Journal of Cancer Therapy
Vol. 3 No. 6A (2012) , Article ID: 24910 , 8 pages DOI:10.4236/jct.2012.326113
ERβ as a Prognostic Factor for Colorectal Cancer Liver Metastasis*
![]()
Department of Colorectal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Email: #zs0771@hotmail.com
Received September 19th, 2012; revised October 19th, 2012; accepted October 29th, 2012
Keywords: Estrogen Receptor β; Survival; Colorectal Cancer; Liver Metastasis
ABSTRACT
Estrogen plays an important role in the development of some cancers. However, previous studies on the influence of estrogen on colorectal cancer (CRC) have had conflicting conclusions, and there have been few reports on estrogens and liver metastasis. The aim of this study was to explore the prognostic impact of estrogens on CRC with liver metastasis. Eighty-six patients with CRC including 43 synchronous liver metastases were studied. Estrogen receptor β (ERβ) levels were assayed by immunohistochemistry in liver metastasis, CRC and adjacent normal tissues. Serum estrogen levels were measured by radioimmunoassay. The correlation between staining, clinicopathological parameters, and prognostic power were analyzed statistically. Significant differences were found in ERβ expression between liver metastasis (P = 0.012) and CRC (P = 0.002) compared to adjacent normal tissues. Serum estrogen levels in patients with liver metastases were significantly lower than those without liver metastasis (P = 0.012). The 1-, 2-, 3- and 5-year survival rates were 80%, 40%, 33% and 22%, respectively for the 43 patients with liver metastasis; and 94%, 63%, 49% and 49%, respectively for patients with positive ERβ in liver metastasis tissues, and 68%, 21%, 21% and 10%, respectively for those with negative ERβ (log-rank; P = 0.018). Cox regression test showed that ER beta (P = 0.029) were detected as the independent prognostic factors for liver metastasis of colorectal cancer. In conclusion, the present study suggests that ERβ may be a prognostic factor for synchronous liver metastasis of colorectal cancer.
1. Introduction
Worldwide, colorectal cancer (CRC) is the third most commonly diagnosed cancer in males, and the second in females, with over 1.2 million new cancer cases and 608,700 deaths estimated to have occurred in 2008 [1] . Although the survival rates in individuals with CRC have increased substantially in the past decade, possibly as a result of early diagnosis and improved treatment [2] , the pathogenesis of this cancer remains poorly understood, and the overall survival is still not satisfactory. The main reasons for poor survival are local recurrence and distant metastases. While the liver is the most common site of metastasis, only about 35% to 55% of patients with CRC develop liver metastases [3] . Most of these patients do not survive for more than 12 months with a median survival of about 8 months without treatment [4] . Surgical resection is the main management approach to cure colorectal liver metastases at present. However, only 10% - 25% of patients are amenable to surgery [5] .
Gender differences in the incidence and prognosis of CRC have been investigated. For example, Nelson et al. studied 38,931 cases of CRC and found that regarding the development of CRC anywhere in the colorectum, male incidence rates adjusted for age and race were greater than rates for females [6] . Koo et al. analyzed 2050 patients (44% women) with CRC and showed that in a young group (aged 50 years and below) women had significantly better overall survival than men (log-rank; P = 0.04) [7] . Moreover, observational epidemiological studies and controlled clinical trials had showed that women who used hormone replacement therapy (HRT) after menopause were at lower risk of developing both colon and rectal cancer [8-10] . Our previous study analyzing 1312 patients with CRC found that compared to females, males were more apt to develop liver metastases from CRC (the ratio 1.9:1, P = 0.006) [11]. All these studies indicate that hormones may be involved in CRC and liver metastasis.
Estrogen receptor (ER) consists of are two forms, α and β, which are part of the nuclear hormone receptor family. They have both been identified in CRC and normal mucosa tissues, but ERβ is the predominant ER. In 1996, Kuiper et al. [12] first found a second estrogen receptor, ERβ, encoded by a gene on chromosome 14q. Since then, at least 5 splice variant isoforms of the ERβ gene product (ERβ1 to ERβ5) have been describedm [13]. ERβ expression was first characterized in prostatic and ovarian tissues ADDIN EN.CITE ADDIN EN.CITE.DATA [12] . Estrogen diffuses freely from serum through the lipid bilayer to the intracellular milieu where estrogen receptors are maintained in a state of transcriptional silence within the cell through interaction with co-repressor molecules [14]. Binding of estrogens causes a conformational change allowing disassociation from co-repressors and recruitment of coactivator molecules [15]. Further investigation of tissue expression in colonic mucosal epithelium found the over-expression of Erβ [16-18] . Although ERβ has been widely investigated in CRCs, little data are available on ERβ expression in liver metastasis from CRC. Moreover, there are no reports are found on the influence of ER on survival in liver metastasis from CRC.
Therefore, the aim of the current study was to evaluate ERβ levels in adjacent normal, CRC and liver metastasis tissues and explored their possible correlations with clinicopathologic features, and prognostic impact on CRC with liver metastasis.
2. Materials and Methods
2.1. Patients
Exclusion criteria: Taking hormone drugs within one year, lesions too small for adequate pathological examination and experimental studies, preoperative radiotherapy and chemotherapy.
