Journal of Cancer Therapy
Vol.3 No.1(2012), Article ID:17213,9 pages DOI:10.4236/jct.2012.31004
Measurements of 5-FU Plasma Concentrations in Patients with Gastrointestinal Cancer: 5-FU Levels Reflect the 5-FU Dose Applied
![]()
Department of Gastroenterology and Endocrinology, University Medicine Göttingen, Göttingen, Germany.
Email: *silke.cameron@med.uni-goettingen.de
Received December 29th, 2011; revised January 30th, 2012; accepted February 14th, 2012
Keywords: 5-FU; Plasma Concentration; Immunolinked Elisa Assay
ABSTRACT
Introduction: 5-Fluorouracil (5-FU) is the basis of most combination chemotherapies for gastrointestinal tumors. It is generally well tolerated, but side-effects might require dose-adjustment. As adverse events are not specific to the 5-FU component of the chemotherapy-combination, i.e. neutropenia, diarrhea or cardiotoxicity, the knowledge of 5-FU serum levels might help to attribute these side effects to the 5-FU compound. The optimal concentration-range (AUC, area under the curve) has been described to be within 20 -25 mgh/l. The aim of this study was to analyse the intraand interindividual variability of 5-FU AUC-levels in patients with 5-FU infusion therapy. Methods: 230 blood samples were obtained from 31 different gastrointestinal cancer patients (esophagus (8), stomach (10), ileum (1), colorectum (12)) treated with 5-FU-infusional regimes, based on a 24- or 48-hour AIO treatment-schedule. 5-FU plasma concentrations were measured using an immunolinked Elisa assay (Saladax 5-FU PCMTM). Intraand interindividual differences were analysed before (0 h; n = 115), at 2 - 3 hours after the start of infusional 5-FU treatment (n = 19) (early sampling) and towards the end of the infusion (n = 96) (late sampling). Results: Early blood sampling resulted in low 5-FU plasma concentrations (541 ± 127 g/ml) due to saline prefilling (2 - 3 ml) of the Baxter pump. Blood sampling at the later time-point resulted in reproducible values (971 ± 81 ng/ml). 5-FU concentrations were dose-dependent with low intraand interindividual variability. However, care has to be taken, as the results can be influenced by inaccurate blood sampling: too early or late sampling (when the folfusor-pump is empty), delayed centrifugation of the tube or hemolysis. Conclusions: With critical analysis of the measurements and correct performance of blood sampling, the measurement of 5-FU plasma concentrations with the immunoassay may in the future allow to optimize 5-FU dosing and to identify the cause of toxicity. Changes of 5-FU clearance in long-term therapy still have to be studied.
1. Introduction
The fluoropyrimidine 5-fluorouracil (5-FU) is the basis of most combination therapies for gastrointestinal tumors. It has been used in daily clinical oncology for nearly 50 years [1].
In Germany the high dose AIO schedule is preferentially applied in a dose of 2000 - 2600 mg/m2 as weekly infusion over 24 hours [2]. In Europe, also the French Folfox-4 regimen is used, which consists of 400 mg/m2 bolus + 600 mg/m2 continuous infusion (CI) over 22 hours at two consecutive days every other week [3]. From this scheme, the Folfox-6 regimen was developed which consists of 400 mg/m2 bolus + 2400 (–3000) mg/m2 as a 46-hour CI with biweekly repetitions [4]. In the United States, mainly the bolus application is given (MAYO regimen: 425 mg/m2 bolus day 1 - 5 with repetitions every 4 - 5 weeks) [5,6].
The efficacy of 5-FU is increased by infusion of leucovorin (folinic acid) prior to 5-FU. Leucovorin acts as co-activator of the thymidylate synthetase, the main target of CI 5-FU action [7].With the aim to increase 5-FU efficacy, combination therapies have been introduced about 10 years ago. 5-FU is generally combined with either oxaliplatin (an alkylating chemotherapy agent which leads to the formation of crosslinks within and between DNA strands) [3,8,9] and/or irinotecan (a camptothecin analogue which requires bioactivation) [10-14]. SN-38 acts as a topoisomerase I poison [15]. Other combinations include docetaxel which blocks mitosis by acting on the microtubules [16], as well as epirubicin which blocks the DNA-polymerase [17].
In the palliative setting, several antibodies can be used in combination with 5-FU therapy to enhance the antitumor effect. These include cetuximab and panitumumab (EGF-R-antibodies) and bevacizumab (VEGFR-antibody). Other site-specific agents are currently being tested. It has been described, that 5-FU plasma concentrations are not affected by the combined agent [18,19].
5-FU is metabolised by the dihydropyrimidine dehydrogenase (DPD), the key enzyme of the pyrimidine catabolism [20]. DPD is found everywhere in the organism, but is mainly expressed in liver, lung, kidney and lymphocytes. DPD activity varies interindividually, however, a complete loss of DPD activity is rare (0.5%) [21-24].
5-FU disappears rapidly from the plasma with a half-life of 10 - 20 minutes. Its total body clearance varies due to the administration schedule: 0.5 - 1.5 l/min for bolus application versus 5 - 58 l/min for continuous infusions [25-28].
The elevated plasma clearance with continuous 5-FU infusion has been explained by lung and kidney as additional sites of catabolism other than the liver [25]. This would explain why continuous infusion allows higher doses of 5-FU than bolus application.
5-FU is known to have a narrow therapeutic index: High levels can lead to severe side effects, whilst low levels will miss a therapeutic effect [29]. Side effects of 5-FU which frequently lead to dose adjustment are diarrhea (CI), neutropenia (bolus) and hand/foot syndrome (CI). If dose adjustment for toxic side effects is necessary, generally all compounds of the anti-cancer therapy are reduced (e.g. 100% to 75%) whilst possibly only 5-FU might have required reduction [30,31]. The measurement of 5-FU levels is important as it can help to identify the cause of treatment toxicity in a combination treatment regime and might in the future help to optimize 5-FU dosing with different treatment schedules.
