Chinese Medicine
Vol.4 No.3(2013), Article ID:37180,10 pages DOI:10.4236/cm.2013.43013
Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on the Differential Expression of GABAA Receptor α Subunits in Hypothalamus of Precocious Puberty Female Rats*
1Department of Integrative Medicine and Neurobiology, Institute of Acupuncture Research, Institutes of Brain Science, State Key Laboratory of Medical Neurobiology, Shanghai Medical College of Fudan University, Shanghai, China
2Department of Integrative Medicine, Children’s Hospital of Fudan University, Shanghai, China
Email: #tianvv@shmu.edu.cn
Copyright © 2013 Jing Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 8, 2013; revised August 9, 2013; accepted August 17, 2013
Keywords: GABA; GABAA receptor α subunit; precocious puberty; Nourishing “Yin”-Removing “Fire” Chinese herb mixture; rat
ABSTRACT
GABAergic input to Gonadotropin-releasing hormone (GnRH) neurons is necessary to initiate the onset of puberty and its action mainly depends on GABAA receptor of which the subunit composition, properties and consequently function varies during this period. Nourishing “Yin”-Removing “Fire” Chinese herb mixture, a Chinese herb-based formulation, has been proved that it may retard the initiation of pubertal development in female precocious puberty rats. Our objective is to investigate the effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on the expression of GABAA receptor α subunits in hypothalamus. Female Sprague-Dawley rats were divided into normal (N), precocious puberty model (M) induced by danazol, model exposed to saline (MS) and model exposed to Chinese herb mixture (CHM) groups. All rats were administered by the Chinese herb mixture from P15 on. Coefficients of reproductive organs and serum gonadotropins and estradiol levels in M were significantly enhanced while they were significantly decreased in CHM. The hypothalamic GnRH mRNA was also significantly increased in M and in CHM, as well as ERα mRNA. At the mean time, the hypothalamic GABAA receptor α1 and α3 subunits mRNA were more significantly decreased in M than those of N, while they were more significantly enhanced in CHM than those in M (p < 0.01), the protein expression of which in hypothalamus had the same trend as the mRNA expression. The evidence suggests that Nourishing “Yin”-Removing “Fire” Chinese herb mixture could significantly retard the sexual development of the precocious rats, and up-regulate the expressions of hypothalamic GABAA receptor α1 and α3 subunits. Our result indicated that GABAA receptor α1 and α3 subunits might involve in the effective treatment of herb mixture on idiopathic precocious puberty.
1. Introduction
Precocious puberty is defined as the onset of puberty before age of 8 years in girls and 9 years in boys. Precocious puberty in general is more frequent in girls, of which the majority have idiopathic precocious puberty whose etiology is unknown [1]. It has been well established that pulsatile GnRH release played a crucial role in triggering the onset of puberty [2]. GABA is one of the primary inhibitory neurotransmitters in the regulatory network of GnRH and contributes a great deal modulating pubertal progress via its receptors in the hypothalamus [3,4]. GABA receptors are widely distributed throughout the central nervous system where they predominantly mediate inhibitory neurotransmission. Specific to the hypothalamus, studies supported that GABA, particularly through its action on GABAA receptor, regulated firing of pubertal GnRH neurons [5].
GABAA receptor includes six α, three β, three γ, one δ, one ε, one π, one θ and three ρ subunits, of which each composition form involves at least one α subunit [6]. It is revealed that the pattern of each GABAA receptor subunit is complex and differs from phase to phase and region to region in mammal species, which ensures the functional heterogeneity of GABA input throughout the life span [7]. There was a normal switch from some GABAA receptor α subunits highly expressed during development (α2, α3, α4) to those subunits highly expressed in adulthood (α1) in rats model [8]. And compared with adult subunits mRNA expression in GnRH neurons of female mice, juvenile GnRH neurons expressed a much more heterogenous population of GABAA receptors that α5, β1, and γ2 were the most frequently co-expressed except α4 and γ1 [9]. In addition, it has been reported that α2 subunit expression is very high before birth to shortly after birth, and decreases gradually to adult levels, whereas α1 expression is minimal before birth and increases after birth until adulthood [10,11]. Examination of GABAA receptor subunit expression in GnRH neurons of hypothalamus supports this trend of age-associated going on during pubertal development [5].
Nourishing “Yin”-Removing “Fire” Chinese herb mixture has been successfully used for the management of idiopathic precocious puberty for a long time. It is reported that it could significantly alleviate the symptoms on precocious patients whose serum gonadotropins and estrogen reduce and secondary sexual characteristics subside. And a series of experiments on female precocious puberty rats model supported that Chinese herb mixture could regulate the function of hypothalamus-pituitarygonadal axis (HPGA) through modulating the gene and protein expressions of GnRH, kisspeptin, GABA and glutamate [12,13]. However, the mechanism of GABA receptor subunits participates in the Chinese herb mixture curative effects of precocious puberty is merely mentioned. We hypothesized that GABAA receptor subunit composition might involve the onset of precocious puberty. The present work was to observe the effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on the expression of GABAA receptor α subunit mRNA in Danazol induced female precocious puberty rats and to explore the possibility of GABAA receptor participating in advance GnRH release in precocious puberty.
2. Methods
2.1. Animal
Female Sprague-Dawley rats at 3 days of age were purchased from Medical Experimental Animals Center of Chinese Academy of Sciences (Shanghai, China). Animals were housed under laminar flow in an isolated room with controlled temperature of about 22˚C under a 12-h light/dark cycle with lights on from 7:00 am to 7:00 pm. The model litters at P5 were given a single subcutaneous injection of 300 µg of danazol (Hualian Pharm Ltd., Shanghai, China) dissolved in 25 µl of propylene glycolethanol (1:1, v/v), and allowed to grow without further treatment, the vehicles were administered with the same volume of propylene glycol-ethanol (1:1, v/v). Animals were weaned on P21, and then vaginal opening was examined daily afterwards, and the daily vaginal smears were examined. All experiments procedures involving the use of animals were conducted in accordance with NIH Guidelines and were reviewed and approved by Animal Use and Care Committee for the Fudan University.
All rats were randomly divided into normal (N), precocious puberty model (M), model exposed to saline (MS) and model exposed to Nourishing “Yin”-Removing “Fire” Chinese herb mixture (CHM) four groups. From P15, rats in CHM and MS were fed with CHM (including crude drug 3.3 g per ml) or the same volume normal saline every day. The intragastric dose increased by about 0.1 ml per day as the rat grew.
