 788-792. doi:10.1021/jm00310a611 [14] K. Ozaki, Y. Yamada, T. Oine, T. Ishizuka and Y. Iwasawa, “Studies on 4(1H)-Quinazolinones. 5. Synthesis and Antiinflammatory Activity of 4(1H)-Quinazolinone Derivatives,” Journal of Medicinal Chemistry, Vol. 28, 1985, pp. 568-576. doi:10.1021/jm50001a006 [15] H. Itahana, T. Kamikubo, E. Nozawa, H. Kaku, M. Okada, T. Toya and A. Nakamura, PCT International Application, 2002, WO 2002062803; Chemical Abstracts, Vol. 137, 2002, p. 169542. [16] L. M. Lima and E. J. Barreiro, “Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design,” Current Medicinal Chemistry, Vol. 12, 2005, pp. 23-49. [17] S. S. Laddha and S. P. Bhatnagar, “A New Therapeutic Approach in Parkinson’s Disease: Some Novel Quinazoline Derivatives as Dual Selective Phosphodiesterase 1 Inhibitors and Anti-inflammatory Agents,” Bioorganic & Medicinal Chemistry, Vol. 17, 2009, pp. 6796-6802. doi:10.1016/j.bmc.2009.08.041 [18] M. S. Manhas, S. D. Sharma and S. G. Sharma, “Hetero- cyclic Compounds. 4. Synthesis and Antiinflammatory Activity of Some Substituted Thienopyrimidones,” Jour- nal of Medicinal Chemistry, Vol. 15, 1972, pp. 106-107. doi:10.1021/jm00271a034 [19] M. J. Kulshreshtha, S. Bhatt, P. Madhuri and N. M. Khanna, “Synthesis of 2-methyl, 3-aryl or arylalkyl 5, 6-dimethyl or Polymethylene Thieno [2,3-d]-Pyrimidine- 4-ones,” Journal of the Indian Chemical Society, Vol. 58, No. 10, 1981, p. 982. [20] S. J. von Niementowski, “Synthesen von Chinazolinver- bindungen,” Journal für Praktische Chemie, Vol. 51, 1895, pp. 564-572. [21] D. J. Brown, Quinazolines, Supplement I. Interscience Publishers, New York, 1996, pp. 1-150. W. L. F. Ar- marego, In: Fused Pyrimidines; D. J. Brown, Ed.; Inter- science Publishers, New York, 1967, pp. 89-218. Copyright © 2011 SciRes. GSC  M. K. KATHIRAVAN ET AL. Copyright © 2011 SciRes. GSC 18 [22] E. S. Lee, J. G. Park and Y. Jahng, “A Facile Synthesis of Simple Alkaloids-Synthesis of 2,3-polymethylene-4(3H)- quinazolinones and Related Alkaloids,” Tetrahedron Let- ters, Vol. 44, 2003, pp. 1883-1886. doi:10.1016/S0040-4039(03)00080-7 [23] S. S. Laddha and S. P. Bhatnagar, “Efficient Niementowski Synthesis of Novel 1,3,10,12-tetra- substituted-8H-pyrido- [2',3':4,5]py rimido[6,1-b]quinazolin-8-ones,” Arkivoc, Vol. (xvi), 2007, pp. 1-11. [24] S. S. Laddha and S. P Bhatnagar, “Efficient Niementowski Synthesis of Novel Derivatives of 1,2,9,11-tetrasub- stituted-7H-thieno[2',3':4,5]pyrimido[6,1-b]-quinazolin-7- one,” Arkivoc, Vol. (xvii), 2008, pp. 212-220. [25] (a) T. Welton, “Room-Temperature Ionic Liquids. Sol- vents for Synthesis and Catalysis,” Chemical Reviews, Vol. 99, 1999, pp. 2071-2083. doi:10.1021/cr980032t (b) P. Wasserscheid and W. Keim, “Ionic Liquids-New ‘Solutions’ for Transition Metal Catalysis,” Ange- wandte Chemie International Edition, Vol. 39, 2000, pp. 3772-3789. (c) J. Dupont, R. F. de Souza and P. A. Z. Suarez, “Ionic Liquid (Molten Salt) Phase Or- ganometallic Catalysis,” Chemical Reviews, Vol. 102, 2002, pp. 3667-3692. (d) N. Jain, A. Kumar, S. Chau- han and S. M. S. Chausan, “Chemical and Biochemical Transformations in Ionic Liquids,” Tetrahedron, Vol. 61, 2005, pp. 1015-1060. doi:10.1016/j.tet.2004.10.070 [26] John S. Wilkes, In: P. Wasserscheid and T. Welton, Eds., Ionic Liquids in Synthesis, 2nd Edition, VCH-Wiley, Weinheim, 2007. [27] I. Newington, J. M. Perez-Arlandis and T. Welton, “Ionic Liquids as Designer Solvents for Nucleophilic Aromatic Substitutions,” Organic Letters, Vol. 9, No. 25, 2007, pp. 5247-5250. [28] S. S. Palimkar, S. A. Siddiqui, T. Daniel, R. J. Lahoti and K. V. Srinivasan, “Ionic Liquid-Promoted Regiospecific Friedlander Annulation: Novel Synthesis of Quinolines and Fused Polycyclic Quinolines,” Journal of Organic Chemistry, Vol. 68, 2003, pp. 9371-9378. [29] S. N. Dighe, K. S. Jain and K. V. Srinivasan, “A Novel Synthesis of 1-aryl Tetrazoles Promoted by Employing the Synergy of the Combined Use of DMSO and an Ionic Liquid as the Solvent System at Ambient Temperature,” Tetrahedron Letters, Vol. 50, 2009, pp. 6139-6142. doi:10.1016/j.tetlet.2009.08.063 [30] M. K. Kathiravan, C. J. Shishoo, S. K. Roy, K. R. Mahadik, S. S. Kadam and K. S. Jain, “Synthesis and An- tihyperlipidemic Activity of Some Novel Condensed 2- chloroalkyl-4-chloro/hydroxy-5,6-disubstituted Pyrimidi- nes,” Arzneimittel-Forschung/Drug Research, Vol. 57, No. 9, 2007, pp. 599-604. [31] M. S. Phoujdar, M. K. Kathiravan, J. B. Bariwal, A. K. Shah and K. S. Jain, “Microwave-based Synthesis of Novel Thienopyrimidine Bioisosteres of Gefitinib,” Tetrahedron Letters, Vol. 49, 2008, pp. 1269-1273. doi:10.1016/j.tetlet.2007.11.135 [32] K. S. Jain, J. B. Bariwal, M. S. Phoujdar, R. D. Amrutkar, M. K. Munde, R. S. Tamboli, S. A. Khedkar, R. H. Khiste, N. C. Vidyasagar, V. V. Dabholkar and M. K. Kathiravan, “A Novel Microwave-assisted Green Synthesis of Condensed 2-substituted-pyrimidin-4(3H)-ones under Solvent-free Conditions,” Journal of Heterocyclic Chemistry, Vol. 46, No. 2, 2009, pp. 178-185.
|