Journal of Water Resource and Protection
Vol. 1 No. 6 (2009) , Article ID: 1073 , 6 pages DOI:10.4236/jwarp.2009.16049
Effects of Light and Monosulfuron on Growth and Photosynthetic Pigments of Anabaena Flos-Aquae Breb
School of Agriculture and Biology, Shanghai Jiaotong University, Shanghai, China
E-mail: jyshen88@sjtu.edu.cn
Received August 16, 2009; revised September 1, 2009; October 12, 2009
Keywords: Anabaena Flos-Aquae, Growth, Light, Monosulfuron, Photosynthetic Pigments
ABSTRACT
The effects of monosulfuron on growth and photosynthetic pigments of the nitrogen-fixing cyanobacterium Anabaena flos-aquae grown exposed to 2000-, 3000-, and 4000-lux light intensity were studied. Exposed to three light intensities, the seven concentrations of monosulfuron tested can significantly inhibit algal growth in a dose-dependent manner. The cell numbers and growth rate were decreased with the increase in monosulfuron concentration, and A. flos-aquae had different degrees of sensitivity to monosulfuron with the most sensitive light intensity being 4000-lux followed by 3000-lux and 2000-lux. The herbicide monosulfuron appeared to have different effects on the synthesis of photosynthetic pigments. The chlorophyll appeared to tackle monosulfuron concentrations. The caroteniod content of algae treated with 0.008 and 0.08 mg/L monosulfuron exposed to 2000-lux had a different stimulatory effect from that of treatments exposed to 3000-lux and 4000-lux, but an inhibitory effect at concentration above 0.8 mg/L. The effect of monosulfuron on biliprotein in cells of A. flos-aquae exposed three light intensities displayed contrary dose dependence.
1. Introduction
Effects of herbicides on non-target organisms in the soil ecosystem such as microorganisms have recently been paid great attention. Soil algae, particularly nitrogenfixing cyanobacteria, are important photosynthetic microoganisms because they contribute to soil fertility by fixing atmospheric nitrogen. They are sensitive to herbicides because they have many characteristics of higher plants [1]. Many effects of herbicides on non-target algae, such as algal growth, photosynthesis, nitrogen fixation, and metabolic activities have been reported [2]. The previous studies, however, were conducted exposed to standard phototoxicity test condition involving only the results of photoautotrophic growth of algae. In fact, in the fields, the algae grown in soil or inland waters is mixotrophic rather than autotrophic [3], and herbicide toxicity to algae is affected by many environmental factors such as nutrient level, temperature, and light. However, little is known about the specific roles of these environmental factors, particularly, the effect of light on herbicide toxicity to algal growth.
Monosulfuron {N-[ (4’-methyl) pyrimidin-2’-yl] – 2 - nitrophenylsulfonyl urea} is a relatively new sulfonylurea herbicide that was developed by the National Pesticide Engineering Research Center in Tianjin, China [4]. This herbicide exhibits low mammalian toxicity and is very effective at post-emergence rates of 15 to 30 g ai./ha in a wide range of crops including corn (Zea mays L.), wheat (Triticum aestivum L.), rice (Oryza sativa L.), and millet (Panicum miliaceum L.) [5]. Currently, over 30 sulfonylurea herbicides are applied worldwide for selective control of weeds in a variety of crops including corn, rice, wheat, and potatoes (Solanum muricatum Oit.) [6]. However, the effect of sulfonylurea herbicides on nitrogen-fixing cyanobacteria capable of enhancing the fertility of agricultural soils has not been investigated. Anabaena flos-aquae (Lyngb.) Breb., a filamentous nitrogen-fixing cyanobacterium, is one of the most common members of the soil algae community, capable not only of photoautotrophic growth but also of mixotrophic growth [7]. This work investigated the mixotrophic growth, chlorophyll a, caroteniod, and biliprotein of A. flos-aquae responded to the herbicide monosulfuron exposed to three light intensity conditions, attempting to compare and assess the effect of light on this herbicide toxicity.
