Paper Menu >>
Journal Menu >>
 Green and Sustainable Chemistry, 2011, 1, 1-6 doi:10.4236/gsc.2011.11001 Published Online February 2011 (http://www.SciRP.org/journal/gsc) Copyright © 2011 SciRes. GSC Lipophilic Optical Supramolecular Nano Devices in the Aqueous Phase Heinz Langhals*, Tim Pust *Department of Chemistry, LMU University of Munich, Munich, Germany E-mail: Langhals@lrz.uni-muenchen.de Received January 5, 2011; revised February 15, 2011; accepted February 19, 20 1 1 Abstract Nano micelles of sodium dodecyl sulphate in water were prepared as local lipophilic media for the organisation of interacting chromophores. Such arrangements were controlled by peripheric substituents to operate either as isolated chromophores or as skew oriented pairs where H-type transitions cause hysochromic absorption and J-type transitions bathochromic fluorescence. As a consequence, large Stokes’ shift could be obtained. Keywords: Lipophilic Optical Supramolecular, Nano Device, Nano Micelle, Sodium Dodecyl Sulphate 1. Introduction The investigation of light-driven processes is an attrac- tive subject of research and will be applied in organic photovoltaic cells [1], photoelectrochemical cells (DSSC) [2], artificial photosynthesis [3] and organic conducting polymers [4]. The controlling of chromophores on the molecular scale is a prerequisite for the development of light-driven devices [4]. Commonly chromophoric sys- tems are applied in media of similar polarity such as lipophilc systems in lipophilic media and hydrophilic system in hydrophilic ones. However, this means not only a restriction for the variety of combinations, but neglects also many possibilities given by the interaction of lipophilic and hydrophilic structures. We used such interactions for the preparation of nano micelles in order to incorporate lipophilic chromohores for their operation in the aqueous phase. Lipophilic organic chromophores in micelles of maleinated linseed oil were previously described [5], however, only isolated chromophores could be incorporated and the intrinsic light absorption of the detergent interferes with some applicatio ns of such systems. The development of more universal micellar dyes systems would bring about an appreciable progress. 2. Results and Discussion We investigated various surfactants such as 1-dodecyl- sulphate or 2,3-dimethylnaphthalene-4-sulphonate [6] (Nekal®) and found the sodium salt of the former (SDS) NO R O O N R O 1 to be the most appropriate for the preparation of suitable nano micelles [7,8]. The lack of light absorption in the UVA and visible is of special advantage for optical ap- plications. Perylene dyes [9] 1 (perylene-3,4:9.10-tetra- carboxylic bisimides) were app lied for incorporation into micelles because of their extraordinarily high stability and light fastness. The surfactant SDS was transformed to a gel [10], doped with dyes and then diluted with wa- ter where the application of ultra sonic is helpful, but not essential. We applied the perylene derivatives 1a … 1h where R are long-chain sec-alkyl groups [11] (“swallow-tail sub- stituents”) for solubilising and used the chain-lengths of R for controlling the arrangement in micelles. Unstruc- tured nano particles could be obtained from all deriva- tives of 1 by means of sodium dodecyl sulphate; see