At the day of vaginal opening in M, rats of all four groups were decapitated with blood and hypothalamus tissue collected, the uterus and ovaries were dissected out of the surrounding fat, and the organ coefficients (mg/100g) were evaluated.
2.2. Hormone Measurement by RIA
At the time of decapitate, the blood of all rats was collected. The serum was separated by centrifugation and stored at −80˚C until assayed. The gonadotropins and oestradiol levels were measured by double-antibody RIA kits purchased from the Beijing Sinouk Institute of Biological Technology (Beijing, China). The samples were assayed in duplicate, and all the subjects’ samples were assayed together. The sensitivity for the E2 was less than 5 pg/ml; the intraand inter-assay coefficients were less than 10% and 15.2%, respectively. For LH, the assay sensitivity was 0.2mIU/ml, and the intraand inter-assay coefficient of variation was 2.0% - 2.4% and 4.2% - 7.5%, respectively. For FSH, the assay sensitivity was 0.25 mIU/ml, and the intraand inter-assay coefficient of variation was 2.2% - 2.5% and 3.7% - 8.7%, respectively.
2.3. Tissue Collection and Total RNA Preparation
The rat brains in every group (n = 5) were rapidly removed and hypothalamus were separated immediately and frozen in liquid nitrogen. The target regions, including mediobasal hypothalamus and the suprachiasmaticpreoptic areas were dissected. Total hypothalamic RNA was extracted by “Trizol Regent” (Invitrogen Inc., America) according to the manufacturer’s instructions. The concentration of RNA was estimated by spectrophotometry with UV absorbance at 260 nm and 280 nm. The purity and integrity of the RNA were checked spectroscopically and by gel electrophoresis before carrying out the analytical procedures.
2.4. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The total RNA from each tissue was reverse transcribed using M-MuLV reverse transcriptase (Superscript II, Promega) and 2.5 mM oligo dT (BioDev-Tech) in the presence of 250 mM of deoxynucleotides triphosphate in a final volume of 12.5 ml at 37˚C for 1 h. The reaction mixture was denatured for 5 min at 95˚C and stored at −70˚C. The transcribed DNA for each subunit was amplified by PCR in a thermal cycler using specific primers for each subunit. The primers were designed in order to amplify DNA sequences (ERα, sense: 5’-AATTCTGACAATCGACGCCAG-3’; antisense: 5’-GTGCTTCAACATTCTCCCTCCTC-3’. β-actin, sense: 5’-AAGCAGGAGTATGACGAGTCCG-3’, Antisens: 5’-GCCTTCATACATCTCAGGTTGG-3’). The amplification mixture contained 1.5 U of Taq PCR Master Mix (Shanghai Lifefeng Biotechnology) and 20 μM of specific primer pairs in a final volume of 20 ml. The mixture was amplified for 30 cycles. Each amplification cycle consisted of 5 min of denaturation at 95˚C to activate the enzyme and 1 min denaturation at 95˚C then 1 min at 58˚C for annealing and 1 min at 72˚C. The final extension step was 15 min at 72˚C. The relative abundance of each product was estimated by the visual intensity of ethidium bromide stained bands under UV light.
2.5. Real-Time Reverse Transcriptase-PCR (qRT-PCR)
Prior to conducting real-time reverse transcriptase-PCR (qRT-PCR), the total RNA was digested with RNase-free DNase I (Invitrogen, Carlsbad, CA), by which possible contamination of genomic DNA. The SuperScrip III reverse transcription system (Invitrogen Corp., Carlsbad, CA, USA) was used for reverse transcription with 2 μg of total RNA according to the manufacturer’s specifi- cations. The primers used for GABAA receptor α subunits and GnRH were designed and synthesized by Invitrogen with standard purity. To determine the sensitivity and efficiency of the amplification, PCR assay linearity ranges were previously established for each gene cDNA. Quantitative Real-Time PCR was carried out in IQ5 Real-time PCR system (Bio-Rad). The amplification protocol was as follows: an initial denaturing step at 95˚C for 2 min followed by 40 cycles of a 95˚C for 10 sec, 60˚C for 30 sec, and 72˚C for 30 sec. Following amplification, a dissociation curve analysis was performed to insure purity of PCR products. All real-time experiments were run in triplicate and a mean value was used for the determination of mRNA levels. The relative linear quantity of the target gene was calculated using the formula 2−ΔΔCt. Therefore, the data were expressed an n-fold change in gene expression normalized to a reference gene (β-actin) and relative to a calibrator sample. The primer sequences and realtime PCR conditions for GABAA receptor α subunits and GnRH mRNA were listed in Table 1.
2.6. Western Blot
Each of six rats in four groups was used to investigate GABAA receptor α1 and α3 subunits protein expression by western blot with a standard procedure. For total protein extraction, hypothalamus was homogenized in lysate (BioDev-Tech. Co., Ltd) and protease inhibitors using a polytron homogenizer. After homogenization, samples were centrifuged at 10,000 rpm for 15 min at 4˚C. The supernatant fraction was the total tissue lysate. Protein concentration in the lysate was determined by a total protein measurement kit from BioDev-Tech. Protein samples were denatured in loading buffer and separated on a sodium dodecyl sulfate-polyacrylamide gel after loading equal amount of protein in each lane. Separated proteins were transferred to PVDF membrane at 100 V for 110 mins in 25 mM Tris-glycine buffer, pH 8.3, 10% methanol using a Transblot apparatus (Bio-Rad Laboratories, Inc.). Following the transfer, the membranes were rinsed five times with TTBS (20 mM Tris, 0.1% Tween- 20) for 10 min each rinse and then incubated with 5% BSA for 4 h at 4˚C to block non specific/unbound surface. The membrane was incubated overnight with polyclonal rabbit anti-rat (1:200; Millipore). The membrane was then washed with TTBS to remove unbound antibody, followed by incubation with secondary HRP-conjugated sheep anti-rabbit IgG (1:2000, Millipore) for 1 h at room temperature. The signal was detected using an ECL detection kit (GE Healthcare) and the membranes were exposed to ImageQuant LAS 4000 mini (GE Healthcare).
2.7. Statistics Analysis
All data are presented as means ± SEM. Statistical analysis was performed on raw data using one-way analysis of variance (ANOVA), with the significance concentrations of P < 0.05 in two-tailed testing chosen. Comparisons among groups were made using the Student’s t-test.
3. Results
3.1. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on the Timing of Vaginal Opening
On P22 at the beginning of observation, one third of the

Table 1. Primer sequences of GABAA receptor α1-6 subunits and GnRH in realtime PCR.
rats in M and MS had showed vaginal opening, while few in N and CHM did. The discrepancy in the proportion of vaginal opening rats in groups sustained from then on till P44 when all rats had steady-state sexual cycles (Figure 1.)