The standard approach for calculating 5-FU dosage is body surface area (mg/m2). However there is a lack of association between body surface area and 5-FU clearance [32,33]. Individual variations regarding absorption, distribution, metabolism and excretion of 5-FU, support the importance of pharmacokinetic dose management within the individual patient. Other factors that should be considered include the genetic background, performance status, age, sex, weight, and circadian diurnal variation [34].
Recently Gamelin et al. [19], using a quite unconventional 5-FU administration schedule, suggested that the optimal AUC (area under the curve) range lies between 20-25 mgh/l. Independent of the 5-FU regimen or schedule, all researchers find nearly the same optimal AUC range [19,34-36].
The AUC value is calculated by multiplying the steady state concentration (Css) of 5-FU with the duration of CI:
AUC24h(mgh/l)= Css (ng/ml) 24 h/1000 [34] (1)
The Gamelin study postulates, that with continuous infusion over 8 hours, an optimal steady state concentration of 5-FU is in the range of 2500 - 3000 ng/ml. These values result in an AUC value of 20 - 25 mgh/L. Gamelin et al. [37,38] have published that the same is true for the FOLFOX 4 regimen. These findings are consistent with an earlier study [39] which measured 5-FU steady state concentrations between 6 - 8 M over an infusion period of 24 hours. This corresponds to an AUC-value of AUC24h = 20 - 25 mgh/l and a steady state level of 830 - 1040 ng/ml.
As many patients develop cancer at a higher age when comorbidities have developed, prevention of overdosing is as important as prevention of underdosing. Routine monitoring of 5-FU concentrations can be facilitated using a nanoparticle antibody-based immunoassay. The assay has been validated [35] and is readily available to clinicians in the community or academic setting. Compared to the high pressure liquid chromatography (HPLC) method used in previous publications [19,39-42], this method is easier to perform and less time-consuming.
The aim of our study was to analyse the intraand interindividual variability of 5-FU plasma-levels in patients with 5-FU infusion therapy. This study reports on the experience under “German” conditions using the different AIO 5-FU-based schedules in an out-patient “reallife” setting. It is the first study using the nanoparticlebased immunoassay for 5-FU concentration measurement in a European population.
2. Methods
5-FU plasma levels were measured in 230 blood samples obtained from 31 consecutive patients (24 male, 7 female, age 63.5 years ± 9, range 45 - 78) with gastrointestinal cancers who received a 5-FU based chemotherapy. 8 patients had esophagus carcinoma, 10 patients had stomach cancer, 1 patient with small bowel cancer, and 12 patients had colon carcinoma (Table 1). This analysis was approved by the ethics committee of the University Medicine Göttingen.
Different chemotherapy combinations (with dose reduction in some cases) had been given using the following 24-hour continuous-infusion schedules: 1500 mg/m2 (75%), n = 28, i.e. 22× FOLFOXIRI, 3× FLP, 3× FOLFOX; 1950 mg/m2 (75%) n = 11, i.e. 11x FLOT; 2000 mg/m2, n = 44, i.e. 6× FOLFOX, 38× FOLFIRI; 2600 mg/m2, n = 17, i.e. 17× FLOT. For the 48-hours continuous-infusion, the following 5-FU doses were given: 2400 mg/m2 (75 %), n = 4, i.e. 4x FOLFOXIRI; 3200 mg/m2, n = 2, i.e. 2× FOLFOXIRI. All patients had adequate liver and kidney function.

Table 1. Patients’ characteristics at baseline.
The 5-FU containing solution was applied by a SV4 folfusor during a 24 hour infusion (Vmax = 130 ml; flow rate 4 ml/h [24 h]) or by a LV5 folfusor (both Baxter, Germany) during a 48 hour infusion (Vmax = 300 ml; flow rate 5 ml/h [48 h]).
The folfusor pump SV4 and its flow profile are displayed in Figure 1. The pump has a maximal volume of 130 ml and the flow is driven by the pressure evoked by filling the elastomeric pump balloon with the infusing solution. The flow rate is regulated by the connecting piece which is fixed to the patients’ skin. The connecting tubing (50 cm) is prefilled with 2 - 3 ml saline solution (0.9% NaCl) for safety reasons. The given flow rate 4 ml/h is adjusted to the following conditions: 33˚C, same level of port and pump, blood pressure within the normal range, and a tube opening of 18 Gauge (instructions manual, Baxter folfusor portable elastomeric infusion system, No. 07-19-61-618 10/2009). For reference, Gamelin et al. [19] have used a battery-operated pump for 5-FU administration.
5 ml EDTA blood were taken before or during 5-FU infusion: early sampling was done at 2 - 3 hours after the start of the infusion. As the SV4 folfusor pumps were depleted earliest after 21.5 und latest after 23.5 hours, late sampling was done at 21 - 23 hours, before the pump was empty. For the 48 hour infusion schedule, 5-FU infusion mostly ended at about 44 hours. Hence late sampling was done at 41 - 43 hours [42]. If the pump was empty at the time point of blood collection, the sample was discarded.