2. Materials and Methods
Culture of Anabaena flos-aquae (FACHB-245) was obtained from the Institute of Hydrobiology of the Chinese
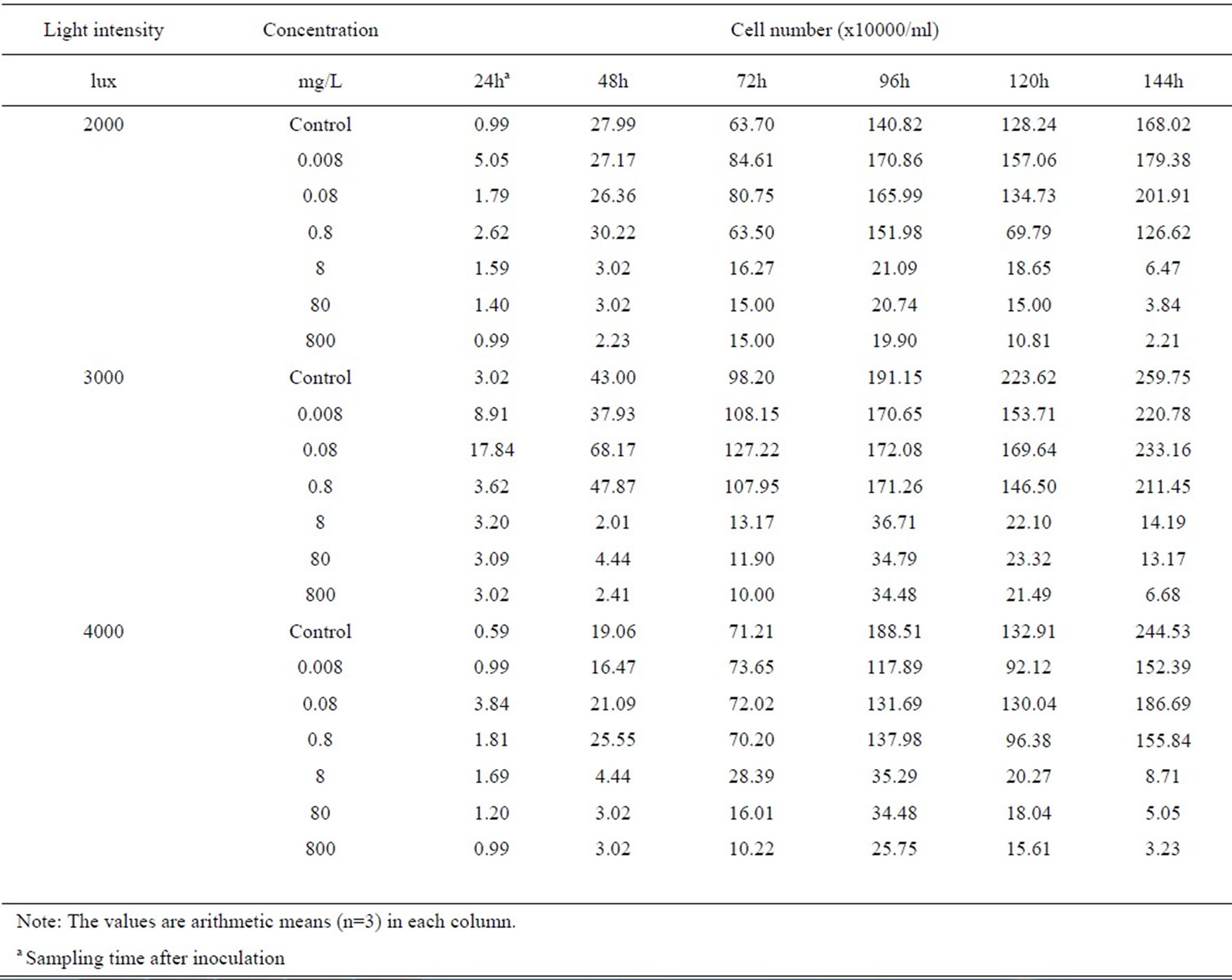
Table 1. Cell numbers of A. flos-aquae treated with monosulfuron exposed to different light intensities.
Academy of Sciences in Wuhan, China. Axenic cultures were grown in a liquid sterilized medium as described by Kratz and Myers (1955) [8] at 30 ± 2 ℃ exposed to fluorescent light at an intensity of 3000-lux (pH 7.2). The experimental cultures were first grown in 250-ml flasks containing 100 ml of medium with 0.5-to-1 million cells per ml exposed to the same conditions as described above. At the exponential growth phase of the algal cultures, monosulfuron from the stock solution was added to the culture medium at 0.008, 0.08, 0.8, 8, 80, and 800 mg/L. Sterilized water was added to some of the culture media rather than the herbicide and these cultures served as controls. Each treatment concentration was replicated four times and all experiments were conducted twice.
