Journal of Cancer Therapy
Vol.5 No.3(2014), Article ID:43910,7 pages DOI:10.4236/jct.2014.53032
Role of Combination Chemotherapy with 5-Fluorouracil, Cisplatin and Paclitaxel for Advanced Gall Bladder Cancer
Mumtaz Ahmad Ansari, Satyendra K. Tiwary, Uday Pratap Shahi, Vijay K. Shukla
Department of General Surgery and Radiotherapy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
Email: vkshuklabhu@satyam.net.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 17 January 2014; revised 15 February 2014; accepted 23 February 2014
ABSTRACT
Aim: The prognosis for patients with advanced Gallbladder carcinoma is poor. Due to unresectability and relatively ineffective chemotherapy available, a need exists for effective chemotherapeutic regimen. The aim of this study was to determine the efficacy and safety profile of 5-fluorouracil, cisplatin and paclitaxel in patients with advanced Gallbladder cancer. Material and Methods: From January 2002 to July 2004, 40 patients of advanced carcinoma Gallbladder received 5-fluorouracil, cisplatin and paclitaxel. On day 1, paclitaxel was given (150 mg/m2), cisplatin was given on day 2 (50 mg/m2) and 5-fluorouracil was given from day 1 to day 3 (500 mg/m2). This cycle was repeated every three weeks and patient assessment was done. Results: Forty patients were enrolled in this study. Thirty-five were assessed for response. Five patients were lost in follow up. There were thirty females and ten males. A median of three cycles of treatment (range one to seven) was administered. Two patients achieved complete response and eleven had partial responses giving an overall response rate of 32.5% in the intention-to-treat population (95% confidence interval 11.1% to 46.5%). The median response duration was 5.3 months. The median time to progression and overall survival was 4.1 months and 11.2 months, respectively. The most common grade 3 adverse effects were neutropenia (30%), nausea (20%), vomiting (15%), diarrhea (10%), stomatitis (5%), and peripheral neuropathy (5%). Only one case had febrile neutropenia. There was no treatment related death. Conclusions: The combination of 5-fluorouracil, cisplatin and paclitaxel has promising anti-tumor activity and is well tolerated in patients with advanced and metastatic Gallbladder cancer.
Keywords:Advanced Gallbladder Carcinoma; Combination Chemotherapy; Prognosis

1. Introduction
Gallbladder cancer is one of the most aggressive human malignancies. It is notorious for clinical detection in advanced stages with unresectability and refractoriness to chemotherapy. When patients come to hospital with pain abdomen, lump abdomen or jaundice is diagnosed of Gallbladder cancer, they have almost lost possibility of a curative resection. Only a small subset of patients of Gallbladder cancer has a curative surgical resection potential. Prognosis for metastatic and advanced disease is dismal with median survival usually <4 months in patients not treated with chemotherapy [1] [2] . Most primitive and most extensively studied chemotherapeutic agent in biliary malignancy to date has been 5-fluorouracil (5-FU), although response rate has been around only 10% - 13% [3] [4] . Similar results are seen for single agent like cisplatin (8%) in advanced cancer of Gallbladder and bile ducts [5] .
Due to poor response to single chemotherapeutic agent, different randomized trials of combination chemotherapy were done which showed improved survival. In phase II studies, 5-day continuous infusion 5-FU and cisplatin have demonstrated a response rate of 24% in patients with advanced biliary cancer [6] . Different trials of Gemcitabine alone or in combination with cisplatin have shown response rate of 10 to 40% [7] -[10] . In other phase II trials of 5-FU, epirubicin, cisplatin (ECF regimen), response rate is around 29% - 40% [11] -[14] . Multi-drug chemotherapy due to its better results motivated us to do a trial of 5-FU + Paclitaxel + Cisplatin for advanced Gallbladder cancer. Patients were evaluated for response rate in terms of time to progression, overall survival and safety of the combination regimen.
2. Material and Methods
All patients had cytologically confirmed advanced or metastatic carcinoma of gall bladder with at least one unidimensionally measurable lesion (diameter ≥ 2 cm as assessed by physical/X-ray/Ultrasound/CT scan examination). Other eligibility criteria were age 20 - 70 years and Eastern Cooperative Oncology Group (ECOG) performance status of 0 - 2 [3] . Patients had received no prior chemotherapy or radiotherapy. Patients with hematological (hemoglobin ≥ 8 gm/dl, total leukocyte count ≥4.0 × 109/L, absolute neutrophil count ≥2.0 × 109/L, platelet count ≥100 × 109/L), hepatic (total bilirubin ≤3 mg/dl, total protein ≥ 6 gm/dl, serum transaminases ≤3 × normal limit or ≤5 × normal limit in cases of hepatic metastases), and renal (serum creatinine ≤1.6 mg/dl) parameters were included. All patients were given proper and adequate information and written consent was taken before enrollment.