3.2. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on Serum E2, LH and FSH Levels
The concentration of serum E2, LH and FSH in M were significantly higher than those of N (p < 0.01). And the serum E2 and LH levels were significantly lower in CHM than those in M (p < 0.01, respectively), and FSH level were also lower in CHM than that in M (p < 0.05). There were not statistically significant differences observed between the M and MS (Figure 2.).
3.3. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on Reproductive Organs’ Coefficients
Table 2 shows changes in reproductive organs’ coefficients of the different rat groups. On P22 the uterus coefficient of M were 80.18 ± 2.19 mg/100g which was significant increased than those of N (59.78 ± 1.30 mg/100g), and the uterus coefficient of CHM were significantly decreased to 58.62 ± 1.72 mg/100g (p < 0.01, respectively). Meanwhile the ovaries coefficient of M were 5.87 ± 0.38 mg/100g which were also increased significantly compared to those of N, and the coefficients of CHM were decreased to 4.36 ± 0.22 mg/100g (p < 0.05, respectively). These reproductive organ coefficients on P26 were similar to those at P22 (p < 0.05, respectively).
3.4. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on Hypothalamic Expression of GnRH mRNA by Realtime PCR
Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on the expression of GnRH mRNA were detected by realtime PCR. Relative mRNA levels for GnRH were determined by the 2−ΔΔCt method and normalized to the β-actin mRNA. Briefly, the mean ΔCt of the N was used as an internal calibrator when comparing the mRNA quantities of GnRH in other groups. Hypothalamic GnRH mRNA expression of M was significantly elevated compared with that of N (p < 0.01). Hypothalamic GnRH mRNA expression was down-regulated in CHM compared to M (p < 0.01). And there were no significant differences between M and MS groups (Figure 3).
3.5. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on the Expression of GABAA Receptor α1-6 Subunits by Realtime PCR
Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on expression of GABAA receptor α1-6 subunits mRNA were detected by realtime PCR. Relative mRNA expression levels for GABAA receptor α1-6 subunits were determined by the 2−ΔΔCt method and normalized to the β-actin mRNA. Briefly, the mean ΔCt of the N was used as an internal calibrator when comparing the mRNA quantities of GABAA receptor α1-6 subunits in other three groups... RT-PCR analysis showed that of all six α subunits of GABAA receptor only α1 and α3 mRNA
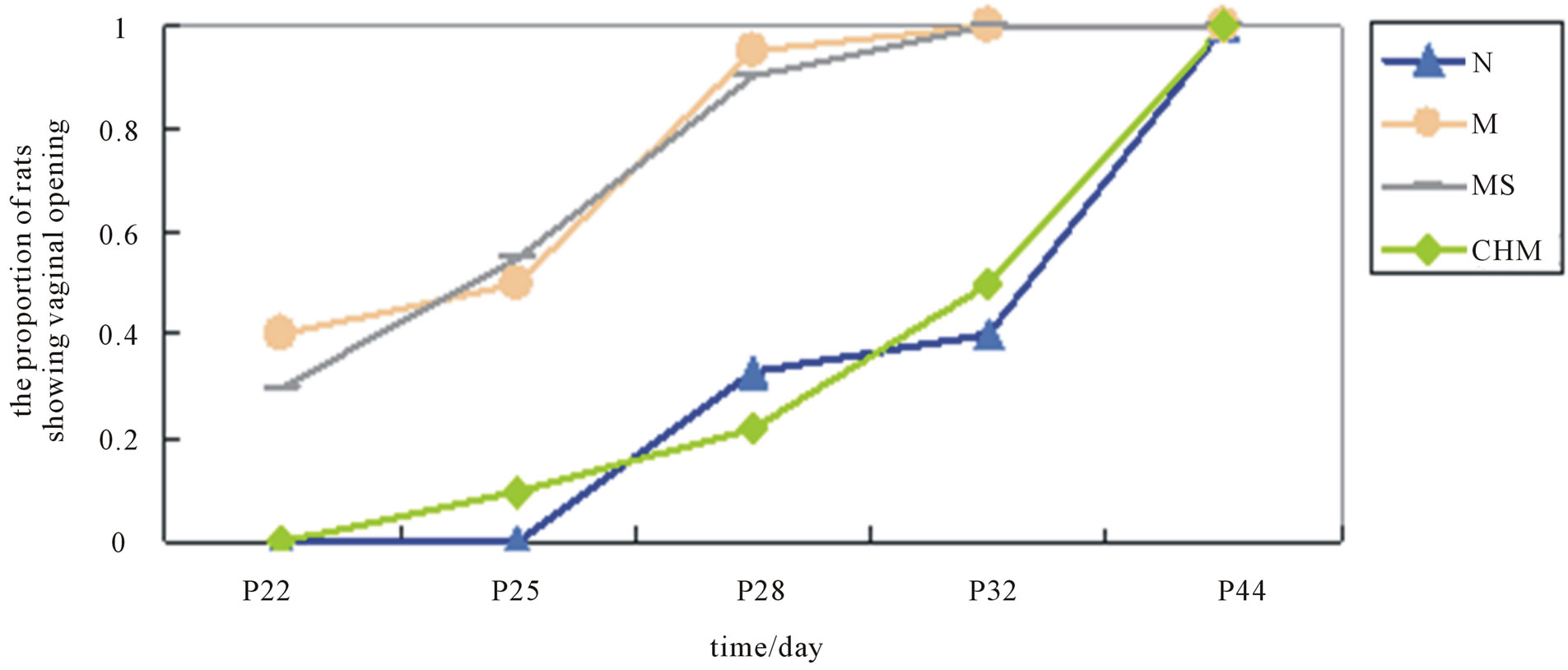
Figure 1. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on the timing of vaginal opening. There were less rats in N and CHM showing vaginal opening than in M and MS at the same time since P22. The discrepancy in the proportion of vaginal opening rats in groups sustained from then on till P44 when all rats had steady-state sexual cycles.