A total of 86 patients with CRC including 43 synchronous liver metastases cases in the First Affiliated Hospital of Guangxi Medical University from January 2005 to December 2006 were enrolled. In the non-liver metastasis group, all patients underwent radical resection and those patients with stage II and III received adjuvant chemotherapy and/or radiotherapy after surgery. In the liver metastasis group, 28 patients underwent curativeintent liver resection and there were 34 cases receiving adjuvant chemotherapy. All patients were confirmed by histological diagnosis. Pathologic staging was determined according to the International Union Against Cancer TNM classification of malignant tumors [19]. All persons gave written informed consent prior to their inclusion in the study. The study was approved by the medical ethics committee of the First Affiliated Hospital of Guangxi Medical University.
2.2. IHC of ERβ
Formalin-fixed (10%) and paraffin-embedded tissue sections from the CRC, adjacent normal and liver metastasis tissues were stained with hematoxylin and eosin using standard histological techniques. IHC staining was performed using a streptavidin-peroxidase technique (ReadyTo-Use SP kit, Fuzhou Maixin Biotechnology Company, China), following the manufacturer’s instructions. Antibody dilutions for IHC were 1:50 for ERβ (monoclonal mouse, Setotec Company, UK). Phosphate-buffered saline (PBS) instead of antibody was used as a negative control. A section of breast carcinoma with ERβ expression was used as a positive control. The staining was semi-quantitatively examined by two independent pathologists who were blinded to the clinical data. The degree of staining was scored for intensity of staining (A) (0 = negative, 1 = weak, 2 = moderate, and 3 = strong) and proportion of positive cells (B) (0 = <5%, 1 = 5% - 25%, 2 = 26% - 50%, 3 = 51% - 75%, 4 = >75%) [20,21] . The score for each section was measured as A × B, and the result was defined as negative (–, 0), weakly positive (+, 1 - 4), positive (++, 5 - 8), and strongly positive (+++, 9 - 12). Representative images of nuclear ERβ in adjacent normal, carcinoma and liver metastasis tissues are depicted in Figure 1.
2.3. Radioimmunoassay for Serum Estrogens
All the serum samples were collected following an overnight fast of at least 8 hours, and were immediately centrifuged and stored at –80˚C. Serum estrogens were measured using a radioimmunoassay (RIA) technique (Estradiol Radioimmunoassay Kit, Beijing North Biotechnology Institute. A GC-911 type γ RIA counter (Zonkia Co., China) was used following the manufacturer’s instructions.
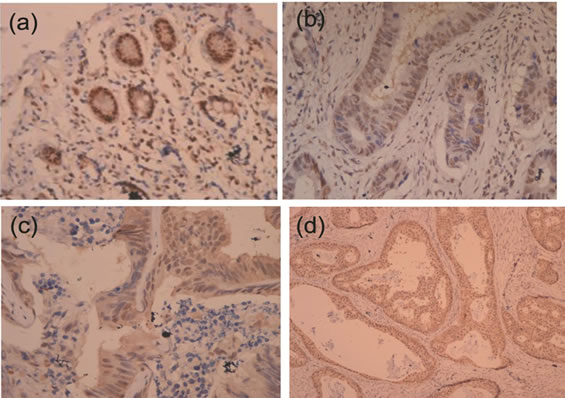
Figure 1. Immunohistochemical (IHC)1 analyses: IHC analyses of colorectal cancer, adjacent normal and liver metastasis tissues were carried out using mouse anti-human ERβ monoclonal antibody. (a) ERβ positive staining in normal intestinal epithelial; (b) ERβ positive staining in colon adenocarcinoma cells; (c) ERβ positive staining in adenocarcinoma cells of liver metastasis; (d) ERβ positive staining in adenocarcinoma cells of liver metastasis (All panels taken at 400×).
2.4. Follow up
The patients were followed every 3 months for the first 2 years and then every 6 months for the next 3 years and then annually. Follow-up visits included physical examination, rectodigital examination, carcinoembryonic antigen (CEA) level, chest X-ray, abdominal sonogram, and/or abdominal CT scanning. Patients were followed up in the outpatient department or by telephone. The last contact was in May 2012. Survival time was calculated from the date of surgery to the date of the last follow up or death. Patients who died from diseases other than CRC or from unexpected events were excluded from the study. There were 51 men and 35 women; and their ages ranged from 23 to 83 years (means: 56.9 ± 13.3 years, median: 58 years). According to the TNM classification, there were 11 stage I patients, 10 stage II patients, 22 stage III patients and 43 stage IV patients. The following clinicopathological features were recorded: gender, age, primary tumor site, histological type, tumor differentiation, lymph node metastasis, depth of invasion, TNM stage and liver metastasis.
2.5. Statistical Analysis
The Chi-square test or Fisher’s exact probability test was used to calculate the rates and examine the association between ERβ expression and various clinicopathological characteristics when appropriate. Comparisons between related groups in serum estrogen levels were performed using a two-sample Student’s t-test. Life table analyses were carried out and the Kaplan-Meier method was used to estimate survival. Univariate analysis of factors thought to influence survival was then made using the Log rank test. Multivariate analysis of the significant univariate factors was conducted using Cox regression analysis. P values of less than 0.05 were considered to indicate statistically significant differences. All statistical analyses were carried out with the SPSS statistical software version 13 (Chicago, Illinois, USA).
