Chinese Medicine
Vol. 3 No. 3 (2012) , Article ID: 22709 , 8 pages DOI:10.4236/cm.2012.33021
Pharmacognostic Evaluation and Antioxidant Activity of Urtica dioica L.
Pharmacognosy & Ethnopharmacology Division, CSIR-National Botanical Research Institute, Lucknow, India
Email: pharmacognosy1@rediffmail.com
Received April 13, 2012; revised June 25, 2012; accepted July 4, 2012
Keywords: Antioxidant Activity; DPPH; Ferulic Acid; HPTLC; U. Dioica
ABSTRACT
Background: Urtica dioica L. is a common Himalayan species which produces allergenic substances causing oedema and inflammation in humans. It has become a source of folk medicine for the treatment of many diseases. The leaves and roots both are used internally as a blood purifier and diuretic and an infusion of the plant is used for nasal and menstrual haemorrhage, diabetes, rheumatism, eczema, anaemia, hair loss, as an expectorant and antidiarrhoeal. Present study includes pharmacognostic evaluation, antioxidant activity and HPTLC analysis of Urtica dioica L. Methods: Pharmacognostic evaluation of aerial part of U. dioica has been performed as per Indian pharmacopoeia. In vitro antioxidant evaluation of U. dioica has been performed using DPPH free radical scavenging activity. Ferulic acid, a potential phenolic antioxidant present in this species, has been studied through HPTLC. Results: U. dioica hydro-alcoholic extract shows positive results for antioxidant activity with IC50 value of 88.33 ± 2.88 µg/ml. Standard ascorbic acid showed IC50 value of 2.8 ± 0.62 µg/ml. Ferulic acid was identified at Rf 0.61 ± 0.01 and quantified to 0.73% in this species through CAMAG HPTLC analysis. Conclusion: The pharmacognostical parameters reported can be considered as quality standards of U. dioica in herbal industry. Hydro-alcoholic extract of U. dioica showed positive in vitro antioxidant activity. Presence of phenolic compound suggests that antioxidant activity may be due to ferulic acid content.
1. Background
U. dioica L. (Urticaceae) or stinging nettle traditionally employed as a folklore remedy for a wide spectrum of ailments [1]. The leaves and roots both are used internally as a blood purifier, diuretic. An infusion of the plant is used for nasal and menstrual haemorrhage, diabetes, rheumatism, eczema, anaemia, hair loss and as an antidiarrhoeal [2]. Leaves of this plant reported to be hypotensive [3,4], anti-inflammatory [5], to be useful in the therapy of prostatic hyperplasia [6,7], diuretic [8], immunomodulatory [9-12], to alleviate rheumatic pain [13], and to serve as an adjuvant therapeutic agent in rheumatoid arthritis [14].
U. dioica herbs are used to treat stomachache in Turkish folk medicine [15]. In addition, this herb is used to treat rheumatic pain and for colds and cough [16] and is used against liver insufficiency [17]. The extracts of U. dioica leaves and seeds are suggested to be useful for patients suffering from neutrophil function deficiency [18]. It was reported that U. dioica prevents damage in the rat liver [19]. Due to its high value medicinal usage, the present study was performed to establish the quality parameters of the herb.
2. Methods
2.1. Chemicals
All the chemicals used were of analytical grade and obtained from E. Merck Limited, India and Hi-Media Laboratories, Mumbai, India.
2.2. Plant Material
Fresh aerial parts of U. dioica were collected from Sankari, Uttarakhand, India. The plant was authenticated by Dr. AKS Rawat, NBRI, Lucknow. A voucher specimen (262543) has been submitted in institute’s repository.
2.3. Macro and Microscopic Evaluation
The macroscopic evaluations involve study of morphological characters and organoleptic studies like colour, odour, taste, texture, etc.
In microscopic evaluation, studies were conducted qualitatively. All the microscopic evaluations were performed under Olympus CX-31 microscope. Photomicrographs were taken using Olympus digital camera Model No. E-420 [20].