After collection, the blood samples were immediately placed on ice and centrifuged within 30 minutes. The plasma was kept at –20˚C. 5-FU levels were measured using the immunolinked Elisa assay developed by Saladax (Bethlehem, USA), following the Saladax’ instructions. This nanoparticle antibody-based immunoassay
 (a)
(a) (b)
(b)
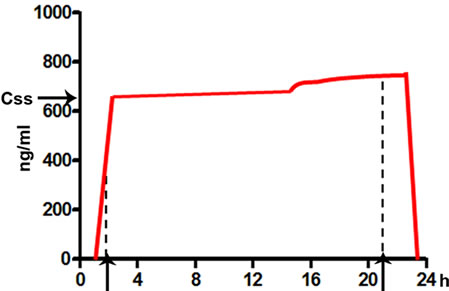
Figure 1. (a) Baxter SV4 pump (130 ml; 4 ml/h) with 0.5 m tubing and white connecting piece for fixation to the patients’ skin to adjust flow velocity. The 5-FU solution is filled into the inner yellow reservoir (Baxter, Groningen). The pumps can be distinguished by their cap color (red = SV4); (b) Diagram of the flow profile of 5-FU plasma concentrations (red line) within a continuous 24 hour infusion. The time points of 5-FU sampling (2 and 21 hours) are marked with dashed lines. The 10% increased flow rate at the end of the infusion is depicted (modified from the incremental flow profile, Baxter folfusor portable elastomeric infusion system, instructions manual).
follows the principle of scattered light and absorption, which occurs with the aggregation of nanoparticles. The aggregation is measured at a wave length between 400 and 650 nm. Multivalent drug-conjugates function as bindingpartner for 5-FU-selective antibodies, which are covalently linked to the surface of nanoparticles. In the 5-FUcontaining sample, the drug-conjugates are competitively displaced, their aggregation is inhibited and the absorption reduced. This displacement is measured as classical inhibition-curve, from which the 5-FU concentration was calculated according to the relevant formula (1). 5-FU plasma concentration measurement has to be performed at steady-state, as the concentrations decrease rapidly (within about 30 min) after the end of the infusion. Since AUC-values are calculated over a time period, we estimated the actual infusion time which was set with a mean of 23 hours for the 24 hour infusion schedule, and 44 hours for the 48 hours schedule. It is to be noted that before the end of the infusion, due to the elastomeric principle of the non-electric-driven pumps, the infusion rate is increased to about 110% (instructions manual, Baxter folfusor portable elastomeric infusion system, No. 07-19-61-618 10/2009, Figure 1. Hence, 10% was subtracted from the measured 5-FU concentration at the late blood sampling time. Assay precision, linearity, calibretion stability and limit of detection of the nanoparticlebased immunoassay have been validated previously, as have interference, cross reactivity and lower limit of quantitation [35]. 5-FU concentrations obtained by this method correlate with the measurements obtained from liquid chromatography-tandem mass spectrometry [35]. Our analyses were performed on a cobas c111 bioanalyser (Roche Diagnostics), as smaller sample numbers can be handled more easily. Test runs for assay stability on this machine were performed previously. None of the substances used as co-medication interfere with the detection of 5-FU [35,43].
3. Results
We studied 31 consecutive patients (Table 1) receiving different AIO 5-FU continuous-infusion regimen over several weeks (mean 13.8 ± 16.1; range 1 - 54 weeks). 5-FU was applied either as single agent or in combination with oxaliplatin (FOLFOX), irinotecan (FOLFIRI), oxaliplatin and docetaxel (FLOT), or oxaliplatin and irinotecan (FOLFOXIRI). Cetuximab, panitumumab, or bevazicumab were initially combined in 2, 1, 6 patients, respectively.
The values obtained before the start of the 5-FU infusion (0 hours) were stable and ranged within 0 - 75 ng/ml. The results for early blood sampling at 2 - 3 hours of continuous infusion or after 21 - 23 hours (before depletion of the pump) are shown in Figure 2. The mean steady-state concentrations (Css) and the standard deviations for 4 different initial 5-FU doses (1500 mg/m2, 1950 mg/m2, 2000 mg/m2 and 2600 mg/m2) using the 24 hour infusion schedule are displayed. The values measured after 2-3 hours were about 56% (range 45% - 74%; mean AUC23h 12.4 ± 2.9 SD; mean plasma concentration 541 ng/ml ± 126.9 SD) of the values obtained after 21 - 23 hours (mean AUC-values of 22.3 ± 1.9 SD; mean plasma concentration 971.9 ng/ml ± 81.1 SD), independent of the administered 5-FU dose. We postulate that the steady state level of 5-FU is not reached after 2 - 3 hours, due to the saline pre-filling of the pump tubing.
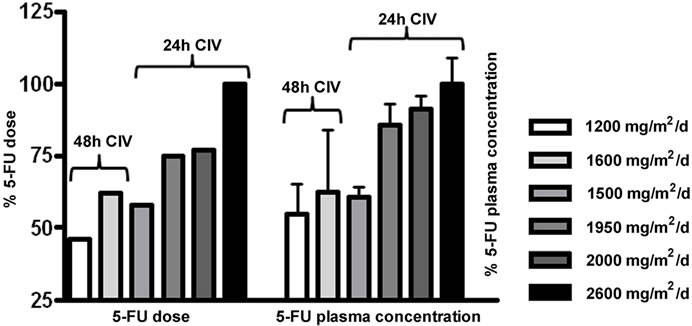
To analyse whether the administered 5-FU dose correlates with the measured 5-FU plasma concentration, the highest 5-FU dose (2600 mg/m2) was normalized to 100 % (Figure 3(a) left panel). The corresponding mean 5-FU concentration was also normalized to 100 % (Figure 3(a)
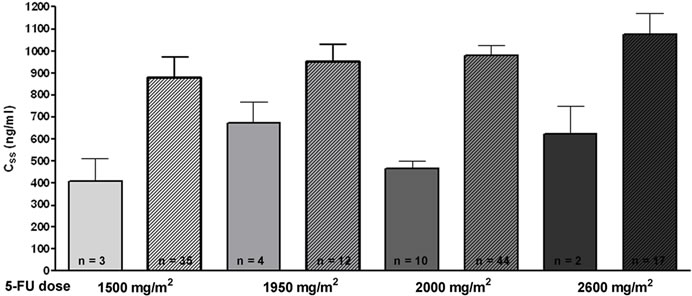
Figure 2. Bar diagram comparing 5-FU plasma levels after early (2 - 3 hours; plain bars) blood sampling and late blood sampling (21 - 23 hours; hatched bars). Bars represent values obtained after application of 4 different 5-FU doses (1500 mg/m2/d, 1950 mg/m2/d, 2000 mg/m2/d and 2600 mg/ m2/d). Error bars show the standard deviation of the mean.