Chemotherapy Schedule
Forty patients were enrolled for this study. All patients received three drugs.
DAY 1: Paclitaxel 150 mg/m2 on day 1 dissolved in 300 ml normal saline and infused over 30 minutes.
DAY 2: Cisplatin 50 mg/m2 dissolved in 150 ml normal saline and infused over 30 minutes.
DAY 1 - 3: 5-Fluorouracil 500 mg/m2 dissolved in 540 ml dextrose normal saline and infused over 4 hours.
Cycle was repeated every 3 weeks. Treatment was continued until development of unacceptable toxicity, progression of disease or patient deciding not to continue the treatment.
Complete blood count and biochemical tests to assess renal and hepatic functions were done before and on day 15 of each cycle of chemotherapy. Tumor size was assessed after completion of each cycle and it was carried on till patient developed progressive disease. Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumor Guidelines [15] . Patients with complete response or partial response required a confirmatory disease assessment at least 4 weeks later.
The trial was conducted according to the two-stage Gehan design [16] with overall response as the primary end point. Assuming a true response rate of at least 15%, initially 19 patients were accrued. Because the probability of obtaining no response in 19 patients was <0.05, the trial was to be abandoned if no response were observed. Study was continued if at least one treatment response was observed and six additional patients were added according to the rules, to obtain an accuracy of 0.10 for the final response rate. According to this design, the probability of completing the trial was >95% if the true response rate was at least 15%. However accrual was continued to a total of 40 patients so that the proportion of patients responding could be better defined.
Duration of response, time taken to progression and survival were calculated as secondary end points by the Kaplan-Meier Method. The duration of response was defined as the interval from the onset of complete response or partial response until evidence of progressive disease was found. Time taken to progression (TTP) was estimated from date of entry to the date of progression of disease. Overall survival was measured from the date of entry to the date of death.
3. Results
A total of 40 patients were registered between January 2002 and July 2004. The median age was 48 years (range 24 to 67 years) (Table 1). Most of the patients (75%) had good performance status (0 or 1 on ECOG scale). Five patients had undergone surgery for biliary decompression and four received endoscopic stents for palliation of obstructive jaundice. Hepatic metastases were present in 40% patients at the time of enrollment for chemotherapy. Abdominal lymph nodes were enlarged in 25% patients. Other metastatic sites were lungs, supraclavicular lymph nodes, and bones.
A total of thirty-five patients were evaluated for response. The remaining five patients were not assessable for response because of loss to follow-up. The overall response rate in intention-to-treat population was 32.5%, including two confirmed complete responses and eleven confirmed partial responses. When eleven patients with stable disease were included, tumor growth control was achieved in 60% of patients (Table 2). The median duration of response in thirteen responding patients was 5.3 months.
Table 1. Patients profile.
Table 2. Anti-tumor activity (Intention-to-treat analysis).
The median time taken to progression for all patients was 4.1 months (95% confidence interval {CI} 2.7 - 5.6) and the median survival was 11.2 months (95% CI 7.2 - 14.1).
A total of 142 treatment cycles (median three, range one to seven) were administered, of which 135 cycles were assessable for safety. The remaining seven cycles in five patients were not assessable for safety. The frequencies of hematological and non-hematological adverse events are shown in Tables 3 and 4 respectively. The most common grade 3 hematological adverse events were neutropenia, which occurred in 30% of the patients. One patient experienced febrile neutropenia. The most common grade 3 non-hematological adverse event was nausea (20%) followed by vomiting (15%), diarrhea (10%), stomatitis (5%), peripheral neuropathy (5%). Grade 3 hand-foot syndromes occurred only in 5% of the patients. There were no treatment related deaths during the study.
Treatment was delayed in 46 cycles and dose was reduced in 8 cycles. Treatment doses were modified in patients suffering from for hematological toxicity, nausea, vomiting, diarrhea, stomatitis, peripheral neuropathy, and hand-foot syndrome.
4. Discussion
Many chemotherapy trials have been focused on biliary tract cancer with mixed results [1] -[6] [8] [10] [11] [13] [16] -[20] . Gall bladder cancer behaves differently from the rest of the biliary tract due to its different etiology, biological behavior and response to modality of treatment. Isolated chemotherapy trials for carcinoma Gallbladder have been difficult due to less number of patients in different geographical regions [7] [9] [12] [14] . However Gangetic belt is a fertile ground for carcinoma Gallbladder [21] .