2.4. Physicochemical and Phytochemical Studies
Physicochemical and Phytochemical studies like extractive values, total ash, acid insoluble ash, total sugar, starch, tannin, and phenols were calculated from the shade-dried and powdered (60 mesh) plant material [21-24].
2.5. Extraction of Plant Material for in Vitro Antioxidant Activity
The dried plant material was powdered using electric grinder. Extraction was performed by soxhlation process in two steps. Firstly the powdered plant material was treated under soxhlet assembly using 250 mL of 98% petroleum ether for 9 hours. This is followed by 6 hours soxhlation of plant material by using 250 mL of 50% hydro-alcohol. The final extract was passed through Whatman No. 1 filter paper. The filtrate obtained was concentrated under vacuum in a rotary evaporator at 40˚C and stored at 4˚C for further use. The crude extract was obtained by dissolving a known amount of dry extract in 98% methanol to obtain a stock solution of 1000 μg/ml. The stock solutions were serially diluted with methanol to obtain lower dilutions of 20, 40, 60, 80, 100, 150 and 200 μg/ml.
2.6. DPPH Free Radical Scavenging Activity
Antioxidant activity of the plant extracts and standard was assessed on the basis of the radical scavenging effect of the stable DPPH free radical. Diluted working solutions of the test extracts were prepared in methanol. Ascorbic acid was used as the standard in solutions ranging from 1 to 50 μg/ml. 0.002% DPPH solution in methanol was prepared, further 2 ml of this solution was mixed with 2 ml of sample solutions (ranging from 50 µg/ml to 250 µg/ml), however, the standard solution to be tested separately. These solution mixtures were kept in dark for 30 min and optical density was measured at 517 nm using double beam spectrophotometer (make: Thermo) against methanol. Blank used was 2 ml of methanol with 2 ml of DPPH solution (0.002%). The optical density was recorded and percentage of inhibition was calculated using the formula given below [25]:
% of inhibition of DPPH activity = (A – B/A) × 100where, A is optical density of the blank and B is optical density of the sample.
2.7. HPTLC Studies
Reagents used were from Merk (Germany) and standard ferulic acid was procured from Sigma-Aldrich (Steinheim).
Chromatography was performed on Merk HPTLC precoated silica gel 60 GF254 (20 × 20 cm) plates. Methanolic solutions of samples and standard compound ferulic acid of known concentrations were applied to the layers as 6 mm-wide bands positioned 15 mm from the bottom and 15 mm from side of the plate, using Camag Linomat V automated TLC applicator with nitrogen flow providing a delivery speed of 150 nl/s from application syringe. These conditions were kept constant throughout the analysis of samples.
Air dried (45˚C - 55˚C) powdered sample of U. dioica (2.0 g) in triplicate were extracted separately with 3 × 10 ml 50% hydro-alcoholic solution. Extracts were concentrated under vacuum and redissolved in methanol, filtered and finally made up to 100 ml with methanol prior to HPTLC analysis.
2.8. Detection and Quantification of Ferulic Acid
Following sample application, layers were developed in a Camag twin trough glass chamber pre-saturated with mobile phase of toluene: ethyl acetate: formic acid (5:4:1) till proper separation of bands up to 8 cm height. After development, layers were dried with an air dryer and ferulic acid was simultaneously quantified using Camag TLC scanner model 3 equipped with Camag Wincats IV software. Following scan conditions were applied:
Slit width: 5 mm × 0.45 mm; wavelength: 325 nm; and absorption-reflection mode.
In order to prepare calibration curves, stock solution of ferulic acid (1 mg/ml) was prepared and various volumes of these solutions were analyzed through HPTLC, calibration curves of peak area vs. concentration were also prepared.