3. Results
3.1. ERβ in Tissues and the Relationship with Clinicopathological Characteristic
The positivity rates of ERβ expression in CRC tissues were 39.5% (34/86), equal to those of liver metastasis tissues, 39.5% (17/43). Both groups had less ERβ expression than in normal mucosa 62.8% (54/86), and the differences were statistically significant (P = 0.002, P = 0.012, respectively) (Table 1). The correlation between ERβ expression in CRC tissues and the clinicopathologic findings are listed in Table 2. Correlations were found between ERβ expression and preoperative CEA, TNM stage and liver metastasis (P = 0.034, 0.003 and 0.009, respectively). Serum estrogens levels were lower in CRC patients with synchronous liver metastases compared to CRC patients without liver metastases (103.44 ± 53.96 pg/mL vs. 164.23 ± 68.49 pg/mL, P = 0.012). The gender differences in the two groups were not statistically significant (P = 0.431) (Table 3).
3.2. ERβ Expression and Survival
The median survival time for the entire population was 52 months (range: 5 - 73 months). The 1-, 2-, 3- and 5-year survival rates were 80%, 40%, 33% and 22%, respectively for the 43 patients with liver metastasis, and the median survival time was 22.0 months. The factors affecting survival which were found to be significant on univariate analysis included number of liver metastasis (P = 0.033), diameter of liver metastasis (P = 0.045), chemotherapy (P = 0.005) (Figure 2), liver metastasis resection (P = 0.001) (Figure 3) and ER beta expression (P = 0.018) (Figure 4). The 1-, 2-, 3- and 5-year survival rates were 94%, 63%, 49% and 49%, respectively for patients with positive ERβ (n = 17 cases) in liver metastasis tissues, and 68%, 21%, 21% and 10%, respectively for those with negative ERβ (n = 26 cases). ERβ positive patients had a significantly higher survival rate compared with negative patients (log-rank; P = 0.018).
In the Cox regression test, chemotherapy (P = 0.037), liver metastasis resection (P = 0.006) and ER beta (P = 0.029) were detected as the independent prognostic factors (Table 4).
4. Discussion
An increasing number of epidemiological studies and controlled clinical trials have suggested a possible protective role of sex hormones against CRC development [8,22-24] . In a large randomized trial (16,608 postmenopausal women), the use of estrogen plus progestin was associated with a statistically significant decrease in
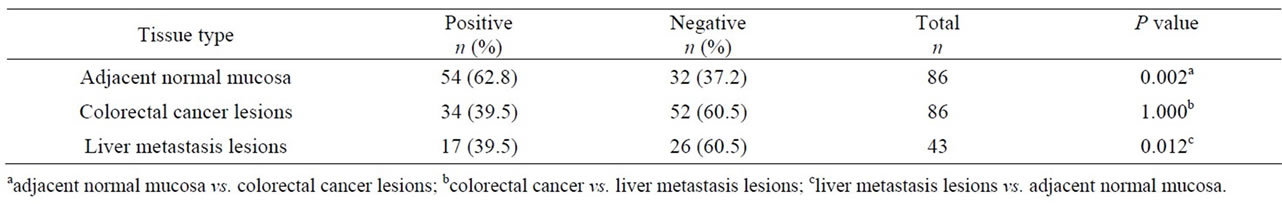
Table 1. The expression of ERβ in colorectal cancer lesions, adjacent normal mucosa and liver metastasis lesions.
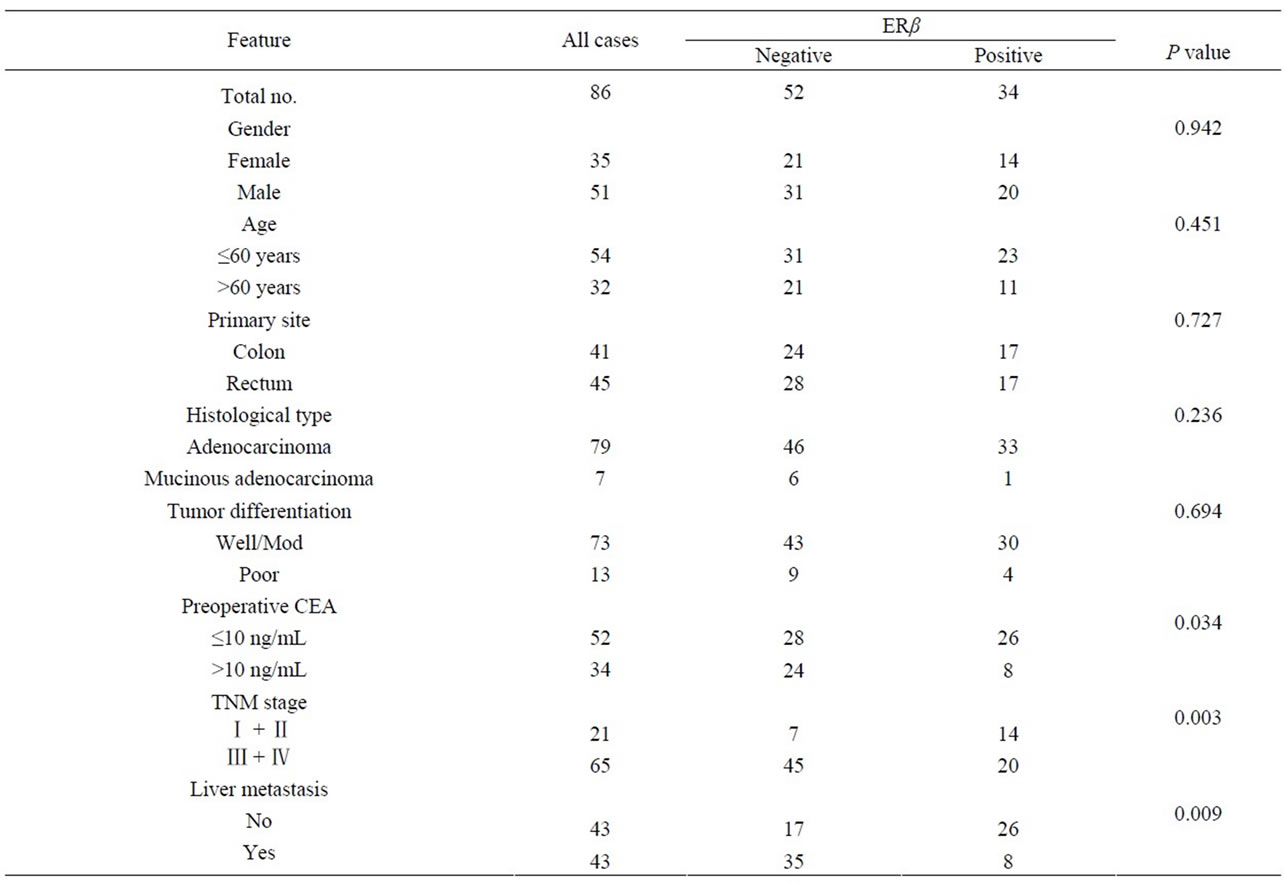
Table 2. Relationship of ERβ expression in primary CRC tissues to clinicopathological characteristics.
