Journal of Biomedical Science and Engineering
Vol.7 No.7(2014), Article ID:46588,8 pages
DOI:10.4236/jbise.2014.77044
A Comparison of Enzyme-Linked Immunosorbent Assay versus Multiplex Methodology Using an in Vitro Model of Pulmonary Hypertension and Inflammation
Yan Zhu1*, Deepthi Alapati1,2, Joanna Costa1,2, Victoria L. Maduskuie3, Paul T. Fawcett3, Thomas H. Shaffer1,2,4
1Nemours Research Lung Center, Biomedical Research, Nemours Alfred I duPont Hospital for Children, Wilmington, USA
2Pediatrics Department, Nemours Alfred I duPont Hospital for Children, Wilmington, USA
3Center for Clinical Diagnostics, Nemours Alfred I duPont Hospital for Children, Wilmington, USA
4Physiology and Pediatrics Department, Temple University School of Medicine, Philadelphia, USA
Email: *yzhu@nemours.org, dalapati@nemours.org, jcosta@nemours.org, vickymaduskuie@gmail.com, pfawcett@nemours.org, tshaffer@nemours.org
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 April 2014; revised 26 May 2014; accepted 2 June 2014
ABSTRACT
Enzyme-linked immunosorbent assay (ELISA) is the most widely used method for measuring a single cytokine. Recent developments in cytokine quantification such as multiple arrays measure multiple cytokines simultaneously. Although good correlations between ELISA and multiplex methods have been observed, side by side comparisons are limited. In the present study we hypothesized that ELISA and Luminex techniques are comparable in detecting cytokines in culture medium when pulmonary artery smooth muscle cells (PASMC) are exposed to stress. Primary human PASMC were cultured in modular chambers and exposed to 21% FiO2 and peak inspiratory and positive end expiratory pressure of 24 and 8 cmH2O respectively, and 95% FiO2. At 24 hours, culture medium was collected and assayed for interleukin-6 (IL-6) and IL-8 by quantitative ELISA and by Human Cytokine 25-Plex Panel using a Luminex 200 analyzer. A comparative analysis of agreement between our ELISA and Luminex data was detailed for control and stress conditions using the Bland-Altman plot analysis. Each assay resulted in comparable increased (p < 0.001) levels of IL-6 and IL-8 as compared to control in response to oxidative and biophysical stress. The Bland-Altman analysis demonstrated that 95% of the differences between ELISA and Luminex values were within ±1.96 SD from the mean difference indicated by the 95% limits of agreement for the measurements of IL-6 and IL-8. There was no systematic bias as a function of inflammation level. We conclude that in this cell culture model, ELISA and Luminex are comparable in detecting the levels of IL-6 and IL-8 in the culture medium. If measurements of multiple cytokines are demanded and the amount of sample is limited, Luminex multi-analyte profiling technology is accurate and sensitive.
Keywords:Enzyme-Linked Immunosorbent Assay (ELISA), Luminex, Pulmonary Artery Smooth Muscle Cells (PASMC), Inflammation, Bland-Altman Plot Analysis

1. Introduction
Cytokines play a key role in host response to infection, inflammation, and trauma [1] . The measurement of cytokines and other inflammatory mediators in human blood and cell culture samples can provide valuable information about in vivo immune status [2] and in vitro biological processes. Different methodologies such as enzyme-linked immunosorbent assay (ELISA), multiplex bead array assays (MBAA), and microarray are currently available for the detection and quantitation of these inflammatory mediators.
