Journal of Cancer Therapy
Vol.07 No.03(2016), Article ID:64705,6 pages
10.4236/jct.2016.73020
ADE as Induction Therapy Results in Treatment of Children with Non-M3 High-Risk Acute Myeloid Leukemia, an Extrapolable Achievement in Developing Countries
Marta Zapata-Tarrés1, Rocío Cárdenas-Cardós1, Liliana Velasco-Hidalgo1, Martín Pérez-García2, Alberto Olaya-Vargas2, Daniela Cárdenas-Pedraza1, Roberto Rivera-Luna3*
1Oncology Department, Instituto Nacional de Pediatría, México City, México
2Hematopoietic Stem Cell Transplantation, Instituto Nacional de Pediatría, México City, México
3Division of Hem-Oncology, Instituto Nacional de Pediatría, México City, México

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 February 2016; accepted 15 March 2016; published 18 March 2016
ABSTRACT
Background: In Mexico, AML survival is referred in 30%. Our aim was to evaluate results with ADE protocol as induction treatment in children with non-M3 AML in a public Mexican institution. Method: We included patients with AML in a single institution between 2005 and 2013. All non- M3-AML patients received ADE as induction therapy (cytarabine 100 mg/m2 in continuous infusion from day 1 to 7, daunorrubicin 30 mg/m2 days 1, 3, 5 and etoposide 100 mg/m2 over days 1 to 5). Patients received antibiotic prophylaxis and strict scheduled appointments to assure adherence and prevent avoidable emergencies. Main Results: Eighteen patients were included. Median age was 106 months. One patient died at diagnosis so 17 were eligible for induction results analysis; eleven needed two cycles and six patients three. Remission rate was 100%. Analyzing non-M3 patients overall survival was 80.2% at 100 months. No fatal complications were observed. Stratifying by number of cycles we observe that patients receiving one ADE cycle had a 0% overall survival at short follow up, with 2 ADE cycles 80% at 100 months and with 3 cycles 100% at 60 months. Conclusion: ADE induction therapy showed improved results in overall survival compared with other standard regimens. Following the protocol we obtained 100% remission. This is an important achievement in our population. Our focus must be to ameliorate maintenance final results. These results should be reproduced in other hospitals in Mexico and other countries.
Keywords:
Acute Myeloid Leukemia, Children, Treatment, ADE

1. Introduction
Acute myeloid leukemia (AML) is the second most common form of leukemia in childhood. Pathogenesis is unknown but frequently AML is associated to Down syndrome and other genetic syndromes. Clinical features are related to intra- and extra-medullary infiltration degree. Main symptoms are fever, bleeding and fatigue. Outcome in pediatric acute myeloid leukemia (AML) has significantly improved over the past decade with remission induction and overall survival rates approaching 90% and 65%, respectively [1] . In the United States during the 1990s, these trials focused on intensively-timed DCTER (dexamethasone, cytarabine, thioguanine, etoposide, and daunomycin)-based regimens [2] . Starting in 2003, the trials shifted to ADE (cytarabine, daunomycin, and etoposide)-based regimens. In Mexico, AML survival is referred in 30% [3] . Results of treatment in children with AML are a challenge. In low income countries toxicity of these regimens is the main cause of death above all in induction period. In a retrospective study including 284 cases of AML in Mexico between 2006 and 2009 overall survival at 36 months was 30.5% (95% 22.8 - 38.5) [4] . Pediatric oncologists in Mexico are using ADE as induction therapy with the purpose to be a National Protocol.
2. Objective
The aim of the study was to describe the socio-demographic, clinical characteristics of patients, and investigate the efficacy and toxicity of ADE induction therapy and evaluate the overall and event free survival in a public single institution.
3. Method
This retrospective cohort study was conducted with data from the period comprised since January 2005 and September 2013. The study population was conformed by children and adolescents with newly diagnosed acute myeloid leukemia according to the French-American-British classification in the Oncology Department of the National Institute of Pediatrics (NIP). M3-AML patients (promyelocytic leukemia) were excluded considering they receive a different treatment strategy. Only patients who had not undergone on a previous treatment and who had a morphological, immunological and cytogenetic diagnosis of AML were included. Exclusion criteria included Down syndrome, immunodeficiency disorders, or a history of serious infections including chronic hepatitis and aspergillus. Patients were seen weekly during the first two months of treatment and monthly thereafter and received prophylactic antibiotics.
