Journal of Cancer Therapy
Vol.06 No.02(2015), Article ID:53684,4 pages
10.4236/jct.2015.62015
Nimotuzumab in Treatment of Infiltrating Squamous Cell Carcinoma of Tongue
Alex A. Prasad
Chennai Cancer Care Hospital, Chennai, India
Email: dralexprasad@gmail.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 January 2015; accepted 29 January 2015; published 30 January 2015
ABSTRACT
A 50-year male patient presented to outpatient department with a mass on tongue associated with pain. A plain computerised tomography scan done on 17/01/13 revealed mild growth on lateral border of tongue with an enlarged jugulo-digastric lymph node measuring 1.8 × 1 cm. Excision of tongue was done and sent for histopathological examination which revealed infiltrating squamous cell carcinoma. The patient was treated with 2 cycles of Induction chemotherapy with cisplatin and paclitaxel administered in q3w schedule. Induction chemotherapy was followed by concur- rent chemoradiotherapy with 6 cycles of nimotuzumab 200 mg and cisplatin 20 mg given weekly along with 60 gy/30 fractions of radiation using IMRT. Latest CT scan done on 20/01/2014 re- vealed no evidence of disease (NED).
Keywords:
Nimotuzumab, Epidermal Growth Factor Receptor (EGFR), Transforming Growth Factor α (TGFα)

1. Background
Carcinoma of the tongue has proved itself to be one of the most difficult types of malignant disease with which to deal. The microscopic anatomy is rather uniform, but the clinical course varies extensively. On the whole, it may be said that growth is rapid and dissemination early. This is probably due to the rich vascular and lymphatic supply and to the constant movement of the organ. While it is relatively accessible, it is, nevertheless, much more fatal than many other more inaccessible types of cancer [1] . It terminates fatally in 75 to 90 percent of cases, according to Warren, Butlin and Meller [2] . In frequency, Jessett [2] places it second only to carcinoma of the cervix, while Jacobsen [3] gives it third place. India stands distinctly claiming that it is the “oral cancer capital of the world” and is the second most frequent tumour among the oral tumors [4] . The average duration of life in advanced untreated cases is less than 8 months. In the treatment of this disease surgery alone has proved inadequate and unsatisfactory for various reasons [5] .
A number of generalities predominate as philosophic tenets of tongue malignancy management. The general beliefs are that superficial lesions are treated with single-modality therapy (e.g., radiation or surgery) and that large lesions are treated with multiple modalities (e.g., combined surgery and radiation). Additionally, cervical nodes are treated with either surgery or radiation therapy, and survival increases if microscopically positive nodes are present [3] . The therapeutic decision must take into consideration the patient’s age, lifestyle, and willingness to participate in the therapeutic regimen. Many cases come to the surgeon in the inoperable stage, thereby leaving a large group in which surgery offers nothing; in fact, offers worse than nothing if an ill-advised operation is attempted. Concomitant chemotherapy has beneficial antiproliferative effects, possible improved locoregional control, and possible improved survival. The 5-year survival rates for patients in Stages III and IV are 30% - 50%, with lymph node metastasis decreasing the survival rate 15% - 30%. Treatment failure often occurs in the cervical lymph nodes, even for patients with an N0 neck. Targeted therapies are the next revolution in the management of head and neck cancers (HNSCC) [3] . Almost 90% of the tumours have over-expressed EGFR receptors and numerous studies have proven a poorer prognosis in such patients [6] . EGFR antagonists are the most researched targeted therapies in the management of HNSCC [6] [7] . Nimotuzumab is a humanised monoclonal antibody approved for advanced head and neck cancer management in India since 2006 [8] . The current case reports rapid resolution of CT findings of infiltrating squamous cell carcinoma of tongue with the help of induction chemotherapy and concurrent chemoradiotherapy with nimotuzumab [8] [9] .