ELISA is the most widely used method for measuring a single cytokine [3] and has become the current “gold standard” in biomedical research and clinical laboratory testing [3] [4] . However, ELISA only measures one analyte at a time from each sample and the assay is time consuming and costly. Furthermore, the dynamic range of ELISA-based assays is narrower than that of other technologies such as multiplex assays. Sample dilution is often required for the assay and consequently creates variations of cytokine level between the neat samples and diluted samples [3] . Based on our experience, any variation in operator, pipetting or washing technique, incubation time, and temperature can generate different values between assays. We have used ELISA techniques to detect changes of the pro-inflammatory cytokines interleukin-6 (IL-6) and IL-8 in the apical washing fluids and culture medium in an in vitro air-liquid interface Calu-3 cell culture model [5] -[7] . In these studies, 280 µl of apical surface fluid was collected from 8 transwell inserts for each experimental condition, which was sufficient for the detection of one or two cytokines only. Although IL-6 and IL-8 play important roles in the initiation and propagation of the inflammatory cascade and pulmonary injury [8] , the process of inflammatory responses to stimuli is complex and highly interactive [9] , usually with multiple cytokines involved. Measurement of multiple cytokines simultaneously in the same sample would provide better evaluation of the complexity and dynamic nature of inflammatory response [3] .
Recent developments in cytokine quantification techniques include the use of multiplex assays to measure large numbers of analytes simultaneously in a small amount of sample. The concept of multiplex bead array assays (MBAA) was first introduced by Horan and Wheeless in 1977 [10] . MBAA simultaneously measures multiple analytes in a single run of the assay, provides more information in small volumes of sample, and saves both money and time compared to traditional ELISA. MBAA also detects analytes in a broad dynamic range of concentrations [3] .
Numerous investigators have compared ELISA with MBAA [3] [4] [11] [12] . Jager et al. found that cytokine multiplex assays were comparable in sensitivity, accuracy, and reproducibility to ELISAs for the same analytes in culture supernatant of human peripheral blood mononuclear cells [13] . In a vaccine antibody testing study, Pickering et al. observed strong correlations between the Luminex multi-analyte assays and ELISAs for quantitation of IgG antibodies to Haemophilus influenza type b polysaccharide and the toxoids of Clostridium tetani and Corynebacterium diphtheriae [12] .
In the present study, PASMC served as an in vitro model of pulmonary hypertension (PH) to evaluate the inflammatory responses of PASMC during oxidative and biophysical stress. Previous studies have demonstrated that inflammation contributes to pulmonary vascular remodeling and the development of PH [14] [15] . PASMC are the most numerous cell types in the vessel wall and play a critical role in vascular remodeling in PH, particularly in response to both oxidative and biophysical stress. In a previous study, fetal pulmonary artery smooth muscle cells exposed to short term high concentration oxygen resulted in significant changes in critical cellular signaling pathways and activated phosphodiesterase 5, demonstrating potential oxygen toxicity [16] . Humbert et al. found elevated proinflammatory cytokines, such as IL-1 beta and IL-6 in serum of patients with primary pulmonary hypertension [17] .
Although most published studies have shown good correlation between ELISA and multiplex methods [4] , side by side comparisons of the two technologies are limited. Using this in vitro model, we designed a study to compare ELISA and Luminex techniques side by side. We hypothesized that ELISA and Luminex techniques are comparable in detecting cytokines, specifically IL-6 and IL-8, in culture medium.