Growth of algae was measured by recording light absorbance of the culture at 448 nm using a spectrophotometer. Standard curves relating spectrophotometric absorbance readings (448 nm) with cell numbers were developed for continuous culture of. A. flos-aquae. These curves were used to determine cell numbers in continuous culture samples used as inoculum for the screening bioassay. Data used to produce the standard curves were obtained from absorbance measurements [9]. Herbicides from stock solution were added to the culture medium to result in the desired concentration and controls (without the herbicide). Each concentration was replicated four times. During the experimental period, samples were withdrawn after herbicide treatment at 24, 48, 72, 96, 120, and 144 hours for measurements of growth. The growth rate (µ) of the algae was calculated by: µ= (lnX1-lnX0)/ ( T1-T0), where X1 represents the number of algal cells in the absorbance at 448 nm at time T1, and X0 represents the number of algal cells in the absorbance at 448 nm at time T0. To determine dry weight of cells, corresponding cultures in triplicate were pelleted; and the pellet washed using distilled water three times before drying to constant weight at 105 ℃ for 8 h [10].
During the experimental period, samples were withdrawn after herbicide treatment at 48, 96, and 144 hours for measurements of photosynthetic pigments. Chlorophyll a was extracted with 90% methanol in dark for 2 h, and centrifuged at 3000g for 3 min; and estimated using absorbance at 665 nm according to Mackinney (1941) [11]. The algal biliproteins were extracted by repeatedly freezing and thawing the pellet in the presence of 0.05 M phosphate buffer (pH 6.7). The solution was centrifuged at 3000g for 15min, and the absorbance at 618 nm measured [12]. The amount of total carotenoid was calculated from the absorbance at 447 nm according to the method described by Jenssen (1978) [13].
A completely randomized design with four replications was used in all experiments. Experiments were repeated twice. The data represent the average of the two trials because of a non-significant trial by treatment interaction and homogeneity of variance according to Bartlett’s test. Analyses of variance (ANOVA) were performed on non-transformed data. Significant differences were determined using Duncan’s test at the P = 0.05 level of significance (PROC GLM, SAS Institute, 2001).
3. Results and Discussion
3.1. Effects of Light on Herbicide Toxicity to Algae Mixotrophic Growth
The growth of A. flos-aquae treated with different concentrations of monosulfuron exposed to 2000-, 3000- and 4000-lux light intensities are listed in Table 1. It can be seen that the herbicide monosulfuron applied stimulated the algal growth at initially 24h, but then markedly inhibited the mixotrophic growth of the algal grown exposed to three light intensities in a dose-dependent manner; namely, the inhibitory effect increased with the increase in monosulfuron concentration. The cell number of A. flos-aquae were reduced by 24 to 99 % exposed to 4000-lux light and by 10 to 97% exposed to 3000-lux, at a concentration range of 0.008 t0 800 mg/L, after inoculation 144h, in comparison with the control (P<0.05). But the reverse was observed at monosulfuron ranging from 0.008 to 0.08mg/L exposed to 2000-lux, the cell number of A. flos-aquae were increased by 7 to 13% after inoculation 144h, in comparison with the control. Thus it is clear that A. flos-aquae had different degrees of sensitivity to monosulfuron with the most sensitive light intensity being 4000-lux followed by 3000-lux and 2000-lux. However, without herbicide, the growth of A. flos-squae exposed to 3000-lux light was better than those exposed to 2000- and 4000-lux light (Figure 1).
The observed transient stimulation effect of monosulfuron on algal growth at initially 24h in this study are similar to those reported by Shen et al. (2005) [14] for butachlor and acetochlor on several Anabaena species. Hormesis, the stimulatory effect of sub-toxic concentrations of a toxin, has been documented following the application of other herbicides and allelochemicals [15].