2. Case Presentation
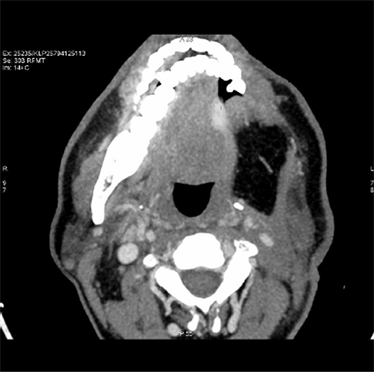
A 49-year male patient presented to outpatient department with a mass on the left lateral border of tongue associated with mild pain and enlarged lymph nodes on the left side of neck. He doesn’t have history of smoking or drinking. A plain computerised tomography (CT) scan done on 17/01/13 (Figure 1) revealed an irregular thickening of left lateral border of tongue with enlarged jugulo digastric lymph node measuring 1.8 × 1 cm. Bilateral level I and II nodes were positive. Patient was staged as T3, N2, M0. Excision of mass on the tongue was done and biopsy sent for histopathological examination which revealed infiltrating squamous cell carcinoma.
2.1. Investigations
Pre-treatment CT scans show irregular thickening of left lateral border of tongue with enlarged jugulodigastric lymphnode.
Histopathological examination of excision biopsy of left lateral mass over tongue revealed malignant infiltrating squamous cell carcinoma.
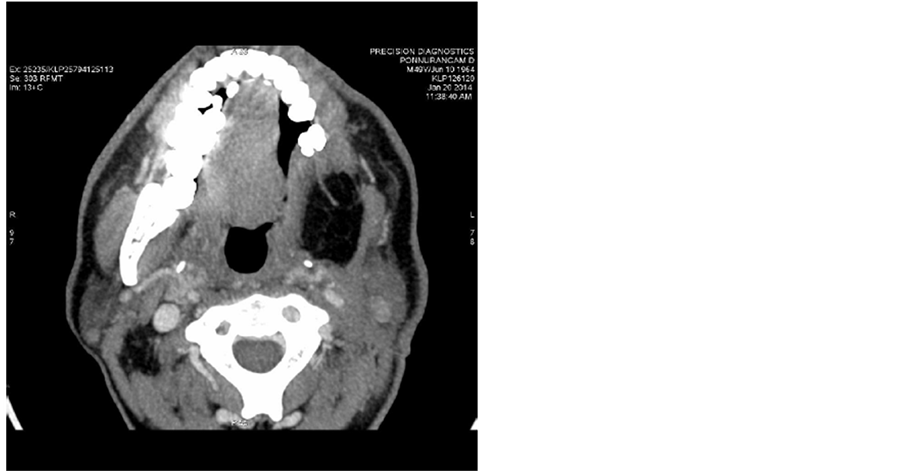
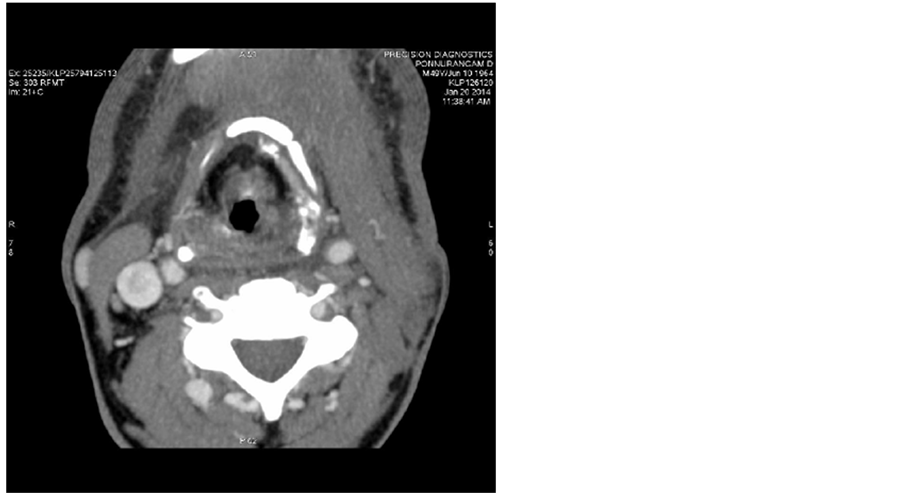
Post treatment CT scans done on 20/01/2014 shows no significant mass (Figure 2) and no lymphadenopathy (Figure 3).