2. Materials and Methods@NolistTemp# Clonetics™ pulmonary artery smooth muscle cell systems (Lonza, Carlsbad, CA) were cultured in temperature and pressure controlled air-tight chambers and exposed to 21% FiO2 at atmospheric pressure (control), 21% FiO2 and peak inspiratory pressure (PIP) of 24 cm H2O and positive end expiratory pressure (PEEP) of 8 cm H2O, or 95% FiO2 at atmospheric pressure. At 24 hours, culture medium was collected and assayed for IL-6 and IL-8 in duplicate by quantitative ELISA using human IL-6 and IL-8 Quantikine ELISA kits (R & D Systems, Minneapolis, MN). The samples were also assayed by Human Cytokine 25-Plex Panel (Invitrogen, Life Technologies Corporation, Frederick, MD) using a Luminex 200 analyzer (Luminex Corporation, Austin, TX).2.1. PASMC Cell Culture Model
Proliferating Clonetics™ pulmonary artery smooth muscle cell systems and reagents were purchased from Lonza (Carlsbad, CA). PASMC were cultured in a humidified incubator maintained at 37˚C and 5% CO2. Clonetics™ SmGM™-2 Smooth Muscle Growth Medium-2 was supplemented with 5% fetal bovine serum, 1 µl/ml human epidermal growth factor, 1 µl/ml insulin, 2 µl/ml human fibroblast growth factor-B, 1 µl/ml Gentamicin Sulfate and Amphotericin-B-1000. Cells were grown in 75 cm2 tissue culture flasks and split when 90% confluent. PASMC in fifth or sixth passage were plated at a density of 10,000 cells/cm2 onto Corning® CellBIND® six well plates (9.5 cm2 Cell Growth Area, Corning® CellBIND® Surface; Corning, Tewksbury, MA) and the medium was changed every other day. When the cells reached 80% confluence, two plates were exposed to 21% FiO2 at atmospheric pressure as control. Remaining four plates were placed in modified modular incubator chambers (MIC-101; Billups-Rothenberg, Del Mar, CA, figure 1) and exposed to one of two conditions: 21% FiO2 and PIP of 24 cm H2O and PEEP of 8 cm H2O (21% FiO2-24/8cmH2O) or 95% FiO2 at atmospheric pressure (95% FiO2). A gas impermeable Mylar sheet of 1 mm thickness separated the chamber into an upper and lower compartment. The modular incubator chamber was then sealed and the O-ring and the Mylar sheet were compressed by a stainless steel ring clamp. To achieve the experimental condition of 21% FiO2-24/8cmH2O, gas from an air compressor was delivered to the upper compartment of the modified modular incubator chambers via a positive pressure ventilator at a rate of 60 cycles per minute with PIP of 24 cm H2O and PEEP of 8 cm H2O and a flow rate of 10 L/min. A PEEP valve was connected with the outlet of the lower compartment of the chamber to maintain the desired PEEP. The pressure was transmitted across the Mylar sheet to the lower compartment, which housed the plated cells. Gas containing 21% FiO2 (FiO2 = 21%, FiCO2 = 5%, balance nitrogen) was passed through a bubble type humidifier and flushed into the lower compartment of the chamber for 24 hours at a flow rate of 1 L/min. The entire chamber was then placed in the incubator. To achieve the experimental condition of 95% FiO2, the gas from 95% FiO2 tank (FiO2 = 95%, FiCO2 = 5%) was warmed and humidified with a MR 730 respiratory humidifier (Fisher & Paykel Healthcare, Laguna Hills, CA) and then flushed through the airtight chamber for 30 min at a flow rate of 10 L/min. The chamber was disconnected from the gas source, sealed and placed in the incubator for 24 hours [18] . For all conditions, chamber oxygen levels were monitored with an oxygen analyzer (MAXO2) (OM-25AE: Maxtec, Inc., Salt Lake City, UT) and the pressure was confirmed by a Rüsch pressure monometer (Teleflexmedical, Duluth, GA) (Figure 1 and Figure 2). At 24 hours, culture medium (n = 6 per condition) was collected for cytokine assay.
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of IL-6 and IL-8 were measured by quantitative ELISA using human IL-6 and IL-8 Quantikine ELISA kits (R & D Systems, Minneapolis, MN). This assay employed the quantitative sandwich enzyme immunoassay technique. The monoclonal antibody specific for IL-6 and IL-8 (mouse) was immobilized onto polystyrene microplate wells in a 96-well plate. Standards and samples were added into the wells and IL-6 or IL-8 present was bound by the immobilized antibody. Polyclonal anti-mouse antibody linked with horseradish pe-
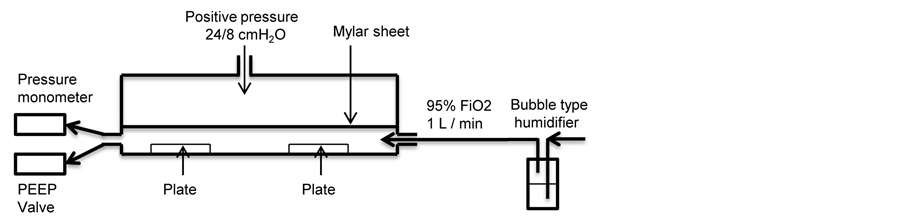
Figure 1. A schematic diagram of modified modular incubator chamber experimental setting. PASMC in six well plates were exposed to one of conditions: 21% FiO2 (control), 21% FiO2-24/8cmH2O, or 95% FiO2, at 37˚C for 24 hours.