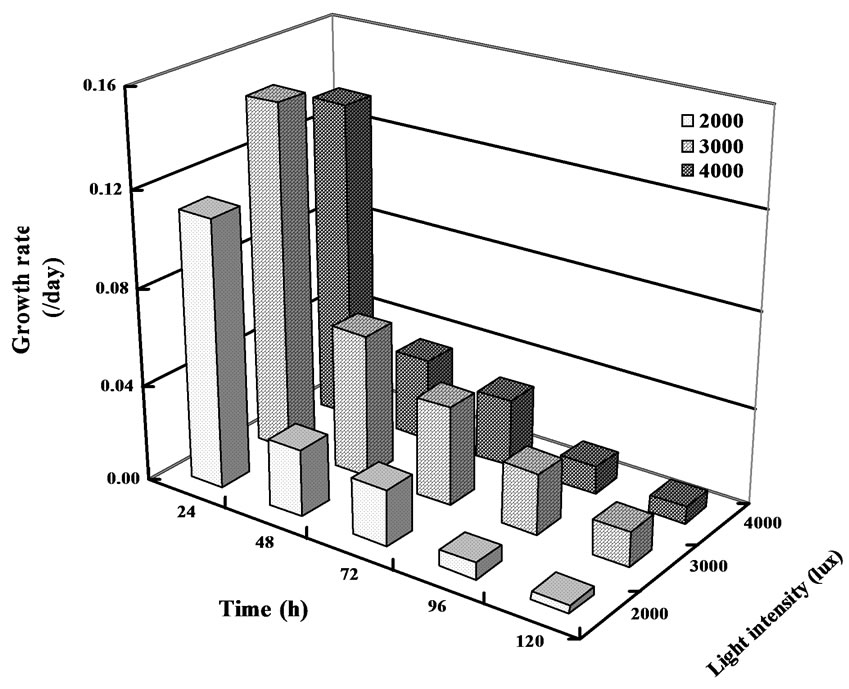
Figure 1. Growth rates of A. flos-aquae treated with monosulfuron exposed to three light intensities.
Many previous reports had been demonstrated to be effects of herbicides on nitrogen-fixing cyanobacteria growth. Exposure of several Anabaena species to prometryne even at the relatively low concentration of 4 mg L-1 reduced growth by 79, 71 and 69 % respectively, while no growth was observed at a concentration of 8 mg L-1 [16] Benthiocarb at concentrations ranging from 6 to 8 mg/L was lethal to some species of Nostoc [17]. The present studies with monosulfuron indicate that A. flos-aquaue maintain a dose-dependent manner algal growth, and the algal grown exposed to 4000-lux light exhibited greater sensitivity toward the same cation monosulfuron than exposed to 3000-, and 2000-lux light. It is possible that monosulfuron applied at higher concentrations exposed to 4000-lux result in stronger toxicity to algae due to enhancing algal efficient photosynthesis and special characteristics metabolism exposed to highlight. The results of stimulation effect on algal growth at lower concentrations (0.008 to 0.08 mg/L) exposed to 2000-lux can be explained in two ways: 1) Monosulfuron self-motionly degraded faster at lower concentrations in this experiment and resulted in reducing toxic to algae. Fan et al. (2004) [18] reported that monosulfuron residues have been shown to dissipate rapidly with half-lives of less than 14 d in field tests using HPLC-UV residue analysis. 2) It was demonstrated that A. flos-aquae is capable not only of growing photoheterotrophically, but also of growing chemoheterotrophically like bacteria to a great extent [19]. Alternatively, at lower monosulfuron concentrations, algal cells may have assimilated more organic carbon and nitrogen [20,21], which interfered with herbicide uptake through the formation of an inactive herbicide complex with organic carbon and nitrogen. This process can reverse herbicide toxicity or can convert the herbicide into hydrate of carbon and amino acid metabolites, thus decreasing the toxicity of the herbicide.
3.2. Effects of Herbicide on the Content of Chlorophyll a in Algal Cells Exposed to Different Light Intensities
The results in Figure 2 indicate the impact of monosulfuron on the content of chlorophyll a in A. flos-aquae. The effect of monosulfuron on chlorophyll a followed a dose-dependence manner, i.e., the chlorophyll a content decreased gradually, as the concentration of monosulfuron increased from 0.008 to 800 mg/L. The content of chlorophyll a was reduced gradually, as the concentration of monosulfuron increased from 0.008 to 0.08 to 0.8 to 8 mg/L, by 8, 14, 17, and 55% exposed to 4000-lux light, and by 15, 28, 62, and 87% exposed to 3000-lux, as well as by 26, 45, 63, and 95% exposed to 2000-lux, after 6days, respectively, in comparison with the control (P<0.05). There was a significant difference in chlorophyll a between each concentration (P < 0.05). Reductions in chlorophyll a rose to 92 to 99% as the concentration of monosulfuron increased to 80 and 800 mg/L exposed to three light conditions. The chlorophyll a synthesis of A. flos-aquae exposed to 2000-lux light was more sensitive to monosulfuron than those exposed to 4000-lux light.
