M. Jiménez-Pérez et al. / Open Journal of Gastroenterology 3 (2013) 307-310 309
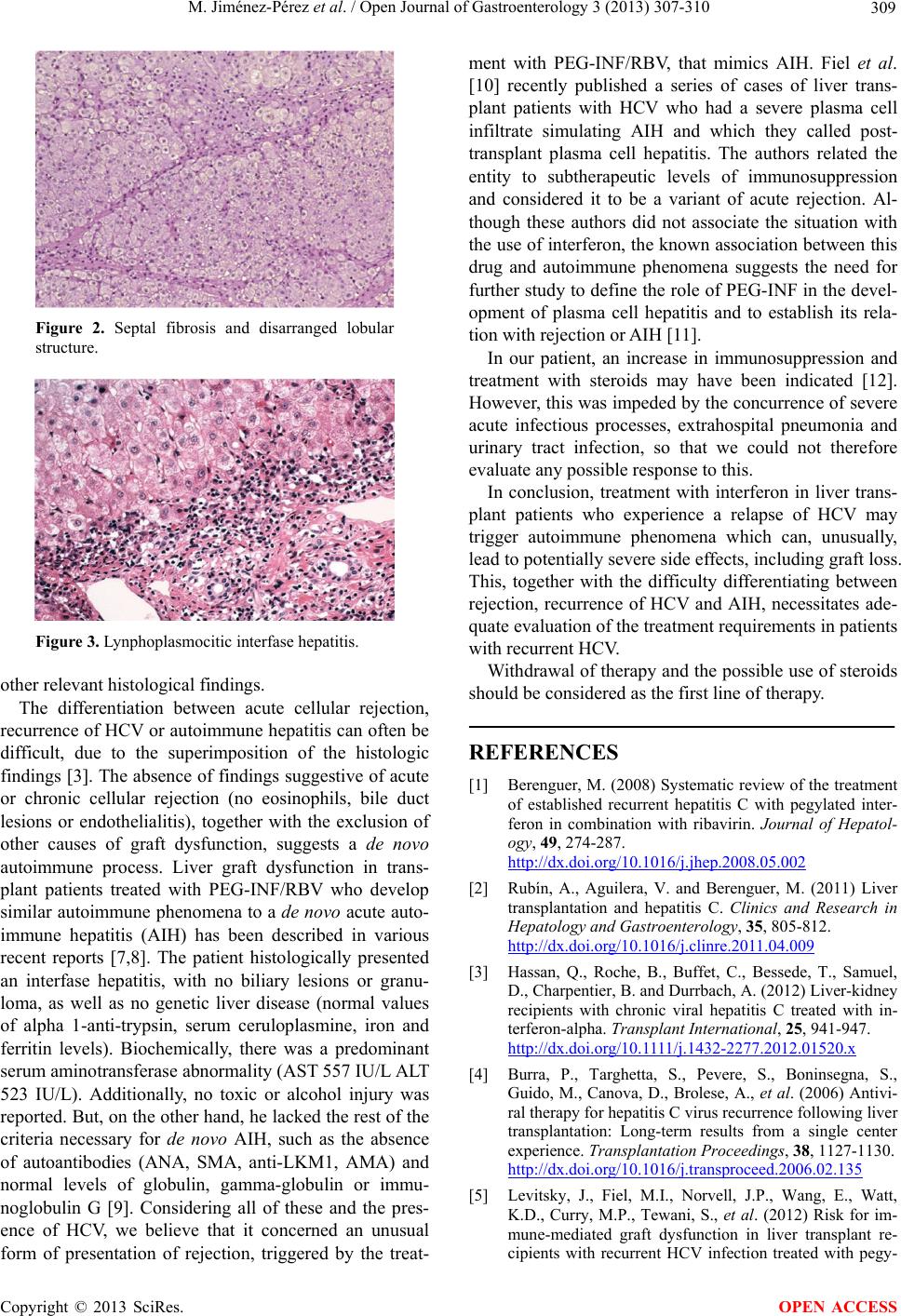
Figure 2. Septal fibrosis and disarranged lobular
structure.
Figure 3. Lynphoplasmocitic interfase hepatitis.
other relevant histological findings.