3. Results
3.1. Macroscopic Evaluation
The organoleptic features of the leaves indicate dark green colour on upper surface and light green colour on lower surface. The powder of leaves appeared green in colour, coarse in texture, slightly aromatic and unpleasant in odour with tingling and burning taste. The stem was brown in colour with bitter taste and smooth texture.
The morphological characters of leaves were observed as deciduous, erect-ascending, annual or perennial, herbaceous, monoecious or dioecious, pubescent, stinging hairs copious. Leaves opposite, 3 to 15 cm long, leaf blades are elliptic, ovate, lanceolate or oblong, leaf bases may be sub-cordate, truncate or acuminate, leaf margin usually crenate or serrate, glabrous except sparsely pubescent below; leaf blades 3 - 7 nerved, stipule lateral, free. Flower mono or dioecious, axillary, uni or bisexual, in cymes which are combined into a paniculi form inflorescence, tapel segment 4, oblong obtuse hairy (Figure 1).
3.2. Microscopic Evaluation
3.2.1. Leaf Microscopy
Diagrammatic transverse section (T.S.) of the leaf passing through the midrib is wavy, convex on the lower side and slightly depressed on the upper side, shows collenchymatous tissue underneath both the epidermii of the midrib and a centrally located meristele with a lateral extension of dorsi-ventral lamina on its either side (Figure 2).
Detailed T.S. shows a layer of upper and lower epidermis embedded with stomata, they being more on the lower side, the cells of the upper being bigger in size than that of the lower one; covered with striated cuticle, unicellular to multicellular, simple and glandular trichomes with unicellular stalk and cylindrical unicellular head are prominent; centrally located meristele shows radially arranged rows of vessels and an arc of phloem, 3 to 5 rows of collenchymatous tissue lie underneath, both the epiermii lamina shows a row of palisade underneath the upper epidermis, the remaining tissue of the mesophyll being occupied by 5 to 6 row of spongy parenchyma embedded with rosette and cluster crystals of calcium oxalate and obliquely cut vascular bundles (Figure 3).
3.2.2. Petiole Microscopy
T.S. of the Petiole is wavy in outline, shows deep groove encircled by ring of 5 vascular bundles identical to that of stem and peripheral discontinuous band of hypodermal collenchymatous tissue encircled by epidermis bearing few trichomes identical to that of lamina, a discontinuous narrow band of collenchymatous hypodermis lies underneath the epidermis (Figures 4 and 5).
3.2.3. Powder Microscopy
The results showed epidermis of leaf in surface view; cluster and rosette crystals of calcium oxalate scattered as such throughout or embedded in parenchymatous cells; stomata are anomocytic; very few simple and glandular unicellular trichomes, stinging emergences have been observed; longitudinally cut fragments of scalariform vessels has been present (Figure 6).
3.2.4. Preliminary Phytochemical Screening
The results showed positive tests for tannins, saponins, flavonoids, carbohydrates and phenolic compounds.
3.3. Physicochemical Studies
Parameters such as moisture content, extractive values (water, alcohol and ether soluble), total ash and acid insoluble ash values, total sugar, starch, and phenolics were determined. Results are shown in Figure 7.

Figure 1. Morphology of U. dioica.
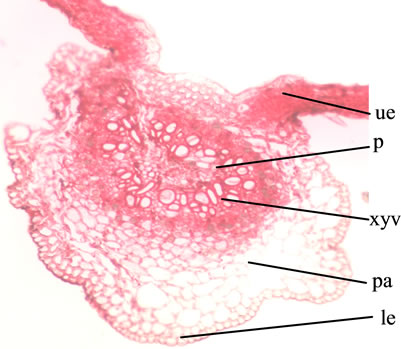
Abbreviations: pa: parenchyma; p: phloem; le: lower epidermis; ue: upper epidermis; xyv: xylem vessel.
Figure 2. T.S. outline of leaf (10×).

Abbreviations: col: cholenchyma; pa: parenchyma; p: phloem; ue: upper epidermis; xyv: xylem vessel.