The dose dependence of photosynthetic pigment affected by the herbicide has been reported to appear several manners. The results in the present study indicate the dose dependence of monosulfuron toxicity on chlorophyll a synthesis is in agreement with that demonstrated by most reports, that the inhibitory effect of herbicide increase with an increase in herbicide concentration, and suggested that the reduction in the growth rate of algae may be due to a decrease in algal photosynthesis caused by the inhibition of synthesis of chlorophyll a, the most important pigment in algal cells for collecting solar energy for photosynthesis [22]. Bhunia et al. (1991) [17] reported that the chlorophyll a content of N. muscorum was reduced by 56, 74, and 97% at 2, 4, and 6 mg/L benthiocarb, respectively, and suggested that the low pigment content may be caused by photooxidation arising from the inability of chlorophyll a to dissipate its absorbed excitation energy when electron transport in inhibited.
3.3. Effects of Herbicide on the Content of Caroteniod in Algal Cells Exposed to Different Light Intensities
The effect of monosulfuron on caroteniod content in A. flos-aquae cells is summarized in Figure 3. The formation of caroteniod was inhibited as the light intensity increased and that the effect of monosulfuron on caroteniod exposed to three light intensities displayed dose dependence. The content of carotenoid in cells of A. flos-aquae was reduced by 30 to 95% and 28 to 87%
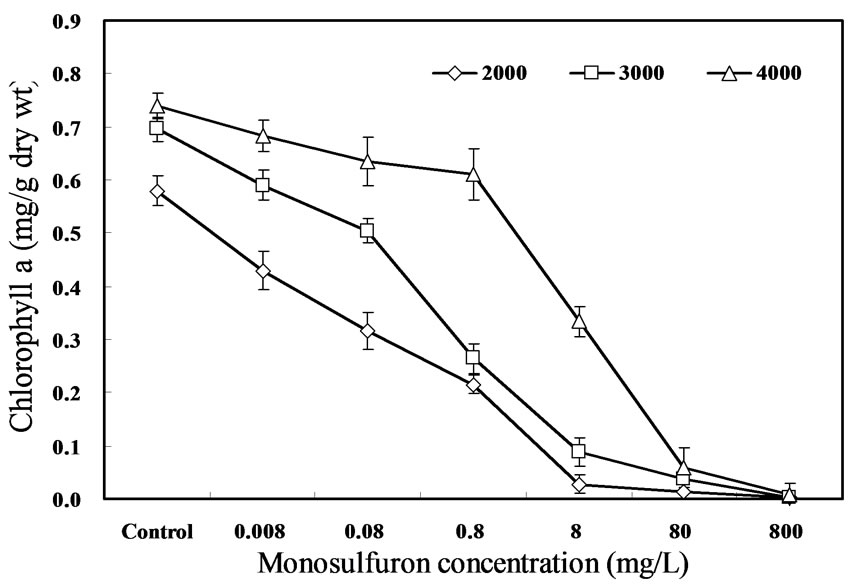
Figure 2. Effects of monosulfuron on the content of chlorophyll a in A. flos-aquae exposed to three light intensities after 6 days.
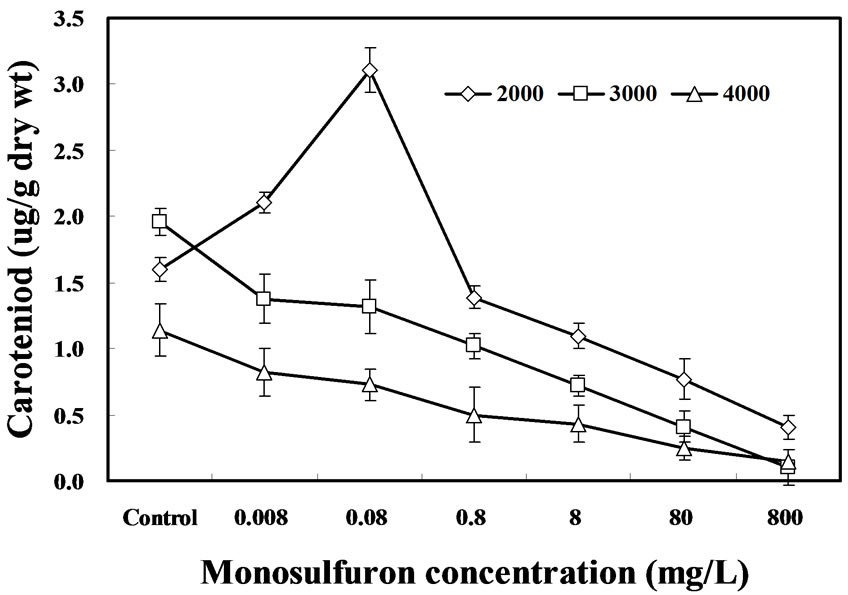
Figure 3. Effects of monosulfuron on the content of carotenoid in A. flos-aquae under three light intensities after 6 days.