 (a)
(a)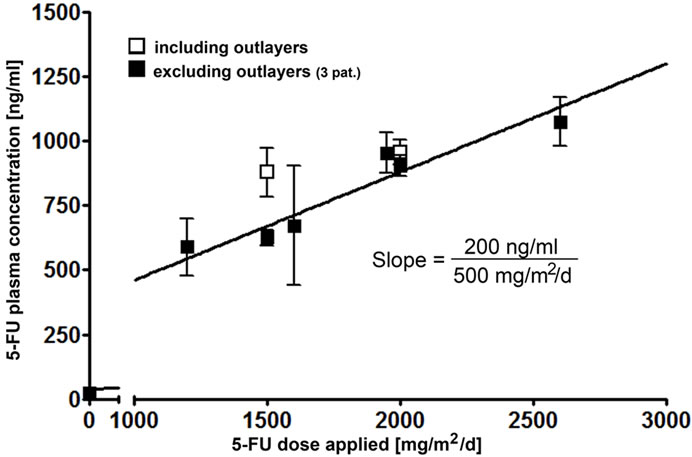 (b)
(b)
Figure 3. Comparison of 5-FU dosage (in %) and resulting 5-FU plasma concentrations. (a) At the left, the administered 5-FU dose is shown in %. The highest 5-FU dose was 2600 mg/m2/d and was set as 100%. At the right, the measured 5-FU plasma concentrations are depicted (in %). The levels obtained with 2600 mg/m2/d were set 100%. The 24 hour and 48 hour infusion-schedules are indicated separately for the 5-FU dose administered per day; (b) Relative increase of 5-FU plasma concentration with 5-FU dose applied: an increase of 500 mg 5-FU/m2/day results in an increase of 200 ng/ml 5-FU plasma concentration.
right panel). When comparing the measured values for each infusion schedule, a clear dose-dependency of the measured 5-FU-concentrations was observed in our system. For instance, the lowest administered 5-FU dose for the 24 h schedule (1500 mg/m2/d) accounts for 58% of the highest dose (2600 mg/m2), and the measured 5-FU concentration corresponds to 61% as compared to the concentration obtained for highest 5-FU dose (2600 mg/m2).
The measured concentrations (in % of the 2600 mg/m2 dose) for the 24 hour infusion schedules were, as expected, significantly higher than those obtained for the 48 hour infusion schedules (1200 mg/m2/d = 2400 mg/m2 over 2 days, and 1600mg/m2/d = 3200 mg/m2 over 2 days) with 55% and 62%, respectively. Figure 3(b) depicts the difference between the 5-FU dose applied and the measured 5-FU plasma concentration as a slope. It shows that with an increase of 500 mg/m2/day, an increase of 200 ng/ml plasma concentration can be expected.
The different concomitant chemotherapy drugs do not seem to influence the measurement, as standard deviations are acceptable.
Although there are inter-individual variations in the 5-FU levels, in general high 5-FU dosage results in high 5-FU concentrations.
To determine the intraand inter-individual 5-FU concentrations for the different 24 hour dose conditions, the individually obtained values are compared in Figure 4. Plasma levels for patients (n = 4) with seven or more available values showed relatively stable concentrations with a variation of 17% (13.9% - 22.4%) around the individual mean. Also inter-individual plasma concentration (after exclusion of the out-layers), showed relatively stable values within a range of 624 ng/ml ±189 SD for the 5-FU dose of 1500 mg/m2; 879 ng/ml ±198 SD for 2000 mg/m2. A slightly higher variation was observed for the higher doses: 897 ng/ml ±273 SD for 1950 mg/m2, and 978 ng/ml ± 389 SD for 2600 mg/m2. In the 1500mg/m2/d treatment schedule, only 3 out of 12 patients showed consistently higher AUC-values. Within the 13 patients receiving 2000 mg/m2/d three patients showed elevated 5-FU concentrations compared to the rest of the group. However, no elevated clinical or hematologic toxicity was observed within these patients.
4. Discussion
Most anticancer drugs are characterised by a narrow therapeutic window: a small change in dose can lead to poor anti-tumor effects or an unacceptable degree of toxicity. But whilst therapeutic drug monitoring is considered standard practice for select antimicrobial drugs and immunosuppressing agents in organ transplants, where the measurement of drug concentrations in the blood allows to balance efficacy and toxicity, therapeutic drug monitoring is not yet a generally available tool in oncological practice.

Figure 4. 5-FU plasma concentrations in 31 different patients. The diagrams show the 5-FU concentrations for 4 different 5-FU dosage schedules (1500 mg/m2/d, 1950 mg/m2/d, 2000 mg/m2/d and 2600 mg/m2/d). The number of obtained measurements per patient varies between 1 and 21. If more than 1 value was obtained the median value is given. The 4 patients who showed higher mean 5-FU concentrations are displayed in red.
The prototype drug for pharmacokinetically guided dose adjustment would not only have a narrow therapeutic index, but would have an efficacy or toxicity that is clearly correlated with the measured drug concentrations. A timely method for assaying drug concentrations is also needed. 5-FU is one of the major drugs used in cancer chemotherapy. It embodies many of these characteristics, making it a good candidate for therapeutic drug monitoring [44]. Already, multiple efforts exist which indicate that it should be possible to define therapeutic regimes wherein 5-FU is administered to achieve a targeted Css determined by the efficacy and the risk and severity of toxicity deemed acceptable [45].