Figure 2. Treated PASMC in the well of six well plate. PASMC were grown at six well plates to 80% confluence before exposure to one of conditions: 21% FiO2 (control), 21% FiO2-24/8cmH2O, or 95% FiO2, at 37˚C for 24 hours.
roxidase (HRP) was used as detection antibody. Stabilized chromagen-hydrogen peroxide served as substrate. The optical density of each well was determined using a microplate reader set to 450 nm within 30 minutes. Quantitative results were calculated from the standard curve using linear regression analysis. Medium samples used in the assay were appropriately diluted. All standards and samples were assayed in duplicate. The test sensitivity for respective immunoassays was as follows: IL-6 (0.7 pg/ml) and IL-8 (7.5 pg/ml). The assay range for respective immunoassays was as follows: IL-6 (3.12 - 300 pg/ml) and IL-8 (31.2 - 2000 pg/ml). Inter-assay and intra-assay coefficients of variance are <10%.
2.3. Multiplex Bead Array Assays (MBAA)
The medium samples were also assayed for IL-6 and IL-8 with a Luminex 200 analyzer (Luminex Corporation, Austin, TX) using the Cytokine Human 25-Plex Panel (Invitrogen, Life Technologies Corporation, Frederick, MD), which was specifically designed for quantifying human cytokines and chemokines in serum, plasma, and tissue culture supernatant for as many as 25 different analytes simultaneously. This kit was a multiplex bead and solid phase immunoassay utilizing specific antibody conjugated microspheres for the detection and quantitation of target proteins by flow cytometric analysis. Each microsphere set specific for an analyte was manufactured with a distinct combination of internal fluorescent red and infrared dye. Multiple bead sets may be combined for the detection of up to 100 different analytes per sample. The Luminex instrument used a red laser (635 nm) to excite the detector and measure fluorescence and side scatter to categorize the bead set location on a “bead map”. The subsequent reporter dye was excited by a green laser (532 nm) and median fluorescence was recorded for the assay result. The amount of surface fluorescence of the reported dye was related to the cytokine concentration of the sample. Quantitative results were determined from the standard curve using the logistic 4-parameter curve fit software. To compare data obtained by ELISA, Human Cytokine 25-Plex Panel values were multiplied by multiplication factors that the manufacturer recommended. The multiplication factors for respective cytokines were as follows: IL-6 (2.10) and IL-8 (0.15). The analytic range for the multiplex assay is 10.78 - 7860 pg/ml.
2.4. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.0 for Windows (GraphPad, San Diego, CA) and Statistical Package of the Social Sciences (SPSS, Version 22) Software. All data were expressed as mean ± SEM. One-way ANOVA was run across different treatments at 24 hours for IL-6 and IL-8 data and post hoc analyses were done using Bonferroni comparisons. The values of IL-6 and IL-8 obtained by ELISA and Luminex techniques were compared using paired t-test (one-tailed). p values less than 0.05 were considered statistically significant. At least 6 samples were used for each group. A comparative analysis of agreement between ELISA and Luminex data was detailed for control and stress conditions using the Bland-Altman plot analysis with GraphPad Prism version 5.
3. Results
IL-6 concentrations analyzed by ELISA and Luminex from the culture medium of PASMCs exposed to 21% FiO2, 21% FiO2-24/8cmH2O, or 95% FiO2 are presented in Figure 3. For ELISA measurement, secretion of IL-6 in the 21% FiO2-24/8cmH2O group was greater than 21% FiO2 group at 24 hrs (p < 0.001), with no significant difference between 95% FiO2 and 21% FiO2-24/8cmH2O (p > 0.05). IL-6 level in 95% FiO2 group was greater than that of 21% FiO2 group, but the difference was not statistically significant. For Luminex measurement, the trend of IL-6 for all three groups was similar to the ELISA results, but there were no significant differences among groups (p > 0.05).