Fig- ure 1 for the size distribution and Figure 2 for the shape and structure. The behaviour of derivatives with short chains in R is complex: Particles with 70 nm (peak) were 1 R a CH(C2H5)2 b CH(n-C3H7)2 c CH(n-C4H9)2 d CH(n-C5H11)2 e CH(n-C6H13)2 f CH(n-C7H15)2 g CH(n-C8H17)2 h CH(n-C9H18)2 i CH(i-C3H7)2 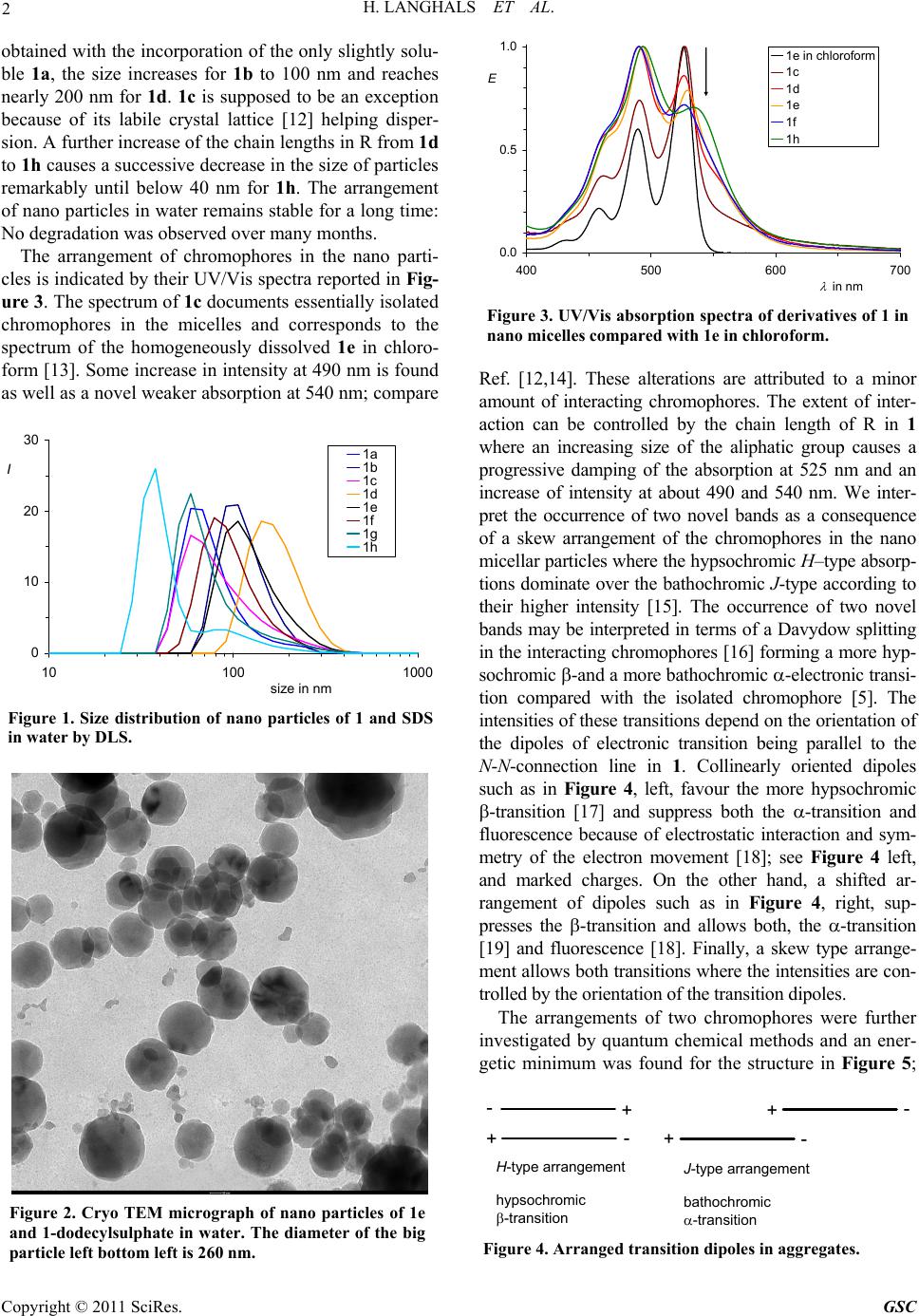 2 H. LANGHALS ET AL. obtained with the incorporation of the only slightly solu- ble 1a, the size increases for 1b to 100 nm and reaches nearly 200 nm for 1d. 1c is supposed to be an exception because of its labile crystal lattice [12] helping disper- sion. A further increase of the chain lengths in R from 1d to 1h causes a successive decrease in the size of particles remarkably until below 40 nm for 1h. The arrangement of nano particles in water remains stable for a long time: No degradation was ob served ove r many months. The arrangement of chromophores in the nano parti- cles is indicated by their UV/Vis spectra reported in Fig- ure 3. The spec trum of 1c documents essentially isolated chromophores in the micelles and corresponds to the spectrum of the homogeneously dissolved 1e in chloro- form [13]. Some increase in intensity at 490 nm is found as well as a novel weaker absorption at 540 nm; compare 0 10 20 30 10100 1000 size in nm I1a 1b 1c 1d 1e 1f 1g 1h Figure 1. Size distribution of nano particles of 1 and SDS in water by DLS. Figure 2. Cryo TEM micrograph of nano particles of 1e and 1-dodecylsulphate in water. The diameter of the big particle left bottom left is 260 nm. 0.0 0.5 1.0 400 500 600 700 in nm E 1e in chlorof o rm 1c 1d 1e 1f 1h Figure 3. UV/Vis absorption spectra of derivatives of 1 in nano micelles compared with 1e in chloroform. Ref. [12,14]. These alterations are attributed to a minor amount of interacting chromophores. The extent of inter- action can be controlled by the chain length of R in 1 where an increasing size of the aliphatic group causes a progressive damping of the absorption at 525 nm and an increase of intensity at about 490 and 540 nm. We inter- pret the occurrence of two novel bands as a consequence of a skew arrangement of the chromophores in the nano micellar particles where the hypsochro mic H–type absorp- tions dominate over the bathochromic J-type according to their higher intensity [15]. The occurrence of two novel bands may be interpreted in terms of a Davydow splitting in the interacting chromophores [16] forming a more hyp- sochromic -and a more bathochromic -electronic transi- tion compared with the isolated chromophore [5]. The intensities of these transitions depend on the orientation of the dipoles of electronic transition being parallel to the N-N-connection line in 1. Collinearly oriented dipoles such as in Figure 4, left, favour the more hypsochromic -transition [17] and suppress both the -transition and fluorescence because of electrostatic interaction and sym- metry of the electron movement [18]; see Figure 4 left, and marked charges. On the other hand, a shifted ar- rangement of dipoles such as in Figure 4, right, sup- presses the -transition and allows both, the -transi tion [19] and fluorescence [18]. Finally, a skew type arrange- ment allows both transitions w here the intensities are con- trolled by the orient ation of the t ransiti on dipol es. The arrangements