The measurement of 5-FU plasma concentrations would help to standardize the treatment of the different 5-FUcontaining regimes. A reason for the lack of implementation of concentration-measurement might be the timeconsuming and expensive HPLC method.
Routine monitoring of 5-FU concentrations can be facilitated using a nanoparticle antibody-based immunoassay. Compared to the HPLC methods used in previous publications [19,39-41] this technique is easier to perform and less time-consuming. The aim of our study was therefore to analyse the feasibility and intraand interindividual variability of 5-FU concentration-measurements in patients with 5-FU infusion therapy using an immunolinked Elisa assay.
We measured 5-FU levels in blood samples taken within the first 3 hours after the start of the infusion. The resulting calculated AUC23h values were low (AUC23h 12.5 ± SD 2.9; plasma concentration 541 ng/ml ± SD 126.9) compared to the proposed AUC range of 20 mgh/l – 25 mgh/l, indicating that every patient would have been underdosed. Low values from at least 2 hours after the start of the infusion have also been described in an earlier publication in American patients receiving 5-FU (2400 mg/m2) as CI, with 51% of the patients below the AUC range of 20 - 25 mgh/L [43]. Re-evaluation of our continuous infusion pump system showed that the tubing was pre-filled with sodium chloride so that steady-state concentrations were delayed compared to other publications [19,46].
When 5-FU concentrations were measured 2 - 3 hours before the end of continuous 5-FU treatment, plasma concentrations where at steady-state and reached the expected AUC-values of 22.3 ± 1.9 SD (plasma concentration 971.9 ng/ml ± 81.1 SD). This was also observed in the individual patients who had lower early blood sampling values for comparison. It also became obvious, that with the elastomeric pump principle, the infusions did not last the expected 24 or 48 hours but were depleted earlier, especially in summer. Sometimes pumps were depleted as early as after 21.5 hours. Earlier sampling (i.e.
between 5 and 21 hours) would be possible, however it would require the patient to wait long hours at the outpatient clinic for blood sample collection or to come back another time during the infusion.
Our results confirm that the measured 5-FU concentrations correlate with the applied 5-FU dose when measured in steady-state conditions. We could further demonstrate that with an increase of 500 mg/m2 5-FU, an increase in the plasma concentration of 200 ng/ml can be expected.
There is further an acceptable inter-individual variation between the different measurements in each patient. This finding supports the use for the measurement of 5-FU plasma concentrations for drug monitoring. Unusual high or low values can be detected reliably, but care has to be taken with the timing of blood sampling.
Some of the patients (14%) had repeatedly higher 5- FU blood levels than other patients with similar dosing schedules (Figure 4). None of these patients did suffer from toxic side effects. Hence there might be individual plasma concentrations for toxic effects. For the future, plasma concentrations should be correlated with toxic effects and efficacy data in our test system.
Taken together, we could show that the measurement of 5-FU plasma concentrations using an immunoassay is a stable and reproducible method to control 5-FU concentrations in patients receiving 5-FU based chemotherapies. Care has to be taken to assure standardized blood sampling, and obtained values should be confirmed with separate measurements, even though intra-individual variability is low.
Although we measured 5-FU concentrations in patients with different chemotherapy schedules and different diseases, intra-individual variation was low, with about 86% of the patients being within the same concentration range. Our study also shows that there are patients, who tolerate higher doses without side-effects.
For the future, the possibility of pharmacokinetic dose monitoring is important. Individual dose adjustment of 5-FU might become practicable. Until then, the current approach for the handling of side-effects is general dose reduction or change of the therapeutic regime, as some side-effects cannot be attributed to a certain compound within the drug-combination.
In view of sequential versus combination chemotherapy for advanced colorectal cancer, which implies noninferiority for the monotherapy with infusional 5-FU/ leucovorin [10,47-50], the measurement of 5-FU plasma concentrations might help to identify those patients who achieve high plasma levels with 5-FU monotherapy and who might therefore benefit from a sequential treatment.
5. Acknowledgements
We would like to thank Saladax for providing the 5-FU immunolinked Elisa Assay, and Roche for the Cobas c111 analyser. We further thank Elke Neumann and Corinna Dunaiski for technical assistance and Kamyar Emami for helpful discussions.
REFERENCES
- B. Ardalan, L. Chua, E. M. Tian, R. Reddy, K. Sridhar, P. Benedetto, et al., “A phase II study of weekly 24-hour infusion with high-dose fluorouracil with leucovorin in colorectal carcinoma,” Journal of Clinical Oncology, Vol. 9, No. 4, 1991, pp. 625-630.
- C. H. Kohne, J. Wils, M. Lorenz, P. Schoffski, R. Voigtmann, C. Bokemeyer, et al., “Randomized Phase III Study of High-Dose Fluorouracil Given as a Weekly 24-Hour Infusion with or without Leucovorin versus Bolus Fluorouracil Plus Leucovorin in Advanced Colorectal Cancer: European Organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952,” Journal of Clinical Oncology, Vol. 21, No. 20, 2003, pp. 3721-3728. doi:10.1200/JCO.2003.11.122
- A. de Gramont, A. Figer, M. Seymour, M. Homerin, A. Hmissi, J. Cassidy, et al., “Leucovorin and Fluorouracil with or without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer,” Journal of Clinical Oncology, Vol. 18, No. 16, 2000, pp. 2938-2947.