Response rate and overall survival with ECF regimen have been reported to be 29% - 40% and 6.4 - 11 months [11] -[14] . Gemcitabine either alone or in combination has shown improved survival rate with median overall survival of 11.5 months [7] -[10] [17] [18] . A phase II study combining fractionated cisplatin and de Gramont (5-FU and Leucovorin) regimen suggested improvement compared with classical 5-FU and cisplatin combination [11] -[13] , and in terms of safety and efficacy [19] . A prospective phase III study using numerous chemotherapy agents has been reported with better results. Large randomized prospective studies on the
Table 3. Hematological adverse effects.
Table 4. Non-hematological adverse effects.
use of adjuvant therapy are lacking, and any recommendations are based on small studies and meta-analysis [22] .
The results of the present study suggest that combination of 5-fluorouracil, cisplatin and paclitaxel is active and well tolerated as first-line chemotherapy for advanced gall bladder cancer. The overall response rate (32.5%), median time taken to progression (4.1 months) (Figure 1) and median survival (11.2 months) (Figure 2) following treatment with 5-fluorouracil, cisplatin and paclitaxel are comparable with results reported previously. Toxicity profile of 5-fluorouracil, cisplatin and paclitaxel is acceptable with the only major grade adverse effect being neutropenia.
5. Conclusion
Carcinoma Gallbladder is one of the most aggressive malignancies and complete resection is the only hope for cure. Most patients come at a very advanced stage with surgically unresectable tumor. Adjuvant therapies with

Figure 1. Kaplan Meier Curve for time to progression.
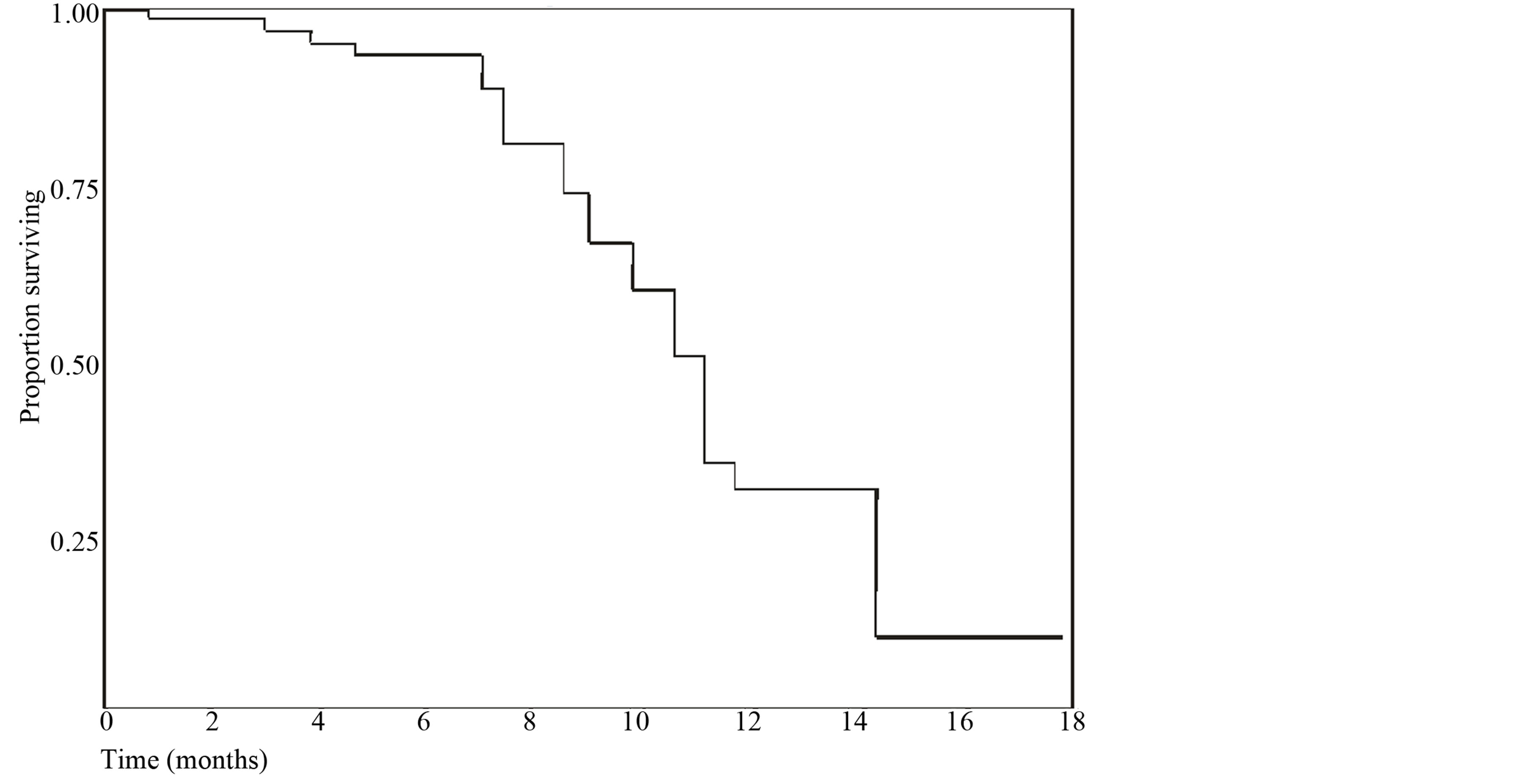
Figure 2. Kaplan Meier Curve for overall survival.