of two chromophores were further investigated by quantum chemical methods and an ener- getic minimum was found for the structure in Figure 5; +- - - - + + + H-type arrangement hypsochromic -transition J-type arrangement bathochromic -transition Figure 4. Arranged transition dipoles in aggregates. Copyright © 2011 SciRes. GSC  H. LANGHALS ET AL. 3 the AM1 method was preferred because of many non- covalent interactions in the pair, whereas less reliable results are expected for DFT B3LYP calculations [20]. The structure of the dimer is presumably stabilised by electrostatic-interaction between electron rich and elec- tron depleted atoms of the two dye molecules. As a con- sequence, a dihedral angle of some 60° between the skew arranged chromophores is formed allowing both the weak and the strong transition because of the domi- nating H-type interaction. This corresponds to the UV/is spectra in Figure 3 where a weaker bathochromic and a stronger hypsochromic absorption was found. The light emission of the -transition is symmetry for- bidden and causes the fluorescence quenching of H-ag- gregates. This can be overcome by skew type arrange- ments such as in Figure 5 where the -transition be- comes allowed. One may expect an internal energy con- version [21] from the excited electronic state of the - transition to the exited state of the -transition causing a bathochromically shifted fluorescence. This shift in fluorescence from isolated chromophores in the lipo- philic chloroform to stacked chromophores in nano mi- celles reaches some 150 nm (5000 cm-1) and is shown in Figure 6. A fine tuning can be achieved by the substitu- ent R in 1, both for the position of the bathochromic band of fluorescence and some residual fluorescence of isolated chromophores at 530 nm. Figure 5. Calculated structure of a typical dimer of 1 with R = CH3. 0.0 0.5 1.0 500 600 700 800 in nm I 1e in chloroform 1b 1c 1d 1e Figure 6. UV/Vis fluorescence spectra of derivatives of 1 in nano micelles compared with 1e in chloroform. The stacking of chromophores according to Figure 5 is further confirmed by a comparison with the UV/Vis spectra of 2 [22]; see Figure 7, compare also Ref. [12,15] for comparable arrangements. The spectra of 1f in mi- celles and 2 in homogeneous solution are very similar and both differ appreciably from 1e and 1f, respectively, in homogeneous solution; see Figure 7. Two chromo- phoric units of 1 are tied together in the cyclophane 2. The twelve membered connecting aliphatic chains are small enough to keep the chromophores close together, but large enough to allow their optimal orientation. Thus a similar orientation as the aggregated 1f in micelles is expected. This is indicated by the similarity of their UV/ Vis spectra. Moreover, the fluorescence quantum yield of 2 is found to be 46% (integration until 900 nm) and is about the same as the fluorescence quantum yield of 1f in the nano particles where 40% are observed. The ap- preciably smaller fluorescence quantum yield of the dimer of 1f in micelles and 2, respectively, compared with close to 100% of isolated dye molecules of 1f in chloroform is interpreted as a consequence of similarly lower transition probability of the -transition compared with the high transition probability for isolated chromo- phores. NO O O N