All non M3-AML patients received ADE as induction therapy (cytarabine 100 mg/m2 in continuous infusion from day 1 to 7, daunorrubicin 30 mg/m2 days 1, 3, 5 and etoposide 100 mg/m2 over days 1 to 5) following the Medical Research Council (MRC) protocol. Patients who needed a third induction cycle received cytarabine 100 mg/m2 in continuous infusion from day 1 to 5 and mitoxantrone 10 mg/m2 days 1, 2 and 3. Obtaining remission patients were considered to received maintenance with average of four cycles of chemotherapy. All chemotherapy drugs were given by social security. M3-AML patients were excluded.
The AML patients in our cohort also received central nervous system preventive therapy including intrathecal therapy in cases with a primary CNS infiltration or hyperleukocytosis. Hematopoietic progenitor stem cell transplantation was indicated in these high-risk patients after remission.
The management of neutropenic febrile events was designed around the patient’s clinical history and the results of physical examinations, blood cultures, urinalysis and cultures, chest radiographs, blood cultures and baseline chemistry profiles. All these interventions are standard. Patients received cefepime and amikacin at standard doses as a first line intervention against neutropenic febrile events (NFE). If the fever persisted for more than 72 hours or the hemodynamic status of the patient worsened, a different antibiotic regimen with meropenem or vancomycin, and possibly an antifungal therapy was considered. Vancomycin was used as a front line antibiotic when the patient presented with hypotension, catheter infection or showed a positive culture.
Patients were included consecutively.
The scientific review board and ethics committee of the NIP reviewed and approved the study protocol. Informed consent forms were signed by patients’ parents or guardians.
We described socio-demographic and clinical characteristics, including remission rate and toxicity. We filled out data on collection instrument and registry information in clinical charts.
The main variables were children with clinical characteristics including leukemia features, remission rate, number of induction cycles needed for remission, number and severity of febrile neutropenia events, risk group, relapse and overall survival.
Diagnosis was established in accordance to the French-American-British classification. Immunophenotype and cytogenetic characterization was done as routine.
4. Results
Data was collected from eighteen patients with AML.
4.1. Clinical Characteristics
Median age was 106 months (16 - 210). Eleven were female. Patients were product of the first pregnancy in 5 cases (27.8%), second in 6 (33.3%), third in 3 (16.7%) and fourth in 4 (22.2%). Fourteen patients (77.7%) were born with normal weight and 3 (16.6%) over 3.5 Kg. No patient had a genetic syndrome.
Symptoms evolution varied from 1 to 90 days (1 - 90, SD 29.4). 8 (44.4%) had fever, 7 (38.9%) bleeding, 9 (50%) extramedulary infiltration. Complete blood count showed hemoglobin under 6 gr/dl in 3 (16.6%) patients, between 6 and 10 gr/dl in 7 patients (38.8%) and over 8 in 8 (44.4%). Only two patients started with hyperleukocytosis. One patient had less than 10,000 platelets, 3 between 10,000 and 20,000, 5 between 20,000 and 50,000, 1 between 50,000 and 150,000 and 8 over 150,000. Eight (44.4%) had hepatomegaly and 4 (22.2%) splenomegaly.
According to the French-American-British classification, 2 (11.1%) were M0, 4 (22.2%) M2, 6 (33.3%) M4, 2 (11.1%) M5, 4 (22.2%) M7. Two patients had inv16, three t (8; 21) and one 11q23. 50% had extramedullary infiltration at diagnosis (Table 1).
4.2. Induction Response
One patient died at diagnosis so seventeen qualify for induction results analysis; eleven needed two cycles and six patients three. Remission rate was 100%.
4.3. Survival Analysis
One died of intracranial hemorrhage at diagnosis and was excluded from the analysis. Two died during treatment (one of septic shock and one pulmonary hemorrhage). Four patients had bone marrow relapse and were rescued with hematopoietic progenitor stem cell transplantation. Analyzing non-M3 patients overall survival was 80.2% at 100 months. Follow up was 110 months (Figure 1). Considering the morphological type we observe that M2, M4 and M5 AML had the best survival. M0 had 50% and M7 AML had 33.3%.