- F. Maindrault-Goebel, C. Louvet, T. Andre, E. Carola, J. P. Lotz, J. L. Molitor, et al., “Oxaliplatin Added to the Simplified Bimonthly Leucovorin and 5-Fluorouracil Regimen as Second-Line Therapy for Metastatic Colorectal Cancer (FOLFOX6). GERCOR,” European Journal of Cancer, Vol. 35, No. 9, 1999, pp. 1338-1342. doi:10.1016/S0959-8049(99)00149-5
- I. P. Popov, S. B. Jelic, M. M. Matijasevic, B. Grbic and D. R. Babic, “Mayo Regimen plus Three Different Second-Line Chemotherapy Regimens in Sequential Therapy in Patients with Advanced Colorectal Cancer (ACRC). Journal of Experimental & Clinical Cancer Research, Vol. 23, No. 3, 2004, pp. 395-401.
- A. Tomiak, M. Vincent, W. Kocha, M. Taylor, E. Winquist, B. Keith, et al., “Standard Dose (Mayo Regimen) 5-Fluorouracil and Low Dose Folinic Acid: Prohibitive Toxicity?” Amrican Journal of Clinical Oncology, Vol. 23, No. 1, 2000, pp. 94-98. doi:10.1097/00000421-200002000-00025
- D. Machover, E. Goldschmidt, P. Chollet, G. Metzger, J. Zittoun, J. Marquet, et al., “Treatment of Advanced Colorectal and Gastric Adenocarcinomas with 5-Fluorouracil and High-Dose Folinic Acid,” Journal of Clinical Oncology, Vol. 4, No. 5, 1986, pp. 685-696.
- C. Louvet, T. Andre, J. M. Tigaud, E. Gamelin, J. Y. Douillard, R. Brunet, et al., “Phase II Study of Oxaliplatin, Fluorouracil, and Folinic Acid in Locally Advanced or Metastatic Gastric Cancer Patients,” Journal of Clinical Oncology, Vol. 20, No. 23, 2002, pp. 4543-4548. doi:10.1200/JCO.2002.02.021
- F. Maindrault-Goebel, G. A. de, C. Louvet, T. Andre, E. Carola, M. Mabro, P. Artru, et al., “High-Dose Intensity Oxaliplatin Added to the Simplified Bimonthly Leucovorin and 5-Fluorouracil Regimen as Second-Line Therapy for Metastatic Colorectal Cancer (FOLFOX 7),” European Journal of Cancer, Vol. 37, No. 8, 2001, pp. 1000-1005. doi:10.1016/S0959-8049(01)00068-5
- D. Cunningham, S. Falk and D. Jackson, “Clinical and Economic Benefits of Irinotecan in Combination with 5-Fluorouracil and Folinic Acid as First Line Treatment of Metastatic Colorectal Cancer,” British Journal of Cancer, Vol. 86, No. 11, 2002, pp. 1677-1683. doi:10.1038/sj.bjc.6600204
- J. Y. Douillard, D. Cunningham, A. D. Roth, M. Navarro, R. D. James, P. Karasek, et al., “Irinotecan Combined with Fluorouracil Compared with Fluorouracil Alone as FirstLine Treatment for Metastatic Colorectal Cancer: A Multicentre Randomised Trial,” Lancet, Vol. 355, No. 9209, 2000, pp. 1041-1047. doi:10.1016/S0140-6736(00)02034-1
- A. Falcone, S. Ricci, I. Brunetti, E. Pfanner, G. Allegrini, C. Barbara, et al., “Phase III trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared with Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) as First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest,” Journal of Clinical Oncology, Vol. 25, No. 13, 2007, pp. 1670-1676. doi:10.1200/JCO.2006.09.0928
- P. Rougier, C. E. Van, E. Bajetta, N. Niederle, K. Possinger, R. Labianca, et al., “Randomised Trial of Irinotecan versus Fluorouracil by Continuous Infusion after Fluorouracil Failure in patients with Metastatic Colorectal Cancer,” Lancet, Vol. 352, No. 9138, 1998, pp. 1407- 1412. doi:10.1016/S0140-6736(98)03085-2
- L. B. Saltz, J. V. Cox, C. Blanke, L. S. Rosen, L. Fehrenbacher, M. J. Moore, et al., “Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer,” The New England Journla of Medicine, Vol. 343, No. 13, 2000, pp. 905-914. doi:10.1056/NEJM200009283431302
- M. K. Ma and H. L. McLeod,, “Lessons Learned from the Irinotecan Metabolic Pathway,” Curr.Med.Chem., Vol. 10, No. 1, 2003, pp. 41-49. doi:10.2174/0929867033368619
- M. A. Jordan and L. Wilson, “Microtubules as a Target for Anticancer Drugs,” Nature Reviews Cancer, Vol. 4, No. 4, 2004, pp. 253-265. doi:10.1038/nrc1317
- S. Spadari, G. Pedrali-Noy, F. Focher, A. Montecucco, T. Bordoni, C. Geroni, et al., “DNA Polymerases and DNA Topoisomerases as Targets for the Development of Anticancer Drugs,” Anticancer Research, Vol. 6, No. 5, 1986, pp. 935-940.
- J. Bertino, M. Fleisher, J. H. Beumer, E. Chu, A. diPaolo, C. Eng, et al., “Highlights from: 5-Fluorouracil Drug Management Pharmacokinetics and Pharmacogenomics Workshop; Orlando, Florida; January 2007,” Clinical Colorectal Cancer, Vol. 6, No. 6, 2007, pp. 407-422. doi:10.1016/S1533-0028(11)70480-7
- E. Gamelin, R. Delva, J. Jacob, Y. Merrouche, J. L. Raoul, D. Pezet, et al., “Individual Fluorouracil Dose Adjustment Based on Pharmacokinetic Follow-Up Compared with Conventional Dosage: Results of a Multicenter Randomized Trial of Patients with Metastatic Colorectal Cancer,” Journal of Clinical Oncology, Vol. 26, No. 13, 2008, pp. 2099-2105. doi:10.1200/JCO.2007.13.3934
- O. Capitain, M. Boisdron-Celle, A. L. Poirier, S. AbadieLacourtoisie, A. Morel and E. Gamelin, “The Influence of Fluorouracil Outcome Parameters on Tolerance and Efficacy in Patients with Advanced Colorectal Cancer,” The Pharmacogenomics Journal, Vol. 8, No. 4, 2008, pp. 256-267. doi:10.1038/sj.tpj.6500476
- E. Bandres, R. Zarate, N. Ramirez, A. Abajo, N. Bitarte and J. Gariia-Foncillas, “Pharmacogenomics in Colorectal Cancer: The First Step for Individualized-Therapy,” World Journal of Gastroenterology, Vol. 13, No. 44, 2007, pp. 5888-5901.