Figure 1. Pre-treatment CT scan showing irregular thickening of left lateral border of tongue.
Figure 2. Post treatment CT scans done on 20/01/2014 shows no significant tongue mass.
Figure 3. Post treatment CT scans done on 20/01/2014 shows no lymphadenopathy.
2.2. Treatment
The patient was treated with 2 cycles of induction chemotherapy with cisplatin and paclitaxel administered in q3w schedule. Induction chemotherapy was followed by concurrent chemoradiotherapy with 6 cycles of BIOMAB 200 mg and cisplatin 20 mg given weekly along with 60 gy/30 fractions of radiation using IMRT.
2.3. Outcome and Follow-Up
At the end of 1 year no malignant mass was noted on post treatment CT scans with no lymphadenopathy.
3. Discussion
Oral squamous cell carcinoma is not a frequent event in young patients. Average age of cases registered in literature for young patients with SCC ranges between 30.8 - 34.2 years, with majority being males [1] . Site of greatest occurrence of OSCC in these age group patients is the tongue [1] . Patients belonging to younger age range groups are considered to bear more aggressive diseases when compared to population in the older age ranges. The factors to be investigated to explain OSCC occurrences in young patients include genetic predispo- sition, previous viral infections, deleterious habits, immune deficiency states, occupational exposure, previous exposure to carcinogens, socioeconomic conditions and oral hygiene [2] .
Our patient who was 49 years of age did not have any smoking or drinking habits. Past medical history did not reveal anything significant. The tumour was seen as an irregular thickening of left lateral border of tongue with enlarged jugulo digastric lymph node measuring 1.8 × 1 cm. Bilateral level I and II nodes were positive. Patient was staged as T3, N2, M0. Excision of mass on the tongue was done and biopsy sent for histopathological examination which revealed infiltrating squamous cell carcinoma [3] .
Primary therapy is largely dictated by the anatomical origin of the cancer and whether distant disease is present. Many patients with localized disease are treated with chemoradiotherapy, either in the definitive or adjuvant setting, and those with metastatic disease are treated with palliative radiotherapy. The chemotherapy used in SCCHN can be toxic, whether given with radiation or alone. The 5-year survival rates for patients in Stages III and IV are 30% - 50%, with lymph node metastasis decreasing the survival rate 15% - 30%. Treatment failure often occurs in the cervical lymph nodes, even for patients with an N0 neck [3] . To better evaluate prognosis and outcome, many studies have looked at the expression of various growth factors and tumor markers. In study- ing the over-expression of epidermal growth factor (EGFR) and cerb-B2, these 2 growth factors may be found useful in predicting outcome and survival [6] [7] .