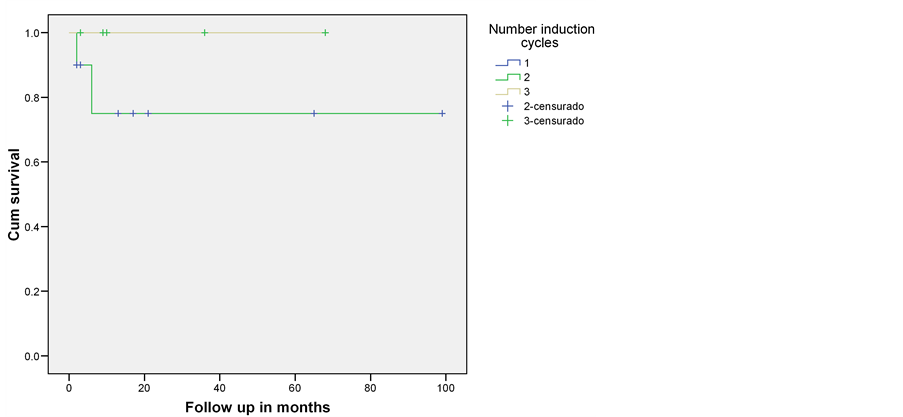
Stratifying by number of cycles we observe that patients receiving one ADE cycle had a 0% overall survival at short follow up, with 2 ADE cycles 80% at 100 months and with 3 cycles 100% at 60 months (Figure 2).
4.4. Analysis of NFE Characteristics in Terms of the Sequence of Presentation
We recorded each clinical event in sequential order and analyzed these occurrences in terms of the time of presentation, the anatomic infectious focus, the infectious agent and the need for intensive care unit (ICU) support. We grouped these events and analyzed them separately. We considered separately the NFE events occurring during induction in order to stablish safety of ADE protocol.
We analyzed 17 patients that received at least one induction cycle. One patient had no NFE, 5 had one, 3 two,
Table 1. Patients features.
DWD: death with disease; AWD: alive without disease; ICH: intracranial hemorrhage.
Figure 1. Overall survival in children with high risk non M3 acute myeloid leukemia. Instituto Nacional de Pediatría-México 2005-2013.
Figure 2. Overall survival stratified by number of ADE induction cycles. Instituto Nacional de Pediatría 2005-2013.
1 three, 4 four, 1 five, 1 seven and 1 nine. From these patients, 6 had one ICU hospitalization, two 2 and 4 one. During induction 10 NFE were registered. The most common anatomic infectious foci was gastrointestinal tract, followed by mucosa, catheter and lung.
Patients had between one and nine infectious events. None had fatal events during de induction treatment. For 6 of first NFEs recorded, we identified 3 cases of pseudomonas aeruginosa, 1 staphilococcus warneri and 1 nolstaniai pibetti.
5. Discussion
There are at least three protocols for the treatment of childhood AML that have shown a continuous improvement, overall outcome with acceptable toxicity from various groups (MRC, BFM and NOPHO) with complete remission rate of 92%, 82% and 83% respectively and overall 5-year survival of 60% [4] [5] . In developing countries like México, mortality associated with toxicity limits their use and results are poor [6] [7] . In a single institution experience considering all infectious aspects, we could obtain similar results in the induction period even if the follow up is not that successful we have better outcomes. These failures in maintenance can be partly explained by the difficulty to make minimal residual disease. We observe that patients with 2 or more ADE induction cycles improve their prognosis what probably reduces cases with leukemic activity that only would be detected molecularly. The dilemma is that morphological remission in our patients must be considered with caution and perhaps we must establish a minimum of two cycles while we have the resource for minimal residual disease, even though in this type of leukemia does not appear to be as useful than in acute lymphoblastic leukemia [8] [9] .
The cause of death due to hemorrhage and bone marrow relapse were the most common findings as described in the literature.
In our milieu, we have non-biological factors that influence outcome like abandonment, low adherence to protocols, distance home-hospital, economic deficiencies etc. that were not evaluated [7] .