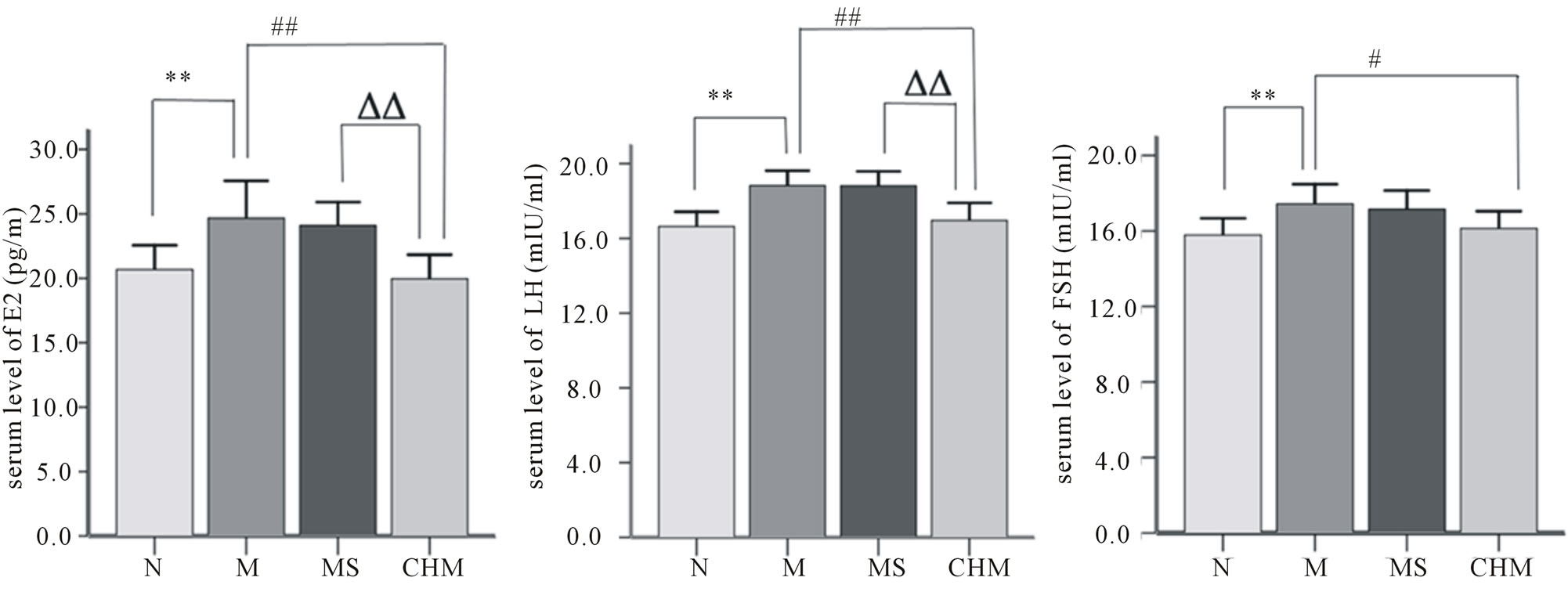
Figure 2. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on serum E2, LH and FSH levels. The serum level of E2 (p < 0.01), LH (p < 0.01) and FSH (p < 0.05) significantly increased in M compared with N, and they were decreased in CHM. N: normal, M: model, MS: saline and CHM: Chinese herb mixture. **p < 0.01 vs N; #p < 0.05 vs M; ##p < 0.01 vs M; ΔΔp < 0.01 vs MS.
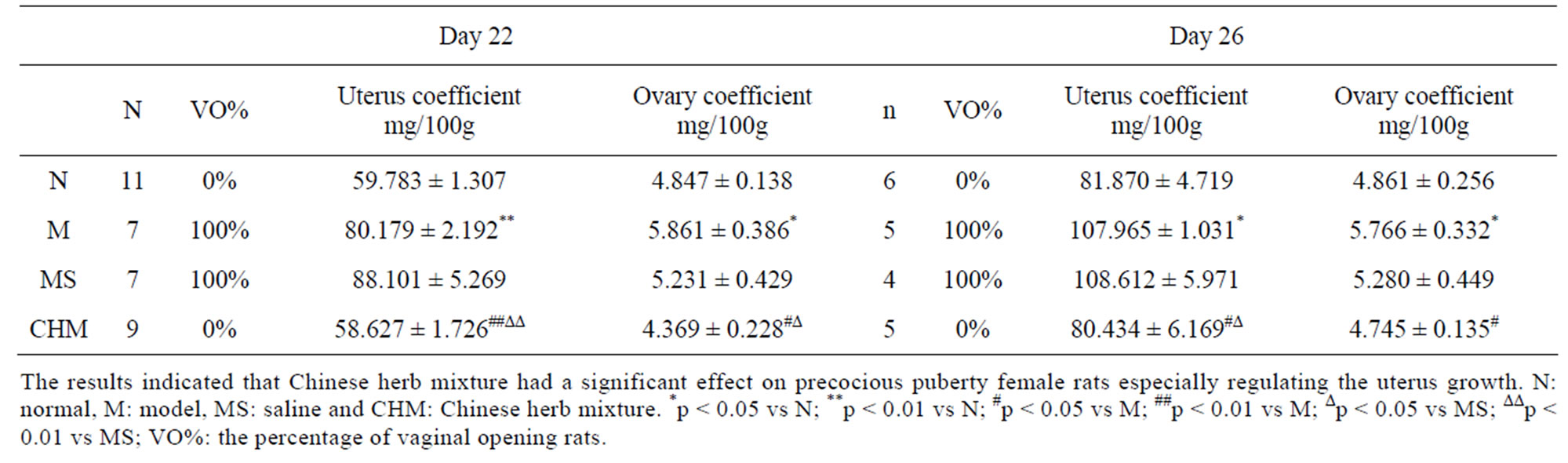
Table 2. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on uterus and ovary coefficient.
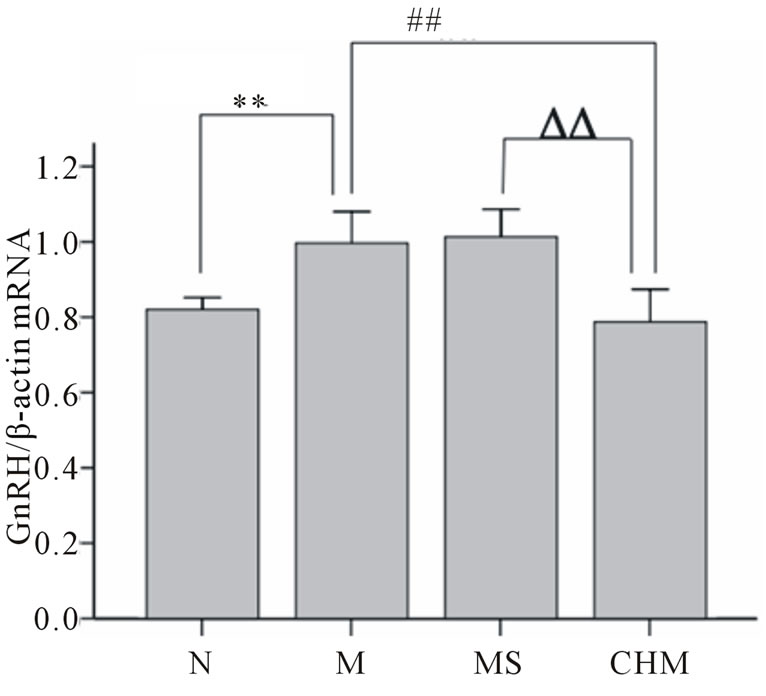
Figure 3. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on GnRH mRNA expression. The expression of GnRH mRNA was decreased in M, and increased in CHM rats. N: normal, M: model, MS: saline and CHM: Chinese herb mixture. **p < 0.01 vs N; ##p < 0.01 vs M; ΔΔp < 0.01 vs MS.