- A. Beck, M. C. Etienne, S. Cheradame, J. L. Fischel, P. Formento, N. Renee, et al., “A Role for Dihydropyrimidine Dehydrogenase and Thymidylate Synthase in Tumour Sensitivity to Fluorouracil,” European Journal of Cancer, Vol. 30, No. 10, 1994, pp. 1517-1522. doi:10.1016/0959-8049(94)00216-R
- G. Bocci, C. Barbara, F. Vannozzi, P. A. Di, A. Melosi, G. Barsanti, et al., “A Pharmacokinetic-Based Test to Prevent Severe 5-Fluorouracil Toxicity,” Clinical Pharmacology & Therapeutics, Vol. 80, No. 4, 2006, pp. 384- 395. doi:10.1016/j.clpt.2006.06.007
- B. E. Harris, J. T. Carpenter and R. B. Diasio, “Severe 5-Fluorouracil Toxicity Secondary to Dihydropyrimidine Dehydrogenase Deficiency. A Potentially More Common Pharmacogenetic Syndrome,” Cancer, Vol. 68, No. 3, 1991, pp. 499-501. doi:10.1002/1097-0142(19910801)68:3<499::AID-CNCR2820680309>3.0.CO;2-F
- H. Bleiberg, N. Kemeny, P. Rougier and H. Wilke, “Colorectal Cancer: A Clinical Guide to Therapy,” Martin Dunitz, London, 2002, pp. 419-429.
- C. Erlichman, S. Fine and T. Elhakim, “Plasma Pharmacokinetics of 5-FU Given by Continuous Infusion with Allopurinol,” Cancer Treatment Reports, Vol. 70, No. 7, 1986, pp. 903-904.
- M. Kawai, J. Rosenfeld, P. McCulloch and B. L. Hillcoat, “Letter: Blood Levels of 5-Fluorouracil during Intravenous Infusion,” British Journal of Cancer, Vol. 33, No. 3, 1976, pp. 346-347. doi:10.1038/bjc.1976.51
- J. G. Wagner, J. W. Gyves, P. L. Stetson, S. C. WalkerAndrews, I. S. Wollner, M. K. Cochran et al., “SteadyState Nonlinear Pharmacokinetics of 5-Fluorouracil during Hepatic Arterial and Intravenous Infusions in Cancer Patients,” Cancer Resarch, Vol. 46, No. 3, 1986, pp. 1499-1506.
- P. A. Di, M. Lencioni, F. Amatori, D. S. Di, G. Bocci, C. Orlandini, et al., “5-Fluorouracil Pharmacokinetics Predicts Disease-Free Survival in Patients Administered Adjuvant Chemotherapy for Colorectal Cancer,” Clin Cancer Resarch, Vol. 14, No. 9, 2008, pp. 2749-2755. doi:10.1158/1078-0432.CCR-07-1529
- B. L. Hillcoat, P. B. McCulloch, A. T. Figueredo, M. H. Ehsan and J. M. Rosenfeld, “Clinical Response and Plasma Levels of 5-Fluorouracil in Patients with Colonic Cancer Treated by Drug Infusion,” British Journal of Cancer, Vol. 38, No. 6, 1978, pp. 719-724. doi:10.1038/bjc.1978.278
- D. I. Jodrell, M. Stewart, R. Aird, G. Knowles, A. Bowman, L. Wall et al., “5-Fluorouracil Steady State Pharmacokinetics and Outcome in Patients Receiving Protracted venous Infusion for Advanced Colorectal Cancer,” British Journal of Cancer, Vol. 84, No. 5, 2001, pp. 600-603. doi:10.1054/bjoc.2000.1664
- E. Gamelin and M. Boisdron-Celle, “Dose Monitoring of 5-Fluorouracil in Patients with Colorectal or Head and Neck Cancer—Status of the Art,” Critical Reviews in Oncology/Hematology, Vol. 30, No. 1, 1999, pp. 71-79. doi:10.1016/S1040-8428(98)00036-5
- G. Milano, M. C. Etienne, E. Cassuto-Viguier, A. Thyss, J. Santini, M. Frenay, et al., “Influence of sex and age on fluorouracil clearance,” Journal of Clinical Oncology, Vol. 10, No. 7, 1992, pp. 1171-1175.
- M. W. Saif, A. Choma, S. J. Salamone and E. Chu, “Pharmacokinetically Guided Dose Adjustment of 5-Fluorouracil: A Rational Approach to Improving Therapeutic Outcomes,” Jouranl of the National Cancer Institure, Vol. 101, No. 22, 2009, pp. 1543-1552. doi:10.1093/jnci/djp328
- J. H. Beumer, M. Boisdron-Celle, W. Clarke, J. B. Courtney, M. J. Egorin, E. Gamelin, et al., “Multicenter Evaluation of a Novel Nanoparticle Immunoassay for 5-Fluorouracil on the Olympus AU400 Analyzer,” Therapeutic Drug Monitoring, Vol. 31, No. 6, 2009, pp. 688-694.