The epidermal growth factor receptor (EGFR) is highly expressed in SCCHN and serves as a logical therapeutic target. EGFR-directed monoclonal antibodies (MOAbs) have higher activity in SCCHN than small molecule tyrosine kinase inhibitors. Novel EGFR MOAbs are being developed with the goal of improving efficacy and tolerability [6] [7] . (Biomab) Nimotuzumab is a humanized IgG1 MOAb-directed at EGFR that is currently approved for use outside of the United States and has demonstrated less skin toxicity than cetuximab or panitu- mumab. Preclinical data have shown nimotuzumab to bind EGFR with high affinity, inhibit cell proliferation, show antitumor activity in the mouse xenograft model, and enhance antitumor efficacy of radiation. Crombet et al. evaluated nimotuzumab in a phase I monotherapy trial whereby 12 patients with advanced epithelial cancers received therapy at four dose levels. No evidence of severe toxicity was seen including anaphylactic or skin reactions, and the MTD was not reached. They further evaluated nimotuzumab in combination with radiation therapy in 24 patients with unresectable SCCHN. Patients received six weekly doses of nimotuzumab, at four dose levels (50 mg, 100 mg, 200 mg, 400 mg), combined with RT. No cases of skin rash were noted. Preliminary efficacy was a primary endpoint of the trial, and it was noted that median OS was significantly increased in the 200 or 400 mg doses (44.3 months) versus the 50 and 100 mg doses (8.6 months, P = 0.03). Pharmacodynamic effect was monitored with pre- and post-treatment tumour biopsies and revealed antiproliferative and antiangiogenic marker modulation by immunohistochemistry correlated with antitumor response [8] [9] .
The current case reports rapid resolution of CT findings of infiltrating squamous cell carcinoma of tongue with the help of induction chemotherapy and concurrent chemoradiotherapy with humanised EGFR antagonsist, ni- motuzumab.
4. Conclusion
Targeted therapies are changing the treatment paradigm of advanced cancers of the tongue. Nimotuzumab which is a humanized monoclonal antibody against the EGFR receptor is a viable and an attractive therapeutic option in these advanced cases. The current case reports rapid resolution of CT findings of infiltrating squamous cell carcinoma of tongue and no evidence of disease was detected even after a year.
References
- Morestein, H. (1919) La Cure Radicale du Cancer de la Langue. Journal Chirurgie, 15, 221-252.
- Butlin, H.T. (1909) An Address on the Results of Operations for Carcinoma of the Tongue, with an Analysis of 197 Cases: Delivered before the International Surgical Society in Brussels. British Medical Journal, 1, 1.b2-1.b6.
- Douglas, Q., et al. (1921) Treatment of Carcinoma of the Tongue. Annals of Surgery, 73, 716-723. http://dx.doi.org/10.1097/00000658-192106000-00005
- Globocan (2015) Fact Sheets by Population―Globocan―IARC. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Iype, E.M., Pandey, M., Mathew, A., Thomas, G., Sebastian, P. and Nair, M.K. (2001) Squamous Cell Carcinoma of the Tongue among Young Indian Adults. Neoplasia, 3, 273-277. http://dx.doi.org/10.1038/sj.neo.7900172
- Ciardiello, F. and Tortora, G. (2008) EGFR Antagonists in Cancer Treatment. The New England Journal of Medicine, 358, 1160-1174. http://dx.doi.org/10.1056/NEJMra0707704
- Akashi, Y., Okamoto, I., Iwasa, T., et al. (2008) Enhancement of the Antitumor Activity of Ionising Radiation by Nimotuzumab, a Humanised Monoclonal Antibody to the Epidermal Growth Factor Receptor, in Non-Small Cell Lung Cancer Cell Lines of Differing Epidermal Growth Factor Receptor Status. British Journal of Cancer, 98, 749-755. http://dx.doi.org/10.1038/sj.bjc.6604222
- Crombet, T., Torres, L., Neninger, E., et al. (2003) Pharmacological Evaluation of Humanized Anti-Epidermal Growth Factor Receptor, Monoclonal Antibody h-R3, in Patients with Advanced Epithelial-Derived Cancer. Journal of Immunotherapy, 26, 139-148. http://dx.doi.org/10.1097/00002371-200303000-00006
- Crombet, T., Osorio, M., Cruz, T., et al. (2004) Use of the Humanized Anti-Epidermal Growth Factor Receptor Monoclonal Antibody h-R3 in Combination with Radiotherapy in the Treatment of Locally Advanced Head and Neck Cancer Patients. Journal of Clinical Oncology, 22, 1646-1654. http://dx.doi.org/10.1200/JCO.2004.03.089