IL-8 concentrations are presented in Figure 4. For both ELISA and Luminex measurements, secretion of IL-8 in the 21% FiO2-24/8cmH2O and 95% FiO2 groups were greater than 21% FiO2 group at 24 hrs (p < 0.001), with no difference between 21% FiO2-24/8cmH2O and 95% FiO2 groups (p > 0.05). The values of IL-8 in Luminex measurement were significantly lower than that in ELISA measurement (p < 0.05).
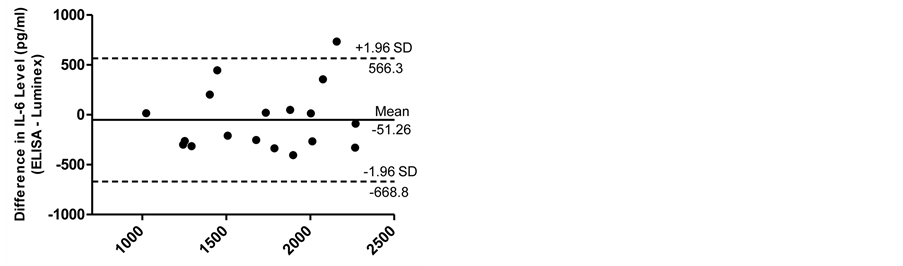
The Bland-Altman analyses for IL-6 and IL-8 measurements are shown in Figure 5(a) and Figure 5(b). In analyzing values over all conditions, Bland-Altman analysis demonstrated that 95% of the differences between ELISA and Luminex values were within ±1.96 SD from the mean difference indicated by the 95% limits of agreement for the measurements of IL-6 and IL-8. There was no systematic bias as a function of inflammation level. The limits of agreement for IL-6 and IL-8 were (−668.8 and 566.3) and (−83.54 and 4142), respectively.
4. Discussion
In the current study, we measured the levels of IL-6 and IL-8 using ELISA and Luminex techniques in culture medium of PASMC exposed to oxidative and biophysical stress. Our results demonstrated that trends for IL-6 and IL-8 levels correlated between ELISA and Luminex techniques. The Bland-Altman analysis showed that ELISA and Luminex techniques are interchangeable in detecting cytokines.
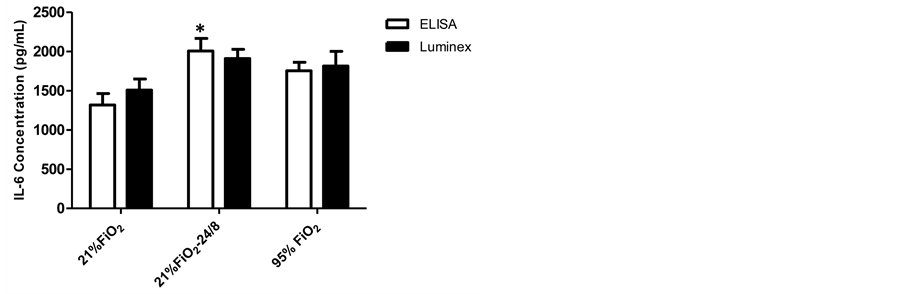
Figure 3. IL-6 concentrations analyzed by ELISA and Luminex. For ELISA measurement, secretion of IL-6 in the 21% FiO2-24/8cmH2O group was greater than 21% FiO2 group at 24 hrs (p < 0.001), with no significant difference between 95% FiO2 and 21% FiO2-24/8cmH2O (p > 0.05). IL-6 level in 95% FiO2 group was greater than that of 21% FiO2 group, but the difference was not significant. Data are mean ± SEM. *Group effect (p < 0.001).