exposed to 3000-lux and 4000-lux after 144 h at the monosulfuron from 0.008 to 800 mg/L, respectively, in comparison with the control (P<0.05). Of particular interest is the observation that the caroteniod content of algae treated with 0.008 and 0.08 mg/L monosulfuron exposed to 2000-lux had a different stimulatory effect from that of treatments exposed to 3000-lux and 4000- lux, but an inhibitory effect at concentration above 0.8 mg/L. The caroteniods synthesis of A. flos-aquae exposed to 4000-lux light was more sensitive to monosulfuron than those exposed to 2000-lux light.
Carotenoids are known to be involved in a number of photosynthetic processes, including light harvesting and energy transfer to chlorophyll a, photoprotection by quenching triplet state chlorophyll a molecules, and scavenging singlet oxygen. Regulation of caroteniod biosynthesis is known to be dependent on external stimuli, including light intensity and oxygen availability in a photosynthetic bacterium Rhodobacter [23], and is in agreement with our findings. These stimulatory effects of monosulfuron on caroteniod formation in A. flos-aquae cells exposed to 2000-lux at lower monosulfuron (0.008 to 0.08 mg/L) are in accordance with that on algal growth.
3.4. Effects of Herbicide on the Content of Biliprotein in Algal Cells Exposed to Different Light Intensities
The effect of monosulfuron concentration on biliprotein in the algal specie tested is shown in Figure 4. Our results showed that the effect of monosulfuron on biliprotein in cells of A. flos-aquae exposed three light intensities displayed contrary dose dependence; it had a stimulatory effect as the concentration effect as the concentration increased from 0.008 to 0.08 mg/L, but an inhibitory effect at concentration above 0.8 mg/L. Subjected to lower monosulfuron concentration (i.e.0.008 to 0.08 mg/L), the content of biliprotein was increased by 11 to 46% exposed to three light intensities, after 6 days, in comparison with the control (P<0.05). But the converse was observed at higher concentrations (i.e. 0.8 to 800 mg/L), the biliprotein content of cells grown exposed to three light intensities was decreased by 33 to 98% after 6 days, relative to the control treatment (P<0.05). The biliprotein formation of A. flos-aquae exposed to 2000-lux light was more sensitive to monosulfuron than those exposed to 3000- and 4000-lux light.
The algal biliprotein function as important light-arvesting components for driving for photosynthetic reactions. In this study, exposure of A. flos-aquae to monosulfuron at the relatively low concentration (i.e. 0.008 to 0.8 mg/L) had a stimulation effect on the synthesis of biliprotein, it may be related to the herbicide monosulfuron, an organic nitrogen compound, algal cells may have assimilated more organic nitrogen. Harrison et al. (1990) [24] observed the increase in nitrogen concentration in medium may stimulate the formation of biliprotein. The inhibitory effect of monosulfuron on the synthesis of biliprotein at higher concentrations (more than 0.8 mg/L) is similar to the previous report by Marco et al. (1990) [25], who found that the organophosphorus insecticide trichlorfon decreased biliprotein content in Anabaena PCC 7119 at higher concentrations ranging from 20 to 300 mg/L. The herbicide monosulfuron appeared to have different effects on the synthesis of photosynthetic pigments. This may be due to the difference in chemical structures and characteristics of three pigments. For example, biliprotein is not a single compound; it is a complex composed of bili pigments (phycobilins) covalently linked to a specific protein, C-phycocyanin [12].