5-fluorouracil, pacliatxel and cisplatin are well tolerated. Large prospective randomized trials are required to compare its efficacy and low toxicity.
References
- Glimelius, B., Hoffman, K., Sjoden, P.O., Jacobsson, G., Sellstrom, H., Enander, L.K., Linne, T. and Svensson, C. (1996) Chemotherapy Improves Survival and Quality of Life in Advanced Pancreatic and Biliary Cancer. Annals of Oncology, 7, 593-600. http://dx.doi.org/10.1093/oxfordjournals.annonc.a010676
- Takada, T., Nimura, Y., Katoh, H., Nagakawa, T., Nakayama, T., Matsushiro, T., Amano, H. and Wada, K. (1998) Prospective Randomized Trial of 5-Flurouracil, Doxorubicin and Mitomycin C for Non-Rescctable Pancreatic and Biliary Carcinoma: Multicenter Randomized Trial. Hepatogstroenterology, 45, 2020-2026.
- Falkson, G., Mac Intyre, J.M. and Moertel, C.G. (1984) Eastern Cooperative Oncology Group Experience with Chemotherapy for Inoperable Gallbladder and Bile Duct Cancer. Cancer, 54, 985-969. http://dx.doi.org/10.1002/1097-0142(19840915)54:6<965::AID-CNCR2820540603>3.0.CO;2-X
- Davis Jr., H.L., Ramirez, G. and Ansfield, F.J. (1974) Adenocarcinomas of Stomach, Pancreas, Liver, and Biliary Tracts. Survival of 328 Patients Treated with Fluoropyrimidine Therapy. Cancer, 33, 193-197. http://dx.doi.org/10.1002/1097-0142(197401)33:1<193::AID-CNCR2820330128>3.0.CO;2-S
- Okada, S., Ishii, H., Nose, H., Okusaka, T., Kyogoku, A., Aoki, K., Iwasaki, M., Furuse, J. and Yoshino, M. (1994) A Phase II Study of Cisplatin in Patients with Biliary Tract Carcinoma. Oncology, 51, 515-517. http://dx.doi.org/10.1159/000227396
- Ducreux, M., Rougier, P., Fandi, A., Yoshimori, T., Wakabayashi, K., Clavero-Fabri, M.C., Villing, A.L., Fassone, F., Fandi, L., Zarba, J. and Armand, J.P. (1998) Effective Treatment of Advanced Biliary Tract Carcinoma Using 5-Fluorouracil Continuous Infusion with Cisplatin. Annals of Oncology, 9, 653-656. http://dx.doi.org/10.1023/A:1008241008379
- Doval, D.C., Shekhon, J.S., Gupta, S.K., Fuloria, J., Shukla, V.K., Gupta, S. and Awasthy, B.K. (2004) A Phase II Study of Gemcitabine and Cisplatin in Chemotherapy—Naive Advanced Unresectable Gall Bladder Cancer. British Journal of Cancer, 90, 1516-1520. http://dx.doi.org/10.1038/sj.bjc.6601736
- Thongprasert, S., Napapan, S., Charoentum, C. and Moonprakan, S. (2005) Phase II Study of Gemcitabine and Cisplatin as First Line Chemotheraphy in Inoperable Biliary Tract Carcinoma. Annals of Oncology, 16, 279-281. http://dx.doi.org/10.1093/annonc/mdi046
- Malik, I.A., Aziz, Z. and Zaidi, Sethumaran, G. (2003) Gemcitabine and Cisplatin Is a Highly Effective Combination Therapy in Patients with Advanced Cancer Gall Bladder. American Journal of Clinical Oncology, 26, 174-177. http://dx.doi.org/10.1097/00000421-200304000-00015
- Eng, C., Ramnathan, R.K., Wong, M.K., Ramick, S.C., Dai, L., Wade-Oliver, K.T., Mani, S. and Kindler, H.L. (2004) A Phase II Trial of Fixed Dose Rate Gemictabine in Patients with Advanced Biliary Carcinoma. American Journal of Clinical Oncology, 27, 565-569. http://dx.doi.org/10.1097/01.coc.0000135924.94955.16
- Di lauro, L., Carpano, S., Capomolla, E., et al. (1997) Cisplatin, Epirubicin and 5-Fluorouracil (PEF) for Advanced Biliary Tract Carcianoma. American Society of Clinical Oncology, 16, 287 a.