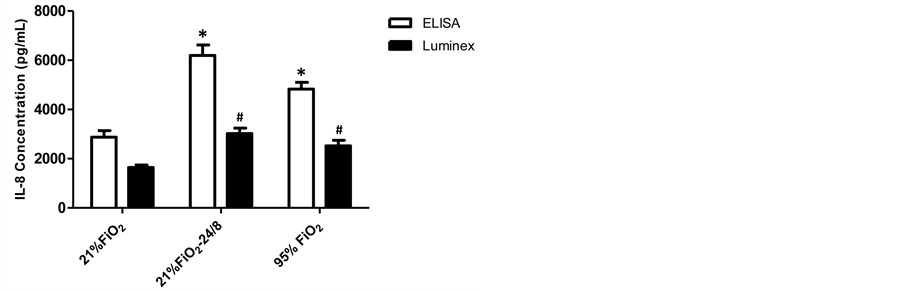
Figure 4. IL-8 concentrations analyzed by ELISA and Luminex. For both ELISA and Luminex measurements, secretion of IL-8 in the 21% FiO2-24/8 cmH2O and 95% FiO2 groups were greater than 21% FiO2 group at 24 hrs (p < 0.001), with no significant difference between 21% FiO2-24/8cmH2O and 95% FiO2 groups (p > 0.05). Data are mean ± SEM and n = 6 for each condition; *Group effect (ELISA) (p < 0.05). #Group effect (Luminex) (p < 0.05).
 (a)
(a)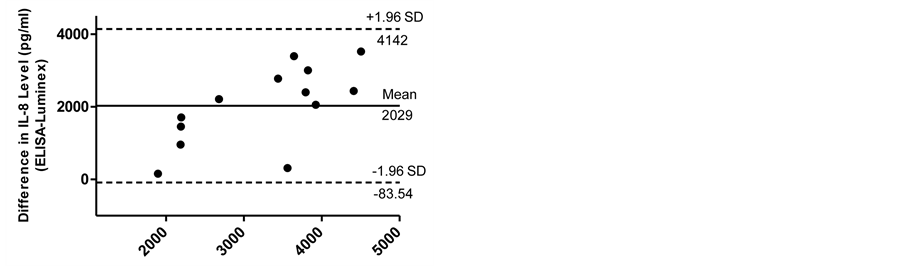 (b)
(b)
Figure 5. The Bland-Altman analyses for IL-6 (a) and IL-8 (b) measurements. Bland-Altman analysis demonstrated that 95% of the differences between ELISA and Luminex values were within ±1.96 SD from the mean difference indicated by the 95% limits of agreement for the measurements of IL-6 and IL-8. There was no systematic bias as a function of inflammation level.
Primary PASMC system from Lonza contains normal human pulmonary artery smooth muscle cells and has been employed as an in vitro cell culture model in vascular pathology and cardiovascular pharmaceutical research [19] . When PASMC are cultured in six well plates, inflammatory mediators are secreted into the culture medium. This mimics the release of inflammatory mediators into the blood in vivo, and the levels of inflammatory mediators in medium should reflect the circulating levels of the inflammatory mediators. The Luminex measurement of inflammatory mediators in culture medium has not been validated against ELISA side by side. Validation of Luminex measurement will allow assessment of inflammatory mediators in patients for clinical use to accurately evaluate disease processes [4] and response to therapy.
In this study, each assay resulted in comparable increased levels of IL-6 and IL-8 in response to hyperoxia and positive pressure stress as compared to control conditions. For Luminex measurement, the trend of IL-6 and IL-8 for all three groups was similar to the ELISA results. The values of IL-8 in Luminex measurement were significantly lower than those in ELISA measurement.
In analyzing values over all conditions, the Bland-Altman analysis demonstrated that 95% of the differences between ELISA and Luminex values were within ±1.96 SD from the mean difference indicated by the 95% limits of agreement for the measurements of IL-6 and IL-8. There was no systematic bias as a function of inflammation level. The limits of agreement for IL-6 and IL-8 were small enough to be confident that Luminex can be used in place of ELISA.
The multiplex bead assay is advantageous for its ability to test multiple targets from a single sample. However, it is important to consider the dynamic range of the assay when choosing a method for analysis. The narrow dynamic range of ELISA makes this method advantageous for detecting low levels of cytokines. The broad analytic range of the multiplex assay is more favorable for measuring higher expression level of cytokines. However, it should be considered that a large panel of analytes does not ensure absolute results for each cytokine in the panel; the possibility exists that one or more analytes will fall outside the analytic range of a particular assay for cytokines. This would require retest on diluted sample for high outliers and alternate testing for low outliers. Furthermore, it has been reported that cytokine values may vary among kits from different manufacturers and quantitative techniques and the kits from the same supplier should be used to compare the absolute values for ELISA and Luminex measurements [12] [20] . Therefore, it is important to realize that absolute cytokine value results obtained by different methods might not be the same, but the trend of the values among experiment groups are comparable.