- M. Ychou, W. Hohenberger, S. Thezenas, M. Navarro, J. Maurel, C. Bokemeyer, et al., “A Randomized Phase III Study Comparing Adjuvant 5-Fluorouracil/Folinic Acid with FOLFIRI In Patients Following Complete Resection of Liver Metastases from Colorectal Cancer,” Annuls of Oncology, Vol. 20, No. 12, 2009, pp. 1964-1970. doi:10.1093/annonc/mdp236
- E. Gamelin, M. Bosdron-Celle, V. Guerin-Meyer, A. Poirier, V. Berger and A. Morel, “5-FU Dose Monitoring and Prevention of Oxaliplatin-Induced Neurotoxicity in FOLFOX 4 Regimen: Results of a Phase II Study,” Proceedings of American Society of Clinical Oncology, Chicago, 30 May-3 June 2008.
- E. Gamelin, O. Capitain, A. Morel, D. Luet, V. GuierinMeyer and M. Boisdron-Celle, “Comparison of Two Patient Cohorts Treated in Parallel for Advanced Colorectal Cancer with a Simplified FOLFOX 4 Regimen with or without 5-FU Therapeutic Dose Management,” Proceedings of American Society of Clinical Oncology, Orlando, 29 May-2 June 2009.
- J. L. Grem, N. McAtee, S. M. Steinberg, J. M. Hamilton, R. F. Murphy, J. Drake, et al., “A Phase I Study of Continuous Infusion 5-Fluorouracil plus Calcium Leucovorin in Combination with N-(phosphonacetyl)-L-aspartate in Metastatic Gastrointestinal Adenocarcinoma,” Cancer Research, Vol. 53, No. 20, 1993, pp. 4828-4836.
- J. M. Kirkwood, W. Ensminger, A. Rosowsky, N. Papathanasopoulos and E. Frei III, “Comparison of Pharmacokinetics of 5-Fluorouracil and 5-Fluorouracil with Concurrent Thymidine Infusions in a Phase I Trial,” Cancer Resaerch, Vol. 40, No. 1, 1980, pp. 107-113.
- M. Ychou, J. Duffour, A. Kramar, C. Debrigode, S. Gourgou, F. Bressolle, et al., “Individual 5-FU Dose Adaptation in Metastatic Colorectal Cancer: Results of a Phase II Study Using a Bimonthly Pharmacokinetically Intensified LV5FU2 Regimen,” Cancer Chemotherapy and Pharmacology, Vol. 52, No. 4, 2003, pp. 282-290. doi:10.1093/annonc/mdp236
- M. Blaschke, S. Cameron, K. Emami, J. Blumberg, U. Wegner, M. Nischwitz, et al., “Measurement of 5-FU Plasma Levels in Patients with Advanced Cancer: Correct Approach to Practical Procedures Is Essential,” International Journal of Clinical Pharmacology, Therapy, Vol. 49, No. 1, 2011, pp. 83-85.
- J. Saam, G. C. Critchfield, S. A. Hamilton, B. B. Roa, R. J. Wenstrup and R. R. Kaldate, “Body Surface AreaBased Dosing of 5-Fluoruracil Results in Extensive Interindividual Variability in 5-Fluorouracil Exposure in Colorectal Cancer Patients on FOLFOX Regimens,” Clinical Colorectal Cancer, Vol. 10, No. 3, 2011, pp. 203- 206. doi:10.1016/j.clcc.2011.03.015
- C. M. Walko and H. L. McLeod, “Will We Ever Be Ready for Blood Level-Guided Therapy?” Journal of Clinical Oncology, Vol. 26, No. 13, 2008, pp. 2078-2079. doi:10.1200/JCO.2007.14.9609
- D. L. Trump, M. J. Egorin, A. Forrest, J. K. Willson, S. Remick and K. D. Tutsch, “Pharmacokinetic and Pharmacodynamic Analysis of Fluorouracil during 72-Hour Continuous Infusion with and without Dipyridamole,” Journal of Clinical Oncology, Vol. 9, No. 11, 1991, pp. 2027-2035.
- J. L. Grem, L. K. Yee, B. Schuler, J. M. Hamilton, A. P. Chen, C. Chabuk, et al., “N-(phosphonacetyl)-L-aspartate and Calcium Leucovorin Modulation of Fluorouracil Administered by Constant Rate and Circadian Pattern of Infusion over 72 Hours in Metastatic Gastrointestinal Adenocarcinoma,” Annals of Oncology, Vol. 12, No. 11, 2001, pp. 1581-1587. doi:10.1023/A:1013185125186
- M. Ducreux, D. Malka, J. Mendiboure, P. L. Etienne, P. Texereau, D. Auby, et al., “Sequential versus Combination Chemotherapy for the Treatment of Advanced Colorectal Cancer (FFCD 2000-05): An Open-Label, Randomised, Phase 3 Trial,” The Lancet Oncology, Vol. 12, No. 11, 2011, pp. 1032-1044. doi:10.1016/S1470-2045(11)70199-1
- M. Koopman, N. F. Antonini, J. Douma, J. Wals, A. H. Honkoop, F. L. Erdkamp, et al., “Sequential versus Combination Chemotherapy with Capecitabine, Irinotecan, and Oxaliplatin in Advanced Colorectal Cancer (CAIRO): A Phase III Randomised Controlled Trial,” Lancet, Vol. 370, No. 9582, 2007, pp. 135-142. doi:10.1016/S0140-6736(07)61086-1
- M. Seymour, “Sequential Chemotherapy for Colorectal Cancer,” The Lancet Oncology, Vol. 12, No. 11, 2011, pp. 987-988. doi:10.1016/S1470-2045(11)70233-9
- M. T. Seymour and C. J. A. Punt, “Sequential Chemotherapy for Advanced Colorectal Cancer: Should We Ever Start with a Sincgle Cytotoxic Agent?” Current Colorectal Cancer Reports, Vol. 4, No. 3, 2009, pp. 130- 138. doi:10.1007/s11888-008-0022-1
NOTES
*Corresponding author.