in hypothalamus decreased significantly in M compared to those of N; and also only α1 and α3 subunits mRNA expression were significantly increased in CHM compared to those in M. there were no statistical differences of other subunits mRNA expression among four groups (Figure 4).
3.6. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on the Expression of GABAA Receptor α1 and α3 Subunits by Western Blot
According to Figure 5, α1 and α3 subunits protein expression by western blot in hypothalamus decreased significantly in M compared to N; and increased in CHM compared to those in M (p < 0.01, respectively). There were no disparities between M and MS.
3.7. Effects of Nourishing “Yin”-Removing “Fire” Chinese Herb Mixture on the Expression of ERα mRNA by RT-PCR
The expression of ERα mRNA in the hypothalamus of precocious rats increased compared to N (p < 0.05) and after the administration of Nourishing “Yin”-Removing “Fire” Chinese herb mixture that of CHM had shown a significant decrease compared to M (p < 0.05). The upregulation of ERα mRNA refers to activation of negative-feedback regulation here (Figure 6).
4. Discussion
Puberty refers to the process of physical changes between childhood and adulthood during which the capacity to reproduce is obtained. Pubertal development occurs before the age of 8 years in girls and the age of 9 years in boys is defined as precocious puberty. Precocious puberty has a profound impact on growth, development and psychosocial well-being of the patients. As is generally accepted, GnRH neurons play an original role in the onset of puberty that represented the final output of the neuronal network controlling sexual maturation in all mammals [14,15]. The regulation of GnRH secretion is associated with a complex interplay with excitatory and inhibitory neurotransmitters and hormones within the hypothalamus included, among which GABA was a major inhibitory neurotransmitter in the hypothalamus [16]. The roles of GABA and GABAA receptor in stimulating GnRH pulsatile release triggering the onset of puberty have been in a general sense studied and established [17]. It has been recognized that the GABAA receptor subunits compositions undergo a series of developmental changes through the life time as well. But how the GABAA receptor subunits participate in this transition remains unclear, which has been rarely studied in a systematic study. Here we focus on the role of GABAA receptor α subunits in the puberty initiation and the effects of herb mixture on precocious puberty.