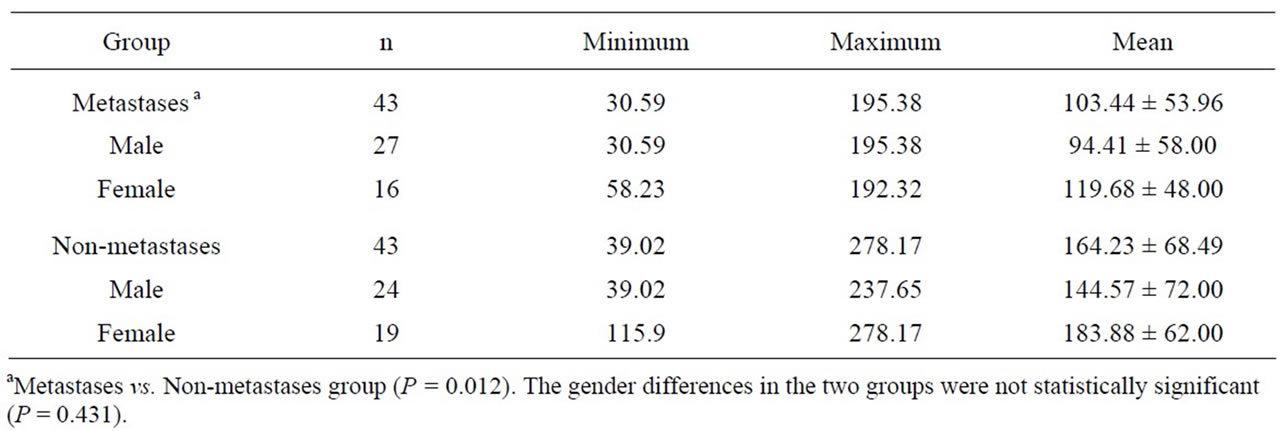
Table 3. Levels of serum estrogens in 86 study patients (pg/mL).
the incidence of CRC among post-menopausal women [25] . Another study showed that the use of oral hormone replacement therapy (HRT) was associated with a 63% relative reduction in the risk of CRC in post-menopausal women after adjustment for other known risk factors [10] . This beneficial effect seems to be specifically mediated by ERβ.
ER α and β have both been identified in CRC and normal mucosa tissues, but ERβ is the predominant ER [26-28] . In 1996, Kuiper et al. first found a second estrogen receptor, ERβ ADDIN EN.CITE ADDIN EN.CITE.DATA [12] . Then further investigations demonstrated that ERβ was expressed in CRC [16-18,27] . More recent studies also found that ERβ was detected in CRC by immunohistochemistry (IHC) [29-31] . In the current study, ERβ expression was found to be significantly lower in CRC tissues compared with normal tissues (P = 0.002). This finding is consistent with data from a previous study made by Foley et al., which reported that colon tumor tissue showed a selective loss of ERβ protein expression when compared to normal colon tissue in the same patient [28]. A reduction of ERβ expression occurred in colon cancer compared with normal colonic epithelium and lower expression was significantly correlated with the degree of tumor differentiation
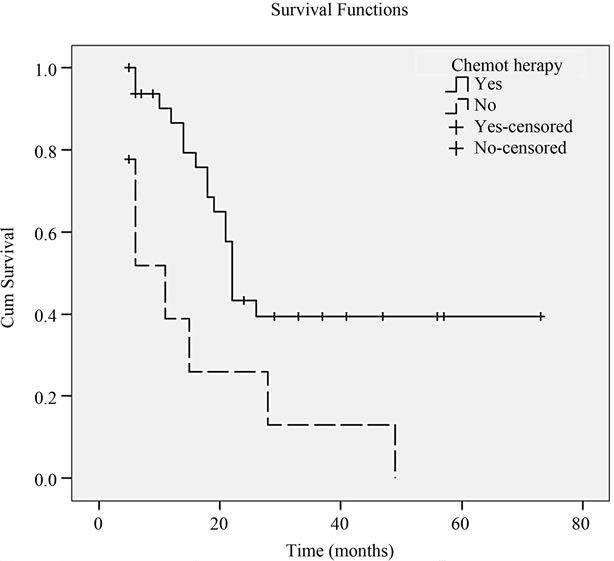
Figure 2. Cumulative survival rates of 43 CRC with liver metastasis patients compared by chemotherapy (P = 0.005).