O NO O O N O 2 0.0 0.5 1.0 400 500 600 700 800 in nm E 0.0 0.5 1.0 I Figure 7. UV/Vis absorption (left) and fluorescence spectra (right). Blue: nano micelles of 1f, magenta: 2 in chloroform, thin black: 1e in chloroform. Copyright © 2011 SciRes. GSC  4 H. LANGHALS ET AL. 3. Conclusion Lipophilic chromohores can be introduced into the aqueous phase by means of micelle forming detergents such as 1-dodecyl sulphate (SDS) where local nano-di- mensioned lipophilic cages were established for organis- ing supramolecular interactions. Peripheral substituents control the interaction of chromophores by their size to form nano devices with special optical properties such as increased Stokes’ shifts. This may be applied, for exam- ple, for solar energy collectors [23]. Even more complex functional structures may be constru cted in such micellar nano devices. 4. Experimental General UV/Vis spectra: Varian Cary 5000; fluorescence spectra: Varian Eclipse. The dyes 1 and 2 were prep ared and purified according to the literature [11,22]. Prepara- tion of nano particles in the aqueous phase: Sodium 1-dodecylsulphate (460 mg) and distilled water (1.7 g) were heated to 50˚C to form a colourless gel. 1 (some 10 mg) and chloroform (60 mg, ca. 10 drops) were added at 50˚C with subsequent ultra sonification for 10 min, treatment with distilled water (30 mL) and filtration (D5 glass filter). The nano particles in water remain unaltered for many months. Neither flocculation nor degradation of the strong fluorescence could be observed. 5. Acknowledgements This work was supported by the Fonds der Chemischen Industrie. T. P. thanks Degussa Evonik for a PhD schol- arship and support by the CIPSM cluster. We thank Prof. Thomas Bein for help with electron microscopy and DLS measurements. 6. References [1] C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Advanced Functional Materials, Vol. 11, 2001, pp. 15-26. S. Günes, H. Neugebauer and N. S. Sariciftci, “Conjugated Polymer-Based Organic Solar Cells,” Chemical Reviews, Vol. 107, 2007, pp. 1324-1338. [2] M. Grätzel, “Photoelectrochemical Cells,” Nature, Vol. 414, 2001, pp. 338-344. H. Choi, S. Kim, S. O. Kang, J. Ko. M, S. Kang, J. N. Clifford, A. Forneli, E. Palomares, M. K. Nazeeruddin and M. Grätzel, “Stepwise Cosensitization of Nanocrystalline TiO2 Films Utilizing Al2O3 Layers in Dye-Sensitized Solar Cells,” Angewandte Chemie, Vol. 120, 2008, pp. 8383-8387; Angewandte Chemie International Edition, Vol. 47, 2008, pp. 8259-8263. F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, “Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells,” Journal of the American Chemical Society, Vol. 130, 2008, pp. 10720-10728. [3] D. Gust, T. A. Moore and A. L. Moore, “Mimicking Photosynthetic Solar Energy Transduction,” Accounts of Chemical Research, Vol. 34, 2001, pp. 40-48. M. R. Wasielewski, “Energy, Charge, and Spin Transport in Molecules and Self-Assembled Nanostructures Inspired by Photosynthesis,” Journal of Organic Chemistry, Vol. 71, 2006, pp. 5051-5066. I. Leray, B. Valeur, D. Paul, E. Regnier, M. Koepf, J. A. Wytko, C. Boudon and J. Weiss, “Photodynamics of Excitation Energy Transfer in Self-Assembled Dyads. Evidence for Back Transfer,” Photochemical and Photobiological Sciences, Vol. 4, 2005, pp. 280-286. [4] L. J. Huo, S. Q. Zhang, H. Y. Chen