4. Conclusions
The data obtained in the present study provide information about the inhibitory effect of the sulfonylurea herbicide monosulfuron on mixotrophic growth of A. flos-aquae exposed to different light intensities, under which the cyanobacterium exhibits different sensitivity to the herbi-
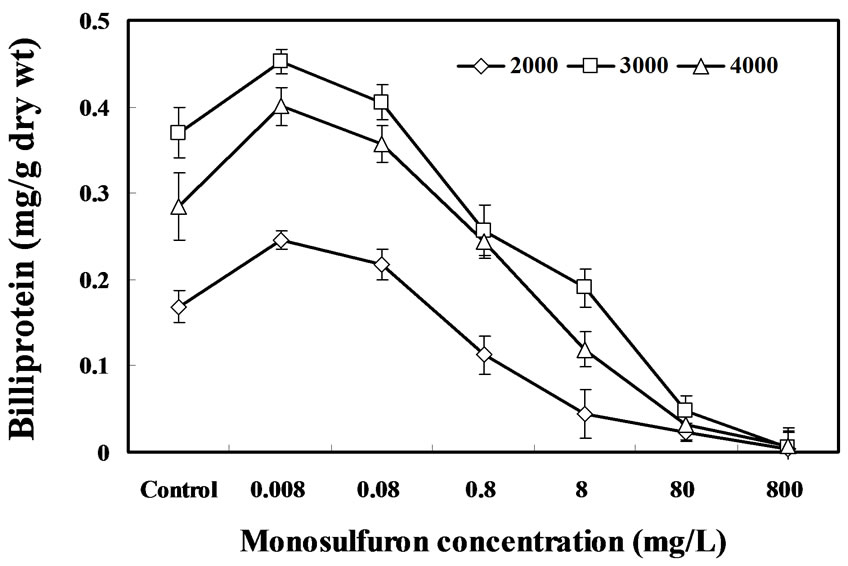
Figure 4. Effects of monosulfuron on the content of biliprotein in A. flos-aquae under three light intensities after 6 days.
cide. These findings suggest monosulfuron be applied at low concentrations (i.e. less than 0.8 mg/L) in the field to minimize the negative impact on these beneficial microorganisms, or postemergence application, owing to its relative lower inhibitory effect on nitrogen-fixing cyanobacteria exposed to low light intensities. The physiological mechanism of monosulfuron toxicity to A. flosaquae was found to be related to not only target enzymes ALS activity but also photosynthesis in algae cells. A better understanding of the mechanism requires further study of the effect of monosulfuron on electronic transfer, ATP, and fatty acid metabolism in photosystem.
5. Acknowledgements
Funding of this work was provided by the Medi-Agriculture Foundation (YG 2009M S24). We also thank all the graduate students and technical support personnel who worked on this project.
REFERENCES
- M. M. EL-Sheekh, H. M. Kotkat, and H. E. Hammouda, “Atrazine herbicide on growth, photosynthesis, protein synthesis, and fatty acid composition in the unicellular green algae Chlorella Kessler,” Ecotocxicol Environ Safety, Vol. 29, pp. 349–358, 1994.
- J. Y. Shen, Z. R.Lu, Y. T. Lu, and X. H. Wang, “Progress on toxicity of nitrogen-fixing cyanobacteria to herbicides,” World Pestic, Vol. 6, pp. 54–61, 2004.
- T. Ogawa and S. C. Aiba, “Bioenergetic analysis of mixotrophic growth in Chlorella vulgaris and Scenedesmus acutus,” Biotechnol Bioeng, Vol. 23, pp. 1121–1132, 1981.
- Z. M. Li, G. F. Jia, and L. X. Wang, “Sulfonylurea compounds and its herbicide usage,” Chinese patent CNI 080 116A, 1994.
- Z. J. Fan, C. F. Qian, and H. B. Dang, “Study on the bioassay of maiguning and safety assessment of maiguning to different maize (Zea mays L.),” Chinese Journal of Pesticide Science, Vol.2, pp. 63–70, 2000.
- C. D. S. Tomilin, “The pesticide manual” (13th Edition). The British Crop Protection Council Press, London, 2003.
- H. Abou-Waly, M. M. Abou-Setta, H. N. Nigg, and L. L. Mallory, “Growth response of freshwater algae, Anabaena flos-aquae and Selenastrum capricornutum, to atrazine and hexazinone herbicides,” Bull Environ Contam Toxicol, Vol.46, pp. 223–229, 1991.
- W. A. Kratz and J. Myers, “Nutrition and growth of several blue-green algae,” American Journal of Botany, Vol. 42, pp. 282–287, 1955.