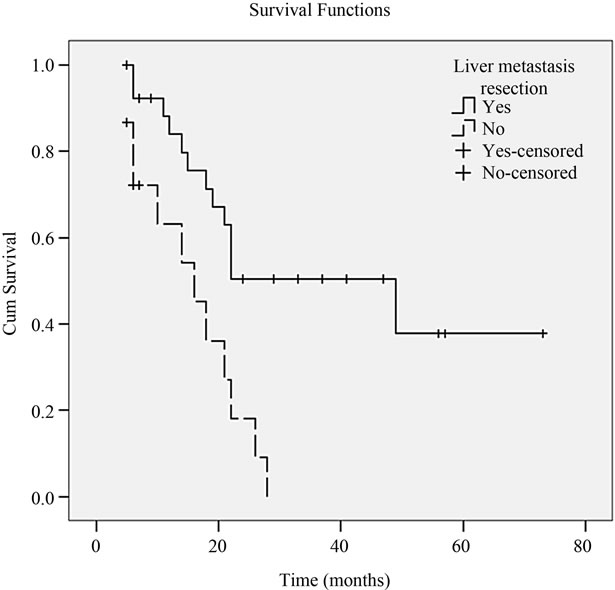
Figure 3. Cumulative survival rates of 43 CRC with liver metastasis patients compared by liver metastasis resection (P = 0.001).
[17] . ERβ mRNA was detected in both normal colon mucosa and tumors by RT-PCR [18,27] . Campbell-Thompson et al. showed that decreased levels of ERβ1 and ERβ2 mRNA were associated with colonic tumorigenesis in females [27] . These findings suggest a protective role for ERβ against colorectal carcinogenesis.
In current study correlations were found between ERβ expression and preoperative CEA, TNM stage and liver metastasis (P = 0.034, 0.003 and 0.009, respectively).The reduction in number of ERβ receptors in colorectal cancer might relate to worsening stage and grade of the tu-
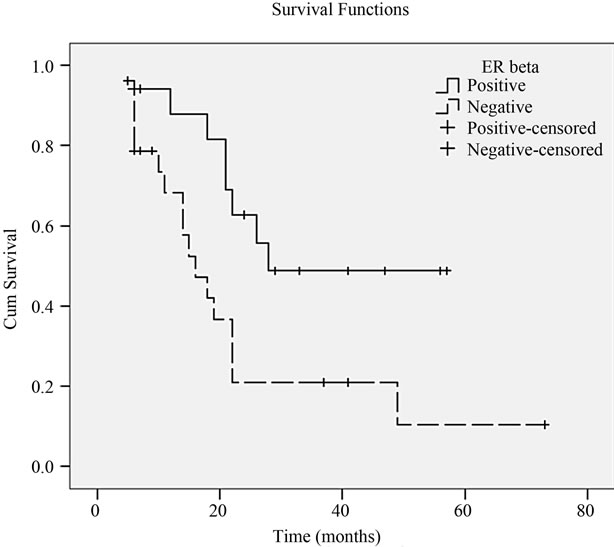
Figure 4. Kaplan-Meier survival curve correlating survival with positive or negative expression of ERβ in liver metastasis tissues (P = 0.018).
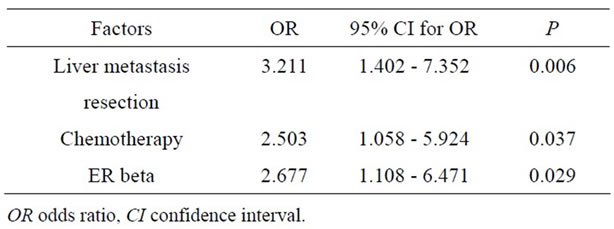
Table 4. Significant factors affecting survival by Cox Regression analysis.
mour [17,32] .
The important finding in the current study was that there was positive expression of ERβ in the liver metastasis tissues (17/43). However, compared to adjacent normal tissues, the ERβ positivity rate was lower in liver metastasis tissues (P = 0.012). We searched the literature and found few studies on the association between CRC with liver metastasis and ERβ protein. However, one study found that there was no ERα positivity expression in 14 CRC with liver metastasis as determined by IHC [33] . Therefore, the current results suggest that ERβ is expressed in primarily liver metastasis.
Many studies have reported that the ERβ status in primary tumors was a significant prognostic predictor in hepatocellular carcinoma [34] , breast cancer [35,36] and gastric cancer [37] . But there have been few studies on the association between liver metastasis and ERβ gene expression. In the liver metastasis group of the current study, Kaplan-Meier analysis showed that negative ERβ expression of metastatic liver tissues was related to decreased survival (P = 0.018). Chemotherapy, liver metastasis resection was an independent prognostic factor on multivariate analyses as already known [4,38-41] . Both univariate and multivariate analyses showed that ER beta was an important prognostic factor in patients with synchronous liver metastases. To our knowledge, this is the first study to investigate the prognostic impact of ERβ levels on CRC with liver metastasis. In a previous study, we analyzed 1312 patients with CRC and found that compared to females, males were more apt to develop liver metastasis [11].