It has been documented that GABAA receptor subunits composition changed resulting in function heterogeneity at the onset of puberty [18], so we hypothesized that α subunits may undergo important changes in this period. In general sense the day of vaginal opening is taken as the token of puberty onset, and uterus and ovaries coefficients as the tokens of sexual development as well as serum levels of estrogens. According to the time of vaginal opening, hormone levels and sexual organ coefficients we supposed that after the regulation of Nourishing “Yin”-Removing “Fire” Chinese herb mixture, precocious rats got remission on the pathological process. The expression of GnRH and ERα mRNA showed significant increase in M compared to N and then decrease in CHM compared to M. What’s important, of all six α subunits, only α1 and α3 subunits were down-regulated in M when compared to those in N, while up-regulated in CHM. These changes showed in both mRNA expression and protein expression of GABAA receptor α1 and α3 subunits. We may conclude that GABAA receptor α subunits, especially α1 and α3 subunits may participate in the initiation of puberty and the regulation of Nourishing “Yin”-Removing “Fire” Chinese herb mixture. In summary, the down-regulation of GABAA receptor α1 and α3 subunits in precocious rat hypothalamus led to the change of affinity between GABA and GABAA receptor, that affected the inhibition process of GABA to GnRH. As a result the increasing level of GnRH referred to precocious puberty. After a period of Nourishing “Yin”- Removing “Fire” Chinese herb mixture administration all the pathological process above got reversed, and the
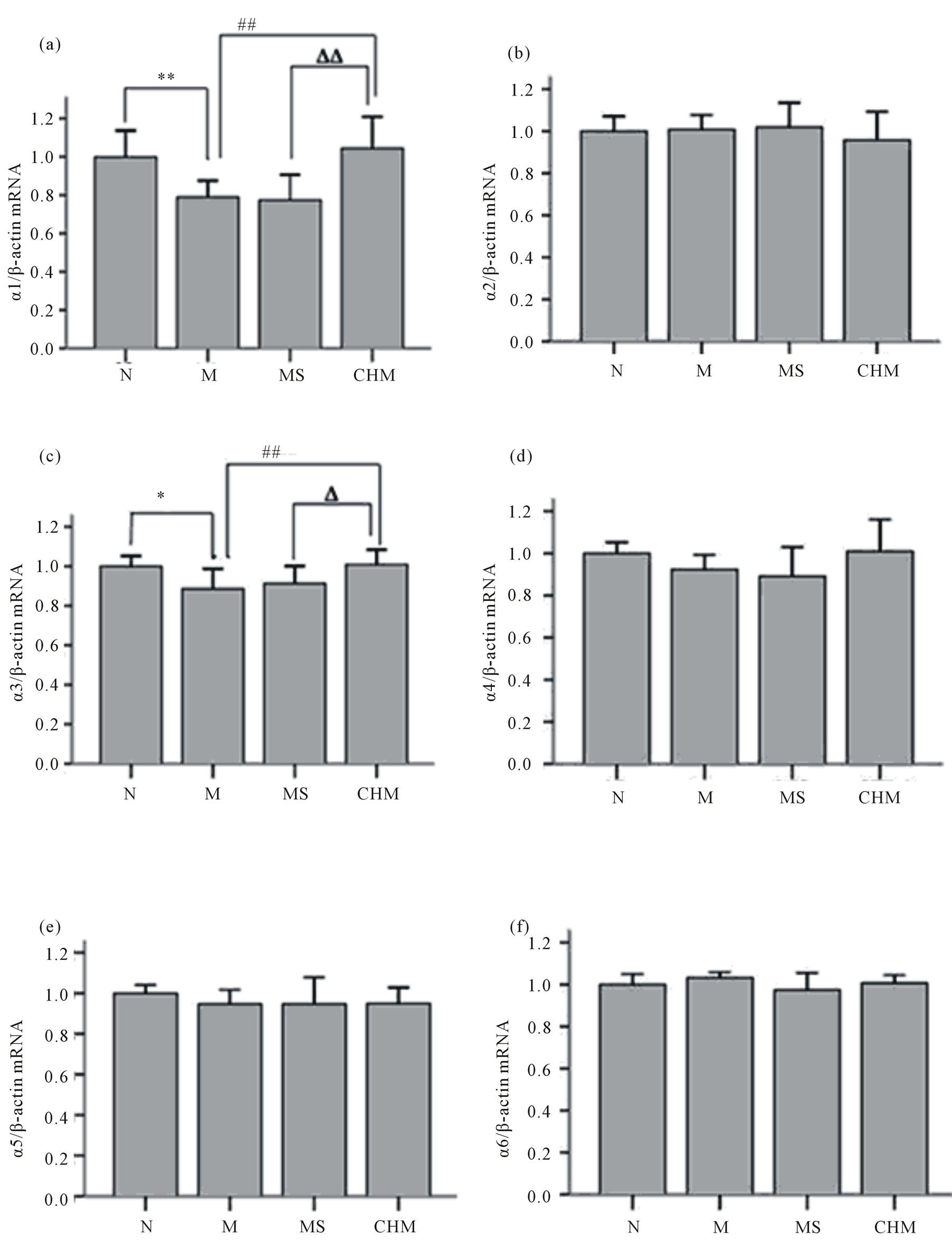
Figure 4. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on GABAA receptor α subunit mRNA expression by realtime PCR. Of all six subunits only α1 and α3 mRNA undergo changes between four groups. α1 and α3 expression in M both showed increased compared to N, and after the intervention of herb mixture that in TCM decreased compared to M. There were no statistical differences of the two mRNA expressions between M and MS. (a) GABAA receptor α1 subunit; (b) GABAA receptor α2 subunit; (c) GABAA receptor α3 subunit; (d) GABAA receptor α4 subunit; (e) GABAA receptor α5 subunit; (f) GABAA receptor α6 subunit. N: normal, M: model, MS: saline and CHM: Chinese herb mixture. *p < 0.05, vs N; **p < 0.01 vs N; ##p < 0.01 vs M; Δp < 0.05 vs MS; ΔΔp < 0.01 vs MS.
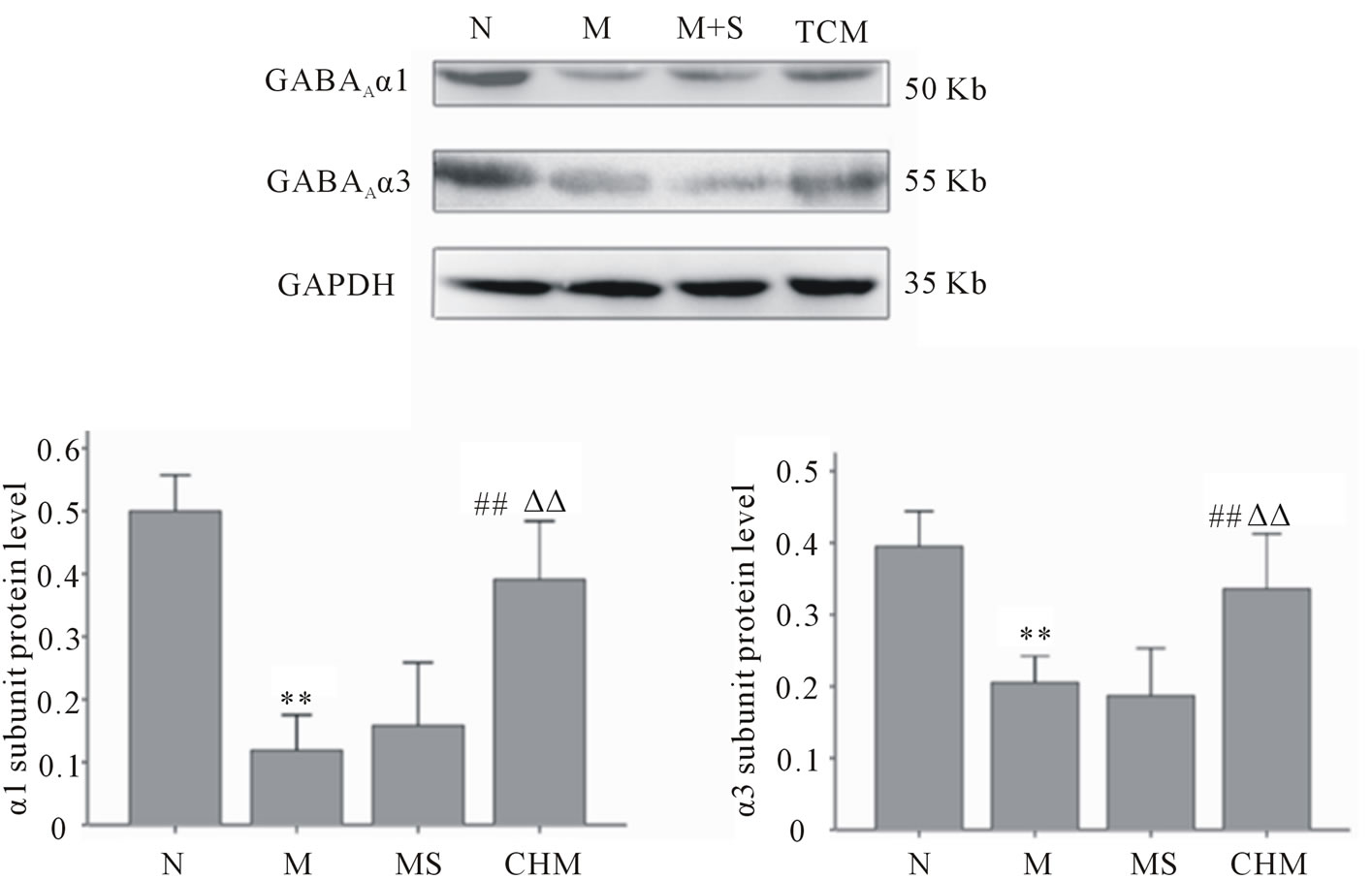
Figure 5. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on GABAA receptor α1 and α3 subunits protein expression by Western Blot. The subunits participated the regulation at onset of puberty including only α1 and α3 according to Figure 4. α1 (p < 0.01) and α3 (p < 0.05) were down-regulated in PP and up-regulated under the influence of Chinese herb mixture. N: normal, M: model, MS: saline and CHM: Chinese herb mixture. **p < 0.01 vs N; ##p < 0.01 vs M; ΔΔp < 0.01 vs MS.