and Y. Yang, “A Polybenzo[1,2-b:4,5-b′]dithiophene Derivative with Deep HOMO Level and Its Application in High-Performance Polymer Solar Cells,” Angewandte Chemie, Vol. 122, 2010, pp. 1542-1545; Angewandte Chemie International Edition, Vol. 49, 2010, pp. 1500-1503. H. Goesmann and C. Feldmann, “Nanoparticulate Functional Materials,” Angewandte Chemie, Vol. 122, 2010, pp. 1402-1437; Angewandte Chemie International Edition, Vol. 49, 2010, pp. 1362-1395 . [5] H. Langhals, “Brightly Shining Nano Particles: Lipophilic Perylene Bisimides in Aqueous Phase,” New Journal of Chemistry, Vol. 32, 2008, pp. 21-23. [6] G. Sonnek and F. Wolf, “Alkyl Naphthalenesulfonic Acids (Nekal BX),” Tenside, Vol. 4, 1967, pp. 325-330; Chemical Abstracts, Vol. 67, 1967, p. 118445. V. Jacopian, “Decrease of the Resin Content of Dissolving Pulps by Means of Surfactants,” Zellstoff und Papier (Leipzig), Vol. 14, 1965, pp. 310-311; Chemical Abstracts, Vol. 64, 1966, p. 36839. J. Figaret, “(Practical Test Methods for) the Effect of Surface-active Agents on the Wettability of Pigments,” Farbe + Lack, Vol. 55, 1949, pp. 234-238; Chemical Abstracts, Vol. 43, 1949, p. 48403. [7] H. Langhals and T. Pust, “Fluorescent Nano pH Indicators Based on Supramolecular Interactions,” Zeitschrift für Naturforschung, Vol. 65b, 2010, pp. 291-294. [8] M. Almgren and S. Swarup, In: K. L. Mittal and B. Lindman, Eds., Surfactants Solution, New York, Vol. 1, 1982, pp. 613-625; Chemical Abstracts, Vol. 101, 1984, p. 28739. A. Patist, S. G. Oh, R. Leung and D. O. Shah, “Kinetics of Micellization: Its Significance to Technological Processes,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 176, 2001, pp. 3-16; Chemical Abstracts, Vol. 134, 2000, p. 152923. [9] For reviews see: H. Langhals, “Control of the Interactions in Multichromophores: Novel Concepts. Perylene Bisimides as Components for Larger Functional Units,” Helvetica Chimica Acta, Vol. 88, 2005, pp. 1309-1343. H. Langhals, “Cyclic Carboxylic Imide Structures as St ructure Elements of Hi gh St ability. Novel Developments in Perylene Dye Chemistry,” Heterocycles, Vol. 40, 1995, pp. 477-500. H. Langhals, Copyright © 2011 SciRes. GSC  H. LANGHALS ET AL. 5 “Molecular Devices. Chiral, Bichromophoric Silicones: Ordering Principles in Complex Molecules,” In: F. Ganachaud, S. Boileau and B. Boury, Eds., Silicon Based Polymers, Springer, 2008, pp. 51-63 (ISBN 978-1-4020-8527-7, e-ISBN 978-1-4020 8528-4). [10] “Dynamics of Surfactant Self-Assemblies: Micelles, Microemulsions, Vesicles, and Lyotropic Phases,” In: R. Zana, Ed., Surfactant Science Series, CRC Press LLC, Florida, 2005, p. 125; Chemical Abstracts, Vol. 143, 2005, p. 200524. A. Rusanov, Micellization in Surfactant Solutions, Harwood, Amsterdam, 1998; Chemical Abstracts, Vol. 134, 2001, p. 257304. F. L. Boschke, “Topics in Current Chemistry,” Micelles, Vol. 87, Springer-Verlag, Berlin, 1980; Chemical Abstracts, Vol. 92, 1980, p. 100018. D. A. Jaeger, “Sodium Dodecyl Sulfate,” Encyclopedia of Reagents for Organic Synthesis, 2001; Chemical Abstracts, Vol. 149, 2008, p. 287761. H. D. Doerfler and A. Grosse, “Characterization of Aque ous Microemulsions by Phase Diagrams and Interface Tension Measurements. Pseudo-binary and Pseudo- ternary Phase Diagrams and Interfacial Tension Measurements of the System