6. Conclusion
As an oncology department, we have a selection bias with almost half of patients with extramedullary infiltration. However, ADE induction therapy showed improved results in overall survival compared with other standard regimens. This regimen must be analyzed in other developing countries’ institutions. Cancer mortality decline is a reality and must be our national goal [10] .
Cite this paper
Marta Zapata-Tarrés,Rocío Cárdenas-Cardós,Liliana Velasco-Hidalgo,Martín Pérez-García,Alberto Olaya-Vargas,Daniela Cárdenas-Pedraza,Roberto Rivera-Luna, (2016) ADE as Induction Therapy Results in Treatment of Children with Non-M3 High-Risk Acute Myeloid Leukemia, an Extrapolable Achievement in Developing Countries. Journal of Cancer Therapy,07,197-202. doi: 10.4236/jct.2016.73020
References
- 1. Stevens, R.F., Hann, I.M., Wheatley, K., et al. (1998) Marked Improvements in Outcome with Chemotherapy Alone y Paediatric Acute Myeolid Leukemia: Results of the United Kingdom Medical Research Council’s 10th AML Trial. British Journal of Haematology, 101, 130-140.
http://dx.doi.org/10.1046/j.1365-2141.1998.00677.x - 2. Woods, W.G., Kobrinsky, N., Buckley, J.D., Lee, J.W., Sanders, J., Neudorf, S., et al. (1996) Timed-Sequential Induction Therapy Improves Postremission Outcome in Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Blood, 87, 4979-4989.
- 3. Creutzig, U., Ritter, J., Zimmerman, M., Reinhardt, D., Hermann, J., Berthold, F., et al. (2001) Improved Treatment Results in High-Risk Pediatric Acute Myeloid Leukemia Patients after Intensification with High-Dose Cytarabina and Mitoxantrone: Results of Study AML-BFM 93. Journal of Clinical Oncology, 19, 2705-2713.
- 4. Creutzig, U., Zimmermann, M., Ritter, J., Reinhardt, D., Hermann, J., Henze, G., et al. (2005) Treatment Strategies and Long-Term Results in Paediatric Patients Treated in Four Consecutive AML-BFM Trials. Leukemia, 19, 2030-2042.
http://dx.doi.org/10.1038/sj.leu.2403920 - 5. Lie, S.O., Abrahamsson, J., Clausen, N., Forestier, E., Hasle, H., Hovi, L., et al. (2005) Long-Term Results in Children with AML: NOPHO-AML Study Group—Report of Three Consecutive Trials. Leukemia, 19, 2090-2100.
http://dx.doi.org/10.1038/sj.leu.2403962 - 6. Pérez-Cuevas, R., Doubova, S., Zapata-Tarrés, M., Flores-Hernández, S., Frazier, L., Rodríguez-Galindo, C., et al. (2013) Scaling up Cancer Care for Children without Medical Insurance in Developing Countries: The Case of Mexico. Pediatric Blood & Cancer, 60, 196-203.
http://dx.doi.org/10.1002/pbc.24265 - 7. Zapata-Tarrés, M., Klunder-Klunder, M., Cicero-Oneto, C., Rivera Luna, R., Ortega-Ríos Velasco, F., Cortés-Gallo, G., et al. (2012) Análisis de la atención de las complicaciones durante el tratamiento de ninos con leucemia linfoblástica aguda. El Boletín médico del Hospital Infantil de México, 69, 218-226.
- 8. Goulden, N., Virgo, P. and Grimwade, D. (2006) Minimal Residual Disease Directed Therapy for Childhood Myeloid Leukaemia: The Time Is Now. British Journal of Haematology, 134, 273-282.
http://dx.doi.org/10.1111/j.1365-2141.2006.06182.x - 9. Campana, D. (2008) Status of Minimal Residual Disease Testing in Childhood Haematological Malignancies. British Journal of Haematology, 143, 481-489.
http://dx.doi.org/10.1111/j.1365-2141.2008.07350.x - 10. Smith, M.A., Altekruse, S.F., Adamson, P.C., Reaman, G.H. and Seibel, N.L. (2014) Declining Childhood and Adolescent Cancer Mortality. Cancer, 120, 2497-2506.
http://dx.doi.org/10.1002/cncr.28748
NOTES

*Corresponding author.