In addition, from the current data we also found that serum estrogens levels in patients with liver metastases was significantly lower than those without liver metastasis (P = 0.012), which suggested that endogenous estrogen levels may be closely related with liver metastasis. Estrogen action is believed to be mediated through ERβ, and high levels of estrogen may be able to induce an increase in ERβ protein levels [42,43] . After 17 beta-estradiol (E2)-induced ERβ up-regulation in DLD-1 colon cancer cells, ERβ mRNA was found to be increased in the short term (2 and 4 h after stimulation) and was followed by a late (24 h after stimulation) enhancement of transcription [44]. This suggests that estrogens exert a protective action by ERβ against CRC with liver metastasis mediated by ERβ. Therefore, low or negative estrogen levels in CRC patients may affect the incidence of liver metastasis.
In conclusion, the present study suggests that CRC liver metastasis patients who have ERβ positive metastatic tumors have a better survival, and that ERβ may be a prognostic factor for synchronous liver metastasis of colorectal cancer.
5. Acknowledgements
This study was supported by grants from Key Scientific Research Project of Guangxi Medical Treatment and Public Health (No: 200404) and Guangxi Natural Science Foundation (No: 0728119) and Innovation Project of Guangxi Graduate Education (No. 2011105981002M208).
REFERENCES
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, “Global Cancer Statistics,” CA: A Cancer Journal for Clinicians, Vol. 61, No. 2, 2011, pp. 69-90. doi:10.3322/caac.20107
- D. Cunningham, W. Atkin, H. J. Lenz, H. T. Lynch, B. Minsky, B. Nordlinger and N. Starling, “Colorectal Cancer,” Lancet, Vol. 375, No. 9719, 2010, pp. 1030-1047. doi:10.1016/S0140-6736(10)60353-4
- T. M. Pawlik and M. A. Choti, “Surgical Therapy for Colorectal Metastases to the Liver,” Journal of Gastrointestinal Surgery, Vol. 11, No. 8, 2007, pp. 1057-1077. doi:10.1007/s11605-006-0061-3
- R. Lochan, S. A. White and D. M. Manas, “Liver Resection for Colorectal Liver Metastasis,” Surgical Oncology, Vol. 16, No. 1, 2007, pp. 33-45. doi:10.1016/j.suronc.2007.04.010
- M. E. Barugel, C. Vargas and G. K. Waltier, “Metastatic Colorectal Cancer: Recent Advances in Its Clinical Management,” Expert Review of Anticancer Therapy, Vol. 9, No. 12, 2009, pp. 1829-1847. doi:10.1586/era.09.143
- R. L. Nelson, T. Dollear, S. Freels and V. Persky, “The Relation of Age, Race, and Gender to the Subsite Location of Colorectal Carcinoma,” Cancer, Vol. 80, No. 2, 1997, pp. 193-197. doi:10.1002/(SICI)1097-0142(19970715)80:2<193::AID-CNCR4>3.0.CO;2-V
- J. H. Koo, B. Jalaludin, S. K. Wong, A. Kneebone, S. J. Connor and R. W. Leong, “Improved Survival in Young Women with Colorectal Cancer,” The American Journal of Gastroenterology, Vol. 103, No. 6, 2008, pp. 1488- 1495. doi:10.1111/j.1572-0241.2007.01779.x
- F. Grodstein, P. A. Newcomb and M. J. Stampfer, “Postmenopausal Hormone Therapy and the Risk of Colorectal Cancer: A Review and Meta-Analysis,” American Journal of Medicine, Vol. 106, No. 5, 1999, pp. 574-582. doi:10.1016/S0002-9343(99)00063-7
- H. D. Nelson, L. L. Humphrey, P. Nygren, S. M. Teutsch and J. D. Allan, “Postmenopausal Hormone Replacement Therapy: Scientific Review,” Journal of the American Medical Association, Vol. 288, No. 7, 2002, pp. 872-881. doi:10.1001/jama.288.7.872
- G. Rennert, H. S. Rennert, M. Pinchev, O. Lavie and S. B. Gruber, “Use of Hormone Replacement Therapy and the Risk of Colorectal Cancer,” Journal of Clinical Oncology, Vol. 27, No. 27, 2009, pp. 4542-4547. doi:10.1200/JCO.2009.22.0764
- S. Zhang, D. Wan, Z. Pan, Z. Zhou, G. Chen, Z. Lu, X. Wu and L. Li, “Multiple Factors Analysis on Liver Metastasis from Colorectal Cancer,” Zhonghua Zhong Liu Za Zhi, Vol. 24, No. 4, 2002, pp. 367-369.
- G. G. Kuiper, E. Enmark, M. Pelto-Huikko, S. Nilsson and J. A. Gustafsson, “Cloning of a Novel Receptor Expressed in Rat Prostate and Ovary,” Proceedings of the National Academy of Sciences of the United States, Vol. 93, No. 12, 1996, pp. 5925-5930. doi:10.1073/pnas.93.12.5925
- D. Elkind, “Cognitive Adaptation in Latency. The Construction of Social Reality,” Canadian Journal of Psychiatry, Vol. 21, No. 4, 1976, pp. 186-191.