5. Conclusion
In conclusion, in this cell culture model, ELISA and Luminex are comparable in detecting the levels of IL-6 and IL-8 in the culture medium over a wide range of stress induced inflammation. If measurements of multiple cytokines are demanded and the amount of sample is limited, Luminex multi-analyte profiling technology is accurate and sensitive. Also, this approach may be more efficient and economic for large sample studies.
Acknowledgements@NolistTemp# We thank Barbara E. Gray, BA, CPM, Administrative Manager, Nemours Research Lung Center, for her contribution to the research effort. Statement of Financial Support@NolistTemp# Funded by Nemours Foundation and NIH COBRE 1P20GM103464 (The Center for Pediatric Research).References
- Dinarello, C.A. (2000) Proinflammatory Cytokines. Chest, 118, 503-508. http://dx.doi.org/10.1378/chest.118.2.503
- Breen, E.C., Reynolds, S.M., Cox, C., Jacobson, L.P., Magpantay, L., Mulder, C.B., Dibben, O., Margolick, J.B., Bream, J.H., Sambrano, E., Martinez-Maza, O., Sinclair, E., Borrow, P., Landay, A.L., Rinaldo, C.R. and Norris, P.J. (2011) Multisite Comparison of High-Sensitivity Multiplex Cytokine Assays. Clinical and Vaccine Immunology, 18, 1229-1242. http://dx.doi.org/10.1128/CVI.05032-11
- Leng, S.X., McElhaney, J.E., Walston, J.D., Xie, D., Fedarko, N.S. and Kuchel, G.A. (2008) ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research. The Journals of Gerontology: Series A, 63, 879-884. http://dx.doi.org/10.1093/gerona/63.8.879
- Elshal, M.F. and McCoy, J.P. (2006) Multiplex Bead Array Assays: Performance Evaluation and Comparison of Sensitivity to ELISA. Methods, 38, 317-323. http://dx.doi.org/10.1016/j.ymeth.2005.11.010
- Zhu, Y., Chidekel, A. and Shaffer, T.H. (2010) Cultured Human Airway Epithelial Cells (CALU-3): A Model of Human Respiratory Function, Structure, and Inflammatory Responses. Critical Care Research and Practice, 2010, Article ID: 394578. http://dx.doi.org/10.1155/2010/394578
- Zhu, Y., Miller, T.L., Chidekel, A. and Shaffer, T.H. (2008) KL4-Surfactant (Lucinactant) Protects Human Airway Epithelium from Hyperoxia. Pediatric Research, 64, 154-158. http://dx.doi.org/10.1203/PDR.0b013e318175dd14
- Zhu, Y., Miller, T.L., Singhaus, C.J., Shaffer, T.H. and Chidekel, A. (2008) Effects of Oxygen Concentration and Exposure Time on Cultured Human Airway Epithelial Cells. Pediatric Critical Care Medicine, 9, 224-229. http://dx.doi.org/10.1097/PCC.0b013e318166fbb5
- Kramer, B.W., Jobe, A.H., Bachurski, C.J. and Ikegami, M. (2001) Surfactant Protein A Recruits Neutrophils into the Lungs of Ventilated Preterm Lambs. American Journal of Respiratory and Critical Care Medicine, 163, 158-165. http://dx.doi.org/10.1164/ajrccm.163.1.2005084
- Allen, G.L., Menendez, I.Y., Ryan, M.A., Mazor, R.L., Wispe, J.R., Fiedler, M.A. and Wong, H.R. (2000) Hyperoxia Synergistically Increases TNF-Alpha-Induced Interleukin-8 Gene Expression in A549 Cells. American Journal of Physiology—Lung Cellular and Molecular Physiology, 278, L253-260.