The differentiation between acute cellular rejection,
recurrence of HCV or autoimmune hepatitis can often be
difficult, due to the superimposition of the histologic
findings [3]. The absence of findings suggestive of acute
or chronic cellular rejection (no eosinophils, bile duct
lesions or endothelialitis), together with the exclusion of
other causes of graft dysfunction, suggests a de novo
autoimmune process. Liver graft dysfunction in trans-
plant patients treated with PEG-INF/RBV who develop
similar autoimmune phenomena to a de novo acute auto-
immune hepatitis (AIH) has been described in various
recent reports [7,8]. The patient histologically presented
an interfase hepatitis, with no biliary lesions or granu-
loma, as well as no genetic liver disease (normal values
of alpha 1-anti-trypsin, serum ceruloplasmine, iron and
ferritin levels). Biochemically, there was a predominant
serum aminotransferase abnormality (AST 557 IU/L ALT
523 IU/L). Additionally, no toxic or alcohol injury was
reported. But, on the other hand, he lacked the rest of the
criteria necessary for de novo AIH, such as the absence
of autoantibodies (ANA, SMA, anti-LKM1, AMA) and
normal levels of globulin, gamma-globulin or immu-
noglobulin G [9]. Considering all of these and the pres-
ence of HCV, we believe that it concerned an unusual
form of presentation of rejection, triggered by the treat-
ment with PEG-INF/RBV, that mimics AIH. Fiel et al.
[10] recently published a series of cases of liver trans-
plant patients with HCV who had a severe plasma cell
infiltrate simulating AIH and which they called post-
transplant plasma cell hepatitis. The authors related the
entity to subtherapeutic levels of immunosuppression
and considered it to be a variant of acute rejection. Al-
though these authors did not associate the situation with
the use of interferon, the known association between this
drug and autoimmune phenomena suggests the need for
further study to define the role of PEG-INF in the devel-
opment of plasma cell hepatitis and to establish its rela-
tion with rejection or AIH [11].
In our patient, an increase in immunosuppression and
treatment with steroids may have been indicated [12].
However, this was impeded by the concurrence of severe
acute infectious processes, extrahospital pneumonia and
urinary tract infection, so that we could not therefore
evaluate any possible response to this.
In conclusion, treatment with interferon in liver trans-
plant patients who experience a relapse of HCV may
trigger autoimmune phenomena which can, unusually,
lead to potentially severe side effects, including graft loss.
This, together with the difficulty differentiating between
rejection, recurrence of HCV and AIH, necessitates ade-
quate evaluation of the treatment requirements in patients
with recurrent HCV.
Withdrawal of therapy and the possible use of steroids
should be considered as the first line of therapy.
REFERENCES
[1] Berenguer, M. (2008) Systematic review of the treatment
of established recurrent hepatitis C with pegylated inter-
feron in combination with ribavirin. Journal of Hepatol-
ogy, 49, 274-287.
http://dx.doi.org/10.1016/j.jhep.2008.05.002
[2] Rubín, A., Aguilera, V. and Berenguer, M. (2011) Liver
transplantation and hepatitis C. Clinics and Research in
Hepatology and Gastroenterology, 35, 805-812.
http://dx.doi.org/10.1016/j.clinre.2011.04.009
[3] Hassan, Q., Roche, B., Buffet, C., Bessede, T., Samuel,
D., Charpentier, B. and Durrbach, A. (2012) Liver-kidney
recipients with chronic viral hepatitis C treated with in-
terferon-alpha. Transplant International, 25, 941-947.
http://dx.doi.org/10.1111/j.1432-2277.2012.01520.x
[4] Burra, P., Targhetta, S., Pevere, S., Boninsegna, S.,
Guido, M., Canova, D., Brolese, A., et al. (2006) Antivi-
ral therapy for hepatitis C virus recurrence following liver
transplantation: Long-term results from a single center
experience. Transplantation Proceedings, 38, 1127-1130.
http://dx.doi.org/10.1016/j.transproceed.2006.02.135
[5] Levitsky, J., Fiel, M.I., Norvell, J.P., Wang, E., Watt,
K.D., Curry, M.P., Tewani, S., et al. (2012) Risk for im-
mune-mediated graft dysfunction in liver transplant re-
cipients with recurrent HCV infection treated with pegy-
Copyright © 2013 SciRes. OPEN ACCESS