Water/n-Undecane/Niotenside/Cotenside for the Cha- racterization of Microemulsions,” Tenside, Surfactants, Detergents, Vol. 36, 1999, pp. 29-37; Chemical Abstracts, Vol. 130, 1999, p. 228076. H. D. Doerfler, Grenzflächen und Kolloidchemie, Wiley CH, Weinheim, 1994 (ISBN-10: 3527290729, ISBN-13: 978-3527290727). [11] S. Demmig and H. Langhals, “Highly soluble, Lightfast Perylene Fluorescent Dyes,” Chemische Berichte, Vol. 121, 1988, pp. 225-230. H. Langhals, S. Demmig and T. Potrawa, “The Relation between Packing Effects and Solid State Fluorescence of Dyes,” Journal für Praktische Chemie, Vol. 333, 1991, pp. 733-748. [12] H. Langhals, Dyes for Fluorescent Immunoassays, in B. Hock, Immunochemical Detection of Pesticides and their Metabolites in the Water Cycle, VCH Verlagsgesellschaft, Weinheim, 1995 (ISBN 3-527- 27137-6); Chemical Abstracts, Vol. 124, 1996, p. 24966z. [13] H. Langhals, “Description of Properties of Binary Solvent Mixtures,” In: R. I. Zalewski, T. M. Kry gowski and J. Shorter, Eds., Similarity Models in Organic Chemistry, Biochemistry and Related Fields. Studies in Organic Chemistry, Elsevier Publishers, Amsterdam, 1991, pp. 283-342 (ISBN 0-444-88161-1). [14] W. E. Ford, “Photochemistry of 3,4,9,10-Perylene- tetracarboxylic Dianhydride Dyes: Visible Absorption and Fluorescence Spectra and Fluorescence Quantum Yields of the Mono(n-octyl)-imide Derivative in Aqueous and Non-aqueous Solutions,” Journal of Photochemistry, Vol. 34, 1986, pp. 43-54. W. E. Ford, “Photochemistry of 3,4,9,10-Perylenetetracarboxylic Dianhydride Dyes: Visible Absorption and Fluorescence of the Di(glycyl)imide Derivative Monomer and Dimer in Basic Aqueous Solutions,” Journal of Photochemistry, Vol. 37, 1987, pp. 189-204. [15] J. Seibt, P. Marquetand, V. Engel, Z. Chen, V. Dehm and F. Würthner, “On the Geometry Dependence of Molecular Dimer Spectra with an Application to Aggregates of Perylene Bisimide,” Chemical Physics, Vol. 328, 2006, pp. 354-362. J. M. Giaimo, J. V. Lockard, L. E. Sinks, A. M. Scott, T. M. Wilson and M. R. Wasielewski, “Excited Singlet States of Covalently Bound, Cofacial Dimers and Trimers of Perylene- 3,4:9,10-bis(dicarboximide)s,” Journal of Physical Chemistry A, Vol. 112, 2008, pp. 2322-2330. R. O. Marcon and S. Brochsztain, “Aggregation of 3,4,9,10- Perylenediimide Radical Anions and Dianions Generated by Reduction with Dithionite in Aqueous Solutions,” Journal of Physical Chemistry A, Vol. 113, 2009, pp. 1747-1752. C. D. Schmidt, C. Böttcher and A. Hirsch, “Synthesis and Aggregation Properties of Water-Soluble Newkome-Dendronized Perylenetetra- carboxdiimides,” European Journal of Organic Chemistry, 2007, pp. 5497-5505. J. M. Giaimo, A. V. Gusev and M. R. Wasielewski, “Excited-State Symme- try Breaking in Cofacial and Linear Dimers of a Green Perylenediimide Chlorophyll Analogue Leading to Ultrafast Charge Separation,” Journal of the American Chemical Society, Vol. 124, 2002, pp. 8530-85 31. M. J. Ahrens, L. E. Sinks, B. Rybtchinski, W. H. Liu, B. A. Jones, J. M. Giaimo, A. V. Gusev, A. J. Goshe, D. M. Tiede and M. R. Wasielewski, “Self-Assembly of Supramolecular Light-Harve sting Arrays from Covalent Multi-Chromophore Perylene-3,4:9,10-bis(dicarboximide) Building Blocks,” Journal of the American Chemical Society, Vol. 126, 2004, pp. 