- Horan, P.K. and Wheeless Jr., L.L. (1977) Quantitative Single Cell Analysis and Sorting. Science, 198, 149-157. http://dx.doi.org/10.1126/science.905822
- Khan, S.S., Smith, M.S., Reda, D., Suffredini, A.F. and McCoy Jr., J.P. (2004) Multiplex Bead Array Assays for Detection of Soluble Cytokines: Comparisons of Sensitivity and Quantitative Values among Kits from Multiple Manufacturers. Cytometry Part B: Clinical Cytometry, 61, 35-39. http://dx.doi.org/10.1002/cyto.b.20021
- Pickering, J.W., Martins, T.B., Schroder, M.C. and Hill, H.R. (2002) Comparison of a Multiplex Flow Cytometric Assay with Enzyme-Linked Immunosorbent Assay for Auantitation of Antibodies to Tetanus, Diphtheria, and Haemophilus Influenzae Type b. Clinical and Diagnostic Laboratory Immunology, 9, 872-876.
- de Jager, W., te Velthuis, H., Prakken, B.J., Kuis, W. and Rijkers, G.T. (2003) Simultaneous Detection of 15 Human Cytokines in a Single Sample of Stimulated Peripheral Blood Mononuclear Cells. Clinical and Diagnostic Laboratory Immunology, 10, 133-139.
- Price, L.C., Wort, S.J., Perros, F., Dorfmuller, P., Huertas, A., Montani, D., Cohen-Kaminsky, S. and Humbert, M. (2012) Inflammation in pulmonary Arterial Hypertension. Chest, 141, 210-221. http://dx.doi.org/10.1378/chest.11-0793
- Soon, E., Holmes, A.M., Treacy, C.M., Doughty, N.J., Southgate, L., Machado, R.D., Trembath, R.C., Jennings, S., Barker, L., Nicklin, P., Walker, C., Budd, D.C., Pepke-Zaba, J. and Morrell, N.W. (2010) Elevated Levels of Inflammatory Cytokines Predict Survival in Idiopathic and Familial Pulmonary Arterial Hypertension. Circulation, 122, 920- 927. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.933762
- Farrow, K.N., Lee, K.J., Perez, M., Schriewer, J.M., Wedgwood, S., Lakshminrusimha, S., Smith, C.L., Steinhorn, R.H. and Schumacker, P.T. (2012) Brief Hyperoxia Increases Mitochondrial Oxidation and Increases Phosphodiesterase 5 Activity in Fetal Pulmonary Artery Smooth Muscle Cells. Antioxidants & Redox Signaling, 17, 460-470. http://dx.doi.org/10.1089/ars.2011.4184
- Humbert, M., Monti, G., Brenot, F., Sitbon, O., Portier, A., Grangeot-Keros, L., Duroux, P., Galanaud, P., Simonneau, G. and Emilie, D. (1995) Increased Interleukin-1 and Interleukin-6 Serum Concentrations in Severe Primary Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine, 151, 1628-1631. http://dx.doi.org/10.1164/ajrccm.151.5.7735624
- Babu, P.B., Chidekel, A. and Shaffer, T.H. (2005) Hyperoxia-Induced Changes in Human Airway Epithelial Cells: The Protective Effect of Perflubron. Pediatric Critical Care Medicine, 6, 188-194. http://dx.doi.org/10.1097/01.PCC.0000154944.67042.4F
- Alapati, D., Rong, M., Chen, S., Hehre, D., Hummler, S.C. and Wu, S. (2014) Inhibition of ß-Catenin Signaling Improves Alveolarization and Reduces Pulmonary Hypertension in Experimental BPD. American Journal of Respiratory Cell and Molecular Biology, Epub ahead of print.
- Ledur, A., Fitting, C., David, B., Hamberger, C. and Cavaillon, J.M. (1995) Variable Estimates of Cytokine Levels Produced by Commercial ELISA Kits: Results Using International Cytokine Standards. Journal of Immunological Methods, 186, 171-179. http://dx.doi.org/10.1016/0022-1759(95)00184-C
Abbreviations
PASMC: Pulmonary Artery Smooth Muscle Cells ELISA: Enzyme-linked immunosorbent assay MBAA: Multiplex bead array assays IL: Interleukin PIP: Peak inspiratory pressure
NOTES

*Corresponding author.