- K. M. Dobrzycka, S. M. Townson, S. Jiang and S. Oesterreich, “Estrogen Receptor Corepressors—A Role in Human Breast Cancer?” Endocrine-Related Cancer, Vol. 10, No. 4, 2003, pp. 517-536. doi:10.1677/erc.0.0100517
- J. M. Hall, J. F. Couse and K. S. Korach, “The Multifaceted Mechanisms of Estradiol and Estrogen Receptor Signaling,” The Journal of Biological Chemistry, Vol. 276, No. 40, 2001, pp. 36869-36872. doi:10.1074/jbc.R100029200
- D. Witte, M. Chirala, A. Younes, Y. Li and M. Younes, “Estrogen Receptor Beta Is Expressed in Human Colorectal Adenocarcinoma,” Human Pathology, Vol. 32, No. 9, 2001, pp. 940-944. doi:10.1053/hupa.2001.27117
- P. A. Konstantinopoulos, A. Kominea, G. Vandoros, G. P. Sykiotis, P. Andricopoulos, I. Varakis, G. Sotiropoulou-Bonikou and A. G. Papavassiliou, “Oestrogen Receptor Beta (Erbeta) Is Abundantly Expressed in Normal Colonic Mucosa, but Declines in Colon Adenocarcinoma Paralleling the Tumour’s Dedifferentiation,” European Journal of Cancer, Vol. 39, No. 9, 2003, pp. 1251-1258. doi:10.1016/S0959-8049(03)00239-9
- L. Q. Xie, J. P. Yu and H. S. Luo, “Expression of Estrogen Receptor Beta in Human Colorectal Cancer,” World Journal of Gastroenterology, Vol. 10, No. 2, 2004, pp. 214-217.
- M. K. G. Leslie H. Sobin and C. Wittekind, “TNM Classification of Malignant Tumours,” 7th Edition, WileyLiss, New York, 2011.
- H. Kawasaki, D. C. Altieri, C. D. Lu, M. Toyoda, T. Tenjo and N. Tanigawa, “Inhibition of Apoptosis by Survivin Predicts Shorter Survival Rates in Colorectal Cancer,” Cancer Research, Vol. 58, No. 22, 1998, pp. 5071- 5074.
- F. A. Sinicrope, S. B. Ruan, K. R. Cleary, L. C. Stephens, J. J. Lee and B. Levin, “bcl-2 and p53 Oncoprotein Expression during Colorectal Tumorigenesis,” Cancer Research, Vol. 55, No. 2, 1995, pp. 237-241.
- R. L. Prentice, M. Pettinger, S. A. A. Beresford, J. Wactawski-Wende, F. A. Hubbell, M. L. Stefanick and R. T. Chlebowski, “Colorectal Cancer in Relation to Postmenopausal Estrogen and Estrogen plus Progestin in the Women’s Health Initiative Clinical Trial and Observational Study,” Cancer Epidemiology Biomarkers & Prevention, Vol. 18, No. 5, 2009, pp. 1531-1537. doi:10.1158/1055-9965.EPI-08-1209
- J. E. Rossouw, G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen and J. Ockene, “Risks and Benefits of Estrogen plus Progestin In Healthy Postmenopausal Women: Principal Results from the Women’s Health Initiative Randomized Controlled Trial,” Journal of the American Medical Association, Vol. 288, No. 3, 2002, pp. 321-333. doi:10.1001/jama.288.3.321
- E. Fernandez, C. La Vecchia, A. Balducci, L. Chatenoud, S. Franceschi and E. Negri, “Oral Contraceptives and Colorectal Cancer Risk: A Meta-Analysis,” British Journal of Cancer, Vol. 84, No. 5, 2001, pp. 722-727. doi:10.1054/bjoc.2000.1622
- R. T. Chlebowski, J. Wactawski-Wende, C. Ritenbaugh, F. A. Hubbell, J. Ascensao, R. J. Rodabough, C. A. Rosenberg, V. M. Taylor, R. Harris, C. Chen, L. L. Adams-Campbell and E. White, “Estrogen plus Progestin and Colorectal Cancer in Postmenopausal Women,” The New England Journal of Medicine, Vol. 350, No. 10, 2004, pp. 991-1004. doi:10.1056/NEJMoa032071
- M. L. Slattery, C. Sweeney, M. Murtaugh, K. N. Ma, R. K. Wolff, J. D. Potter, B. J. Caan and W. Samowitz, “Associations between ERalpha, ERbeta, and AR Genotypes And Colon and Rectal Cancer,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 14, No. 12, 2005, pp. 2936-2942. doi:10.1158/1055-9965.EPI-05-0514
- M. Campbell-Thompson, I. J. Lynch and B. Bhardwaj, “Expression of Estrogen Receptor (ER) Subtypes and ERbeta Isoforms In Colon Cancer,” Cancer Research, Vol. 61, No. 2, 2001, pp. 632-640.
- E. F. Foley, A. A. Jazaeri, M. A. Shupnik, O. Jazaeri and L. W. Rice, “Selective Loss of Estrogen Receptor Beta in Malignant Human Colon,” Cancer Research, Vol. 60, No. 2, 2000, pp. 245-248.
- Y. J. Fang, Z. H. Lu, F. Wang, X. J. Wu, L. R. Li, L. Y. Zhang, Z. Z. Pan and D. S. Wan, “Prognostic Impact of ERbeta and MMP7 Expression on Overall Survival in Colon Cancer,” Tumor Biology, Vol. 31, No. 6, 2010, pp. 651-658. doi:10.1007/s13277-010-0082-0
- H. G. Elbanna, M. A. Ebrahim, A. M. Abbas, K. Zalata and M. A. Hashim, “Potential Value of Estrogen Receptor Beta Expression in Colorectal Carcinoma: Interaction with Apoptotic Index,” Journal of Gastrointestinal Cancer, 2010.