8284-8294. A. D. Schaller, W. Wang, H. Y. Gan and A. D. Q. Li, “Tunable Molecular Assembly Codes Direct Reaction Pathways,” Angewandte Chemie, Vol. 120, 2008, pp. 7819-7823; Angewandte Chemie International Edition, Vol. 47, 2008, pp. 7705-7709. [16] A. S. Davydov, “Theory of Absorption Spectra of Molecular Crystals,” Zhur. Eksptl. i Teoret. Fiz., Vol. 18, 1948, pp. 210-218; Chemical Abstracts, Vol. 43, 1949, p. 4575f. A. S. Davydow, Theory of Molecular Excitations, McGraw-Hill, New York, 1962. M. Kasha, H. R. Rawls and M. A. El-Bayoumi, “The Exciton Model in Molecular Spectroscopy,” Pure and Applied Chemistry, Vol. 11, 1965, pp. 371-392. [17] G. Scheibe, “Variability of the Absorption Spectra of some Sensitizing Dyes and its Cause,” Angewandte Chemie, Vol. 49, 1936, p. 563. [18] T. Förster, “Migration of Energy and Fluorescence,” Naturwissenschaften, Vol. 33, 1946, p. 166-175; Chemical Abstracts, Vol. 41, 1947, p. 36668. T. Förster, “Electron Spectra of Coupled Molecules,” Pure and Applied Chemistry, Vol. 4, 1962, pp. 121-134. [19] E. Jelley, “Spectral Absorption and Fluorescence of Dyes in the Molecular State,” Nature, Vol. 138, 1936, p. 1009. [20] S. M. Bachrach, Computational Organic Chemistry, John Wiley & Sons, New York, 2007, pp. 394-395 (ISBN 978-0-471-71342-5). R. Villar, M. J. Gil, J. I. Garcia and V. Martinez-Merino, “Are AM1 Ligand-protein Binding Enthalpies Good Enough for Use in the Rational Design of New Drugs?,” Journal of Computational Chemistry, Vol. 26, 2005, pp. 1347-1358; Chemical Abstracts, Vol. 143, 2005, p. 410902. (We thank Prof. R. de Vivie-Riedle and Dr. H. U. Wagner for helpful discussions.) Copyright © 2011 SciRes. GSC  H. LANGHALS ET AL. Copyright © 2011 SciRes. GSC 6 [21] T. Förster, “Migration of Energy and Fluorescence,” Naturwissenschaften, Vol. 33, 1946, pp. 166-175; Chemical Abstracts, Vol. 41, 1947, p. 36668. T. Förster, “Intermolecular energy transference and fluorescence,” Annals of Physics, Vol. 6, 1948, Folge, No. 2, pp. 55-75; Chemical Abstracts, Vol. 43, 1949, p. 31172. T. Förster, “Investigations of the Intermolecular Transfer of the Energy of Electronic Exitation,” Zeitschrift für Elektrochemie, Vol. 53, 1949, pp. 93-99; Chemical Abstracts, Vol. 43, 1949, p. 33629. T. Förster, “ Experimental and Theoretical Investigation of Intermolecular Transfer of Electron Activation Esnergy,” Zeitschrift für Naturforschung, Vol. 4a, 1949, pp. 321-327; Chemical Abstracts, Vol. 44, 1950, p. 43074. D. L. Dexter, “A Theory of Sensitized Luminescence in Solids,” Journal of Chemical Physics, Vol. 21, 1953, pp. 836-850. Review: T. Woggon, M. Punke, M. Stroisch, M. Bruendel, M. Scheib, C. Vannahme, T. Mappes, J. Mohr and U. Lemmer, “Organic Semiconductor Lasers as Integrated Light Sources for Optical Sensors,” In: J. Shinar and R. Shinar, Eds., Organic Electronics in Sensors and Biotechnology, McGraw-Hill, New York, 2009, pp. 265-298 (ISBN 0-07-159675-5). [22] H. Langhals and R. Ismael, “Cyclophanes as Model Compounds for Permanent, Dynamic Aggregates: Induced Chirality with Strong CD Effects,” European Journal of Organic Chemistry, Vol. 1998, No. 9, 1998, pp. 1915-1917. [23] H. Langhals, “Dyes for Fluorescent Solar Collectors,” Nachrichten aus Chemie, Technik und Laboratorium, Vol. 28, 1980, pp. 716-718; Chemical Abstracts, Vol. 95, 1981, p. R9816q. |