- K. K. Schrader, M. Q. Regt, P. D. Tidwell, C. S. Tucker, and S. O. Duke, “Compounds with selective toxicity towards the off-flavor metabolite-producing cyanobacterium Oscillatoria cf. chalybea,” Aquaculture, Vol. 163, pp. 85‑91, 1998.
- K. K. Schrader, M. Q. Regt, P. D. Tidwell, C. S.Tucker, and S. O. Duke, “Compounds with selective toxicity towards the off-flavor metabolite-producing cyanobacterium Oscillatoria cf. chalybea,” Aquaculture, Vol. 163, pp. 85–91, 1998.
- G. Mackinney, “Absorption of light by chlorophyll solutions,” Journal of Biological Chemistry, Vol. 140, pp. 315–322, 1941.
- A. N. Glazer and C. S. Hixson, “Characterization of R-phycocyanin chromophore content of R-phycocyanin and C-phycoerythrin,” Journal of Biological Chemistry, Vol. 250, pp. 5487–5495, 1975.
- A. Jenssen, “Chlorophyll and carotenoids,” In: Handbook of Phycological Methods. Physiological & Biochemical Methods (Ed. By J. A. Hellebust & J. S. Craigie), Cambridge University Press, Cambridge, UK, pp. 59–70, 1978.
- J. Y. Shen and Y. T. Lu, “Effects of herbicides on biodiversity of rice fields in China,” Pages 56‑67 in Proc. Impact Assessment of Farm Chemicals Run Off from Paddy Conference, Japan: National Institute for Agro-Environmental Sciences, Japan (NIAES) and National Institute of Agricultural Science and Technology, Korea (NIAST), 2005.
- B. Prithiviraj, L. G. Perry, D. V. Badri, and J. M. Vivanco, “Chemical facilitation and induced pathogen resistance
- mediated by a root-secreted phytotoxin,” New Phytol. Vol. 173, pp. 852–860, 2007.
- J. Y. Shen, Y. T. Lu, and G. H. Cheng, “Effects of chemical herbicides on toxicity of non-target nitrogenfixing Cyanobacteria in paddy fields,” Pages 665-670 in Proc. 20th Asian-Pacific Weed Science Conference Vietnam: Weed Science Society, Asian-Pacific, 2005.
- A. K. Bhunia, N. K. Basu, D. Roy, A. Chakrabarti, and S. K. Banerjee, “Growth, chlorophyll a content, nitrogen-fixing ability, and certain metabolic activities of Nostoc muscorum: effect of methylparathion and benthiocarb,” Bull Environ Contam Toxicol, Vol. 471, pp. 43–50, 1991.
- Z. J. Fan, J. Y. Hu, Y. W. Ai, C. F. Qian, W. Q. Yu, and Z. M. Li, “Residue analysis and dissipation of monosulfuron in soil and wheat,” Journal Environmental Science (China), Vol. 16, pp. 717–721, 2004.
- C. Y. Jin, L. R. Song, and S. H. Li, “The mixotrophic growth of Anabaena sp. HB1017,” Acta Hydrobiologica Sinica, Vol. 2, pp. 134–137, 1996.
- A. Kamiya and W. Kowallik, “Photoinhibition of glucose uptake in Chlorella,” Plant and Cell Physiology, Vol. 28, pp. 611–619, 1987.
- J. Y. Shen, D. T. Antonio, M. Q. Shen, W. Luo and Z. M. Li, “Molecular basis for differential metabolic responses to monosulfuron in three nitrogen-fixing cyanobacteria,” Weed Science, Vol. 57, pp. 178‑188, 2009.
- A. K. Pandey, “Effect of propanil on growth and cell constituents of Nostoc calcicoia,” Pestic Biochem Physiol. Vol. 23, pp. 157–162, 1985.
- G. A. Armstrong, B. S. Hundle, and J. E. Hearst, “Evolutionary conservation and structural similarities of carotenoid biosynthesis gene products for photosynthetic and nonphotosynthetic organisms,” Methods Enzymol, Vol. 214, pp. 297–311, 1993.
- P. J. Harrison, P. A. Thompson, and G. S. Calderwood, “Effects of nutrient and light limitation on the biochemical composition of phytoplankton,” Journal of Applied Phycology, Vol. 2, pp. 45‑46, 1990.
- E. Marco, F. Martinez, and M. I. Orus, “Physiological alterations induced by the organophosphorus insecticide trichlofon in Anabaena PCC 7119 grown with nitrates,” Environmental and Experimental Botany, Vol. 30, pp. 119–126, 1990.