- P. D. Grivas, V. Tzelepi, G. Sotiropoulou-Bonikou, Z. Kefalopoulou, A. G. Papavassiliou and H. Kalofonos, “Estrogen Receptor Alpha/Beta, AIB1, and TIF2 in Colorectal Carcinogenesis: Do Coregulators Have Prognostic Significance?” International Journal of Colorectal Disease, Vol. 24, No. 6, 2009, pp. 613-622. doi:10.1007/s00384-009-0647-9
- N. Jassam, S. M. Bell, V. Speirs and P. Quirke, “Loss of Expression of Oestrogen Receptor Beta in Colon Cancer and Its Association with Dukes’ Staging,” Oncology Reports, Vol. 14, No. 1, 2005, pp. 17-21.
- J. W. Nash, C. Morrison and W. L. Frankel, “The Utility of Estrogen Receptor and Progesterone Receptor Immunohistochemistry in the Distinction of Metastatic Breast Carcinoma from Other Tumors in the Liver,” Archives of Pathology & Laboratory Medicine, Vol. 127, No. 12, 2003, pp. 1591-1595.
- M. Iavarone, P. Lampertico, C. Seletti, M. Francesca Donato, G. Ronchi, E. del Ninno and M. Colombo, “The Clinical and Pathogenetic Significance of Estrogen Receptor-Beta Expression in Chronic Liver Diseases and Liver Carcinoma,” Cancer, Vol. 98, No. 3, 2003, pp. 529- 534. doi:10.1002/cncr.11528
- H. Sugiura, T. Toyama, Y. Hara, Z. Zhang, S. Kobayashi, Y. Fujii, H. Iwase and H. Yamashita, “Expression of Estrogen Receptor Beta Wild-Type and Its Variant ERbetacx/Beta2 Is Correlated with Better Prognosis in Breast Cancer,” Japanese Journal of Clinical Oncology, Vol. 37, No. 11, 2007, pp. 820-828. doi:10.1093/jjco/hym114
- L. Nakopoulou, A. C. Lazaris, E. G. Panayotopoulou, I. Giannopoulou, N. Givalos, S. Markaki and A. Keramopoulos, “The Favourable Prognostic Value of Oestrogen Receptor Beta Immunohistochemical Expression in Breast Cancer,” Journal of Clinical Pathology, Vol. 57, No. 5, 2004, pp. 523-528. doi:10.1136/jcp.2003.008599
- C. Y. Xu, J. L. Guo, Z. N. Jiang, S. D. Xie, J. G. Shen, J. Y. Shen and L. B. Wang, “Prognostic Role of Estrogen Receptor Alpha and Estrogen Receptor Beta in Gastric Cancer,” Annals of Surgical Oncology, Vol. 17, No. 9, 2010, pp. 2503-2509. doi:10.1245/s10434-010-1031-2
- J. Cassidy, J. Tabernero, C. Twelves, R. Brunet, C. Butts, T. Conroy, F. Debraud, A. Figer, J. Grossmann, N. Sawada, P. Schoffski, A. Sobrero, E. Van Cutsem and E. Diaz-Rubio, “XELOX (Capecitabine plus Oxaliplatin): Active First-Line Therapy for Patients with Metastatic Colorectal Cancer,” Journal of Clinical Oncology, Vol. 22, No. 11, 2004, pp. 2084-2091. doi:10.1200/JCO.2004.11.069
- J. Cassidy, S. Clarke, E. Diaz-Rubio, W. Scheithauer, A. Figer, R. Wong, S. Koski, M. Lichinitser, T. S. Yang, F. Rivera, F. Couture, F. Sirzen and L. Saltz, “Randomized Phase III Study of Capecitabine plus Oxaliplatin Compared with Fluorouracil/Folinic Acid Plus Oxaliplatin as First-Line Therapy for Metastatic Colorectal Cancer,” Journal of Clinical Oncology, Vol. 26, No. 12, 2008, pp. 2006-2012. doi:10.1200/JCO.2007.14.9898
- M. A. Choti, J. V. Sitzmann, M. F. Tiburi, W. Sumetchotimetha, R. Rangsin, R. D. Schulick, K. D. Lillemoe, C. J. Yeo and J. L. Cameron, “Trends in LongTerm Survival following Liver Resection for Hepatic Colorectal Metastases,” Annals of Surgery, Vol. 235, No. 6, 2002, pp. 759-766. doi:10.1097/00000658-200206000-00002
- G. Chong and D. Cunningham, “Improving Long-Term Outcomes for Patients with Liver Metastases from Colorectal Cancer,” Journal of Clinical Oncology, Vol. 23, No. 36, 2005, pp. 9063-9066. doi:10.1200/JCO.2005.04.4669
- V. Cappelletti, P. Miodini, G. Di Fronzo and M. G. Daidone, “Modulation of Estrogen Receptor-Beta Isoforms by Phytoestrogens in Breast Cancer Cells,” International Journal of Oncology, Vol. 28, No. 5, 2006, pp. 1185- 1191.
- M. J. Weyant, A. M. Carothers, N. N. Mahmoud, H. L. Bradlow, H. Remotti, R. T. Bilinski and M. M. Bertagnolli, “Reciprocal Expression of ERalpha and ERbeta Is Associated with Estrogen-Mediated Modulation of Intestinal Tumorigenesis,” Cancer Research, Vol. 61, No. 6, 2001, pp. 2547-2551.
- F. Caiazza, P. Galluzzo, S. Lorenzetti and M. Marino, “17Beta-Estradiol Induces ERbeta Up-Regulation via p38/ MAPK Activation in Colon Cancer Cells,” Biochemical and Biophysical Research Communications, Vol. 359, No. 1, 2007, pp. 102-107. doi:10.1016/j.bbrc.2007.05.059
NOTES
*The authors of this article declare that there are no conflicts of interest.
#Corresponding author.