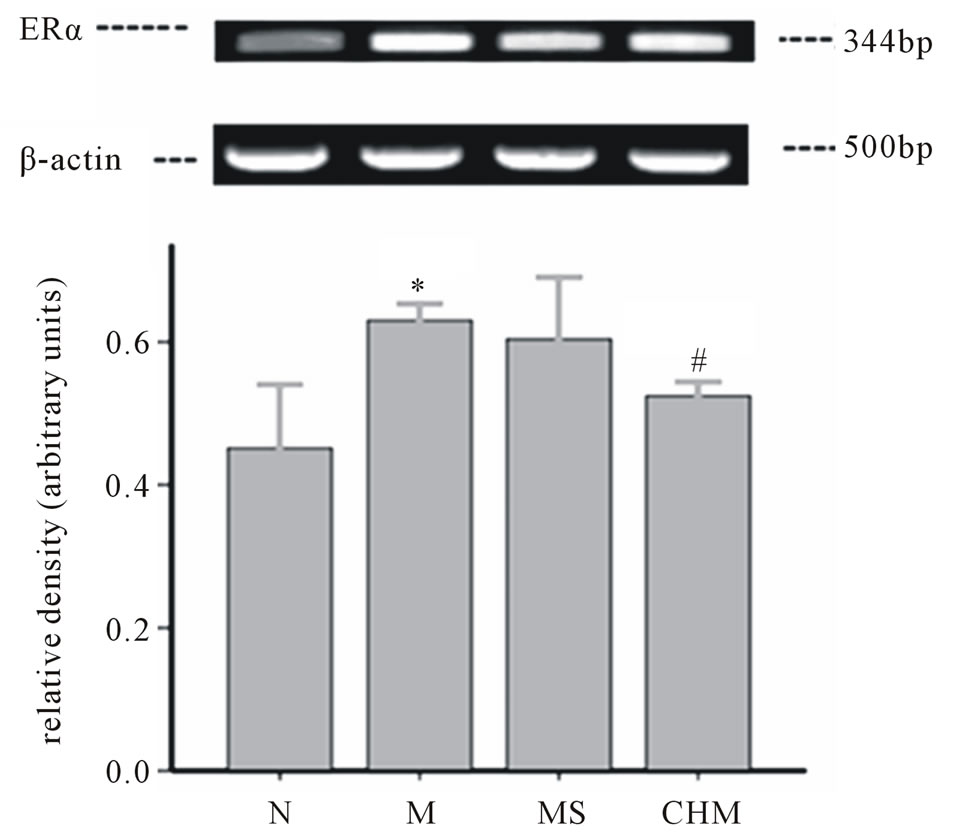
Figure 6. Effects of Nourishing “Yin”-Removing “Fire” Chinese herb mixture on ERα mRNA of female precocious pubertal rats. The expression of ERα mRNA increased in M (p < 0.05) compared to N, and that in TCM decreased compared to M (p < 0.05). N: normal, M: model, MS: saline and CHM: Chinese herb mixture. *p < 0.05 vs N; #p < 0.05 vs M.
symptoms relieved.
Nourishing “Yin” -removing “Fire” Chinese herb mixture was used for the management of IPP since last 80s’ [19,20]. And experiments on precocious puberty rats also achieved great progresses on the mechanism of Nourishing “Yin”-Removing “Fire” Chinese herb mixture regulating precocious puberty. It was reported that Chinese herb mixture might regulate the abnormal function of HPGA in precocious puberty through down-regulate the hypothalamus kiss-1/PGR54 expression, and inhibit GnRH expression and release [21,22]. Another paper showed that the Chinese herb mixture could regulate the gonadotrophic and somatotrophic functions of HPGA through modulating the neuroendocrine regulation and the gene expressions of GnRH in hypothalamus, growth hormone, FSH and LH in pituitary, and IGF2 Ι in metaphysic that may be the mechanisms of herb mixture in modulating the course of pubertal development and ameliorating the skeletal development in precocious puberty children [23,24]. Different from the experimental results about the GABAA receptor α expression changing during the puberty, now our experiment concluded that the GABAA receptor α1 and α3 subunits may take part in the mechanism of the Nourishing “Yin”-Removing “Fire” Chinese herb mixture on precocious puberty. All those studies could help us to know more about how the Chinese herb medicine takes effects on precocious patients.
5. Conclusion
The nourishing “Yin”-Removing “Fire” Chinese herb mixture may significantly delay the sexual development of the precocious puberty rat, through up-regulated the expression of GABAA receptor α1 and α3 subunits in precocious puberty rat. Based on above, GABAA receptor might be involved in the effective treatment of herb mixture on IPP.
REFERENCES
- S. A. DiVall and S. Radovick, “Endocrinology of Female Puberty,” Current Opinion in Endocrinology, Diabetes & Obesity, Vol. 16, No. 1, 2009, pp. 1-4. doi:10.1097/MED.0b013e3283207937
- S. R. Ojeda, A. Lomniczi, A. Loche, V. Matagne, G. Kaidar, U. S. Sandau and G. A. Dissen, “The Transcriptional Control of Female Puberty,” Brain Research, Vol. 1364, 2010, pp. 164-174. doi:10.1016/j.brainres.2010.09.039
- D. Mitsushima, D. L. Hei and E. Terasawa, “GABA Is an Inhibitory Neurotransmitter Restricting the Release of Luteinizing Hormone-Releasing Hormone before the Onset of Puberty,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 91, No. 1, 1994, pp. 395-399. doi:10.1073/pnas.91.1.395
- C. Roth, H. Schmidberger, M. Lakomek, O. Witt, W. Wuttke and H. Jarry, “Reduction of GABAergic Neurotransmission as a Putative Mechanism of Radiation Induced Activation of the Gonadotropin Releasing-Hormone-Pulse Generator Leading to Precocious Puberty in Female Rats,” Neuroscience Letters, Vol. 297, No. 1, 2001, pp. 45-48. doi:10.1016/S0304-3940(00)01663-3
- J. A. Maffucci and A. C. Gore, “Hypothalamic Neural Systems Controlling the Female Reproductive Life Cycle: Gonadatropin-Releasing Hormone, Glutamate and GABA,” International Review of Cell and Molecular Biology, Vol. 274, 2009, pp. 69-127. doi:10.1016/S1937-6448(08)02002-9
- E. A. Mitchell, M. B. Herd, B. G. Gunn, J. J. Lambert and D. Belelli, “Neurosteroid Modulation of GABAA Receptors: Molecular Determinants and Significance in Health and Disease,” Neurochemistry International, Vol. 52, No. 4-5, 2008, pp. 588-595. doi:10.1016/j.neuint.2007.10.007
- D. B. Matthews, L. L. Devaud, J. M. Fritschy, W. Sieghart and A. L. Morrow, “Differential Regulation of GABAA Receptor Gene Expression by Ethanol in the Rat Hippocampus versus Cerebral Cortex,” Journal of Neurochemistry, Vol. 70, No. 3, 1998, pp. 1160-1166. doi:10.1016/j.neuint.2007.10.007
- M. O. Poulter, L. A. Brown, S. Tynan, G. Willick, R. William and D. C. McIntyre, “Differential Expression of a1, a2, a3, and a5 GABAA Receptor Subunits in SeizureProne and Seizure-Resistant Rat Models of Temporal Lobe Epilepsy,” The Journal of Neuroscience, Vol. 19, No. 11, 1999, pp. 4654-4661.