- Okada, S., Okusaka, T., Ishii, H., et al. (1997) Phase II Trial of Cisplatin, Epirubicinss and Continuous Infusion 5-Fluorouracil (5-FU) (CEF Fherapy) for Advanced Gall Bladder Cancer (GBC). American Society of Clinical Oncology, 16, 301 a.
- Ellis, P.A., Norman, A., Hill, A., O’Brien, M.E., Nicolson, M., Hickish, T. and Cunningham, D. (1995) Epirubicin, Cisplatin and Infusional 5-Fluorouracil (5-FU) (ECF) in Hepatobiliary Tumours. European Journal of Cancer, 31A, 1594-1598.
- Ishii, H., Furuse, J., Yonemoto, N., Nagase, M., Yoshino, M. and Sato, T. (2004) Chemotherapy in Treatment of Advanced Gallbladder Cancer. Oncology, 66, 138-142. http://dx.doi.org/10.1159/000077440
- Therasse, P., Arbuck, S.G., Eisenhauer, E.A., Wanders, J., Kaplan, R.S., Rubinstein, L., Verweij, J., Van Glabakke, M., Van Oosterom, A.T., Christian, M.C. and Gyther, S.G. (2000) New Guidelines to Evaluate the Response to Treatment in Solid Tumours. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute, 92, 205-221. http://dx.doi.org/10.1093/jnci/92.3.205
- Gehan, E.A. (1961) The Determination of the Number of Patients Required in a Preliminary and Follow-Up Trial of a New Chemotherapeutic Agent. Journal of Chronic Diseases, 13, 346-353. http://dx.doi.org/10.1016/0021-9681(61)90060-1
- Penz, M., Kornek, G.V., Raderer, M., Ulrich-Pur, H., Fiebiger, W., Lenauer, A., Depish, D., Krauss, G., Schneweiss, B. and Scheithauer, W. (2001) Phase II Trial of Two Weekly Gemcitabine in Patients with Advanced Biliary Tract Cancer. Annals of Oncology, 12, 183-186. http://dx.doi.org/10.1023/A:1008352123009
- Lin, M.H., Chen, J.S., Chen, H.H. and Su, W.C. (2003) A Phase II Trial of Gemictabine in Treatment of Advanced Bile Duct and Periampullary Carcinomas. Chemotherapy, 49, 154-158. http://dx.doi.org/10.1159/000070622
- Taieb, J., Mitry, E., Boige, V., Artru, P., Ezenfis, J., Lecomte, T., Claverno-Fabri, M.C., Vaillant, J.N., Rougier, P. and Ducreux, M. (2002) Optimization of 5-Flurouraicl (5-FU)/Cisplatin Combination Chemotherapy with a New Schedule of Leucovorin, 5-FU and Cisplatin (LV5FU 2-P Regimen) in Patients with Biliary Tract Carcinoma. Annals of Oncology, 13, 1192-1196. http://dx.doi.org/10.1093/annonc/mdf201
- Patt, Y.Z., Hassan, M.M., Lozano, R.D., Waugh, K.A., Hoque, A.M., Frome, A.I., Lahoti, S., Ellis, L., Vauthey, J.N., Curley, S.A., Schnirer, I.I. and Raijman, I. (2001) Phase II Trial of Cisplatin, Interferon Alpha-2 b, Doxorubicin and 5- Fluorouracil for Biliary Tract Cancer. Clinical Cancer Research, 7, 3375-3380.
- Gupta, S.K., Ansari, M.A. and Shukla, V.K. (2005) What Makes the Gangetic Belt a Fertile Ground for Gallbladder Cancers? Journal of Surgical Oncology (In press), http://dx.doi.org/10.1002/jso.20292
- Parikh, P.M., Agarwalla, D.K., Malhotra, H., Govindbabu, K., Ranade, A.A., Vaid, A.K., Basade, M. and Bhattacharyya, G.S. (2005) Gallbladder and Biliary Tract Carcinoma—A Comprehensive Medical Management Update. Indian Co-Operative Oncology Network.