- J. A. Sim, M. J. Skynner, J. R. Pape and A. E. Herbison, “Late Postnatal Reorganization of GABAA Receptor Signaling in Native GnRH Neurons,” European Journal of Neuroscience, Vol. 12, No. 10, 2000, pp. 3497-3504. doi:10.1046/j.1460-9568.2000.00261.x
- A. Hendrickson, D. March, G. Richards, A. Erickson and C. Shaw, “Coincidental Appearance of the α1 Subunit of the GABAA-Receptor and the Type I Benzodiazepine Receptor near Birth in Macaque Monkey Visual Cortex,” Neuroscience, Vol. 12, No. 4, 1994, pp. 299-314. doi:10.1016/0736-5748(94)90078-7
- J. M. Fritschy and H. Mohler, “GABAA-Receptor Heterogeneity in the Adult Rat Brain: Differential Regional and Cellular Distribution of Seven Major Subunits,” Journal of Comparative Neurology, Vol. 359, No. 1, 1995, pp. 154-194. doi:10.1002/cne.903590111
- H. Shen, D. P. Cai and B. Y. Chen, “Effects of KidneyTonifying Chinese Herbal Medicine on HypothalamusPituitary Gonadotrophic Function,” Chinese Journal of Integrative Medicine, Vol. 2, No. 1, 2004, pp. 53-57. doi:10.3736/jcim20040120
- Z. Z. Tian, H. Zhao, Y. Sun, D. Cai and B. Y. Chen, “Evaluation of the True Precocious Puberty Rats Induced by Neonatal Administration of Danazol: Therapeutic Effects of Nourishing ‘Yin’-Removing ‘Fire’ Chinese Herb Mixture,” Reproductive Biology and Endocrinology, Vol. 3, 2005, p. 38. doi:10.1186/1477-7827-3-38
- T. Iwasa, T. Matsuzaki, M. Murakami, S. Fujisawa, R. Kinouchi, G. Gereltsetseg, A. Kuwahara, T. Yasui and M. Irahara, “Effects of Intrauterine Undernutrition on Hypothalamic Kiss1 Expression and the Timing of Puberty in Female Rats,” The Journal of Physiology, Vol. 588, No. 5, 2010, pp. 821-829. doi:10.1113/jphysiol.2009.183558
- A. M. Izquierdo, F. D. Mishima, V. C. Carrard, M. Farina and M. da C. G. Nojima, “Effects of Induced Precocious Puberty on Cranial Growth in Female Wistar Rats,” European Journal of Orthodontics, Vol. 34, No. 2, 2011, pp. 133-140.
- E. Terasawa, “Role of GABA in the Mechanism of the Onset of Puberty in Non-Human Primates,” International Review of Neurobiology, Vol. 71, 2005, pp. 113-129. doi:10.1016/S0074-7742(05)71005-9
- P.-F. Justyna and S. M. Moenter, “Kisspeptin Increases γ-Aminobutyric Acidergic and Glutamatergic Transmission Directly to Gonadotropin-Releasing Hormone Neurons in an Estradiol-Dependent Manner,” Endocrinology, Vol. 151, No. 1, 2011, pp. 291-300.
- H. Shen and S. S. Smith, “Plasticity of the α4βδ GABAA Receptor,” Biochemical Society Transactions, Vol. 37, No. 6, 2009, pp. 1378-1384. doi:10.1042/BST0371378
- X. H. Qiao, J. Yu and X. T. Xie, “Case-control Study on Behavioral Problem of Girls with Earlier Onset of Sexual Development,” Chinese Mental Health Journal, Vol. 22, No. 4, 2008, pp. 249-252.
- R. Huang, Y. H. Wang and J. Yu, “Exploration of Regularity of TCM Syndrome Differentiation and Treatment of Precocious Puberty,” China Journal of Traditional Chinese Medicine and Pharmacy, Vol. 26, No. 2, 2011, pp. 347-349.
- Z. Z. Tian, H. Zhao and B. Y. Chen, “Effects of Chinese Herbal Medicine for Nourishing Yin and Purging Fire on mRNA Expression of Gonadotropin-releasing Hormone and Its Receptor in Precocious Puberty Model Rats,” Zhongguo Zhong Xi Yi Jie He Za Zhi, Vol. 23, No. 9, 2003, pp. 695-701.
- Y. Sun, G. N. Perry, J. Yu, B. Y. Chen and Z. Z. Tian, “Effect of Nourishing “Yin”-Removing “Fire” Chinese Herbal Mixture on Hypothalamic Kisspeptin Expression In Female Precocious Rats,” Journal of Ethnopharmacology, Vol. 127, No. 2, 2010, pp. 274-279. doi:10.1016/j.jep.2009.11.009
- D. Cai and W. Zhang, “Regulative Actions of the Chinese Drugs for Tonifying the Kidney on Gene Expression of the Hypothalamic GnRH, Pituitary FSH, LH and Osteoblastic BGP,” Journal of Traditional Chinese Medicine, Vol. 25, No. 1, 2005, pp. 58-61.
- D. P. Cai, B. Y. Chen, W. Zhang and P. Li, “Effects of Chinese Herbal Medicine on Modulating the Course of Puberty Development in Children with Precocious Puberty,” Journal of Chinese Integrative Medicine, Vol. 4, No. 2, 2006, pp. 166-174. doi:10.3736/jcim20060212
NOTES
*This project was financially supported by the National Natural Science Foundation (No. 30973814).
#Corresponding author.