Figure 3. T.S. cellular of leaf (40×).
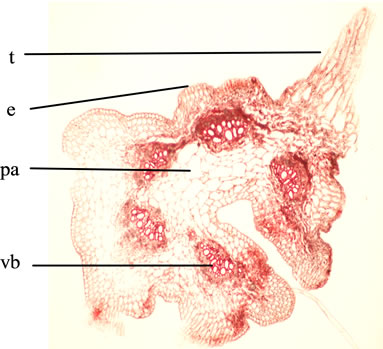
Abbreviations: e: epidermis; pa: parenchyma; t: trichome; vb: vascular bundle.
Figure 4. T.S. outline of Petiole (10×).
3.4. Antioxidant Activity
In-vitro antioxidant study of U. dioica was performed using hydro-alcoholic extract. Ascorbic acid (AA) was used as standard. In this study U. dioica extract showed IC50 value of 88.33 ± 2.88 µg/ml. Standard ascorbic acid showed IC50 value of 2.8 ± 0.62 µg/ml (Figure 8).
3.5. HPTLC Studies
Quantitative HPTLC analysis of ferulic acid in hydroalcoholic extract of U. dioica has been performed. A
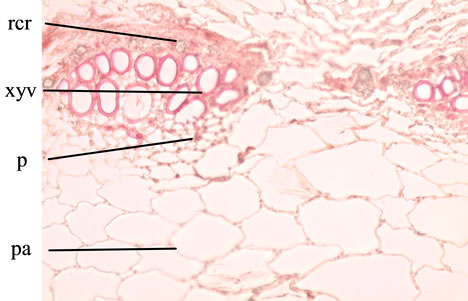
Abbreviations: pa: parenchyma; p: phloem; rcr: rosette crystals of calcium oxalate; xyv: xylem vessel.
Figure 5. T.S. cellular of Petiole (40×).

Abbreviations: e: epidermis; glt: glandular trichome; rcr: rosette crystals of calcium oxalate; sclxyv: sclariform xylem vessel; s: stomata; t: trichome.
Figure 6. Powder microscopy of aerial part.
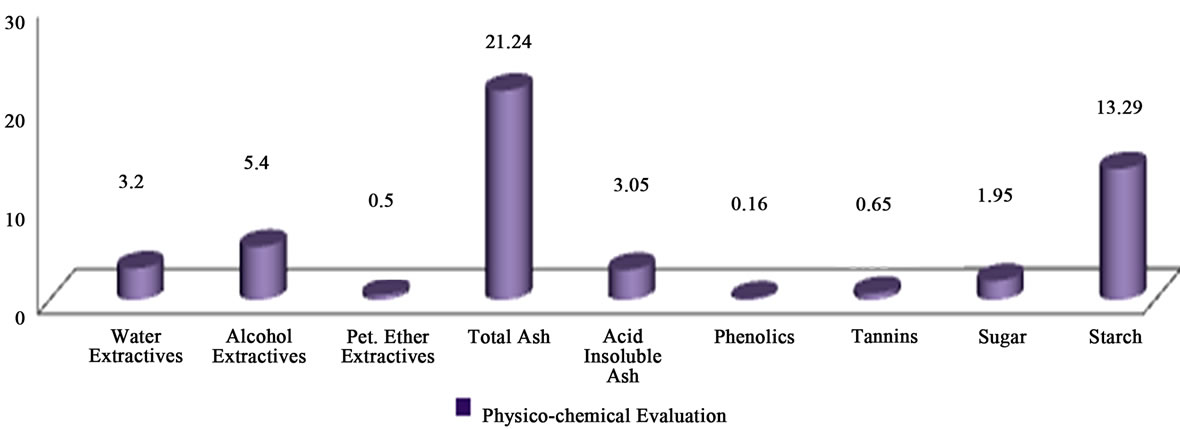
Figure 7. Physico-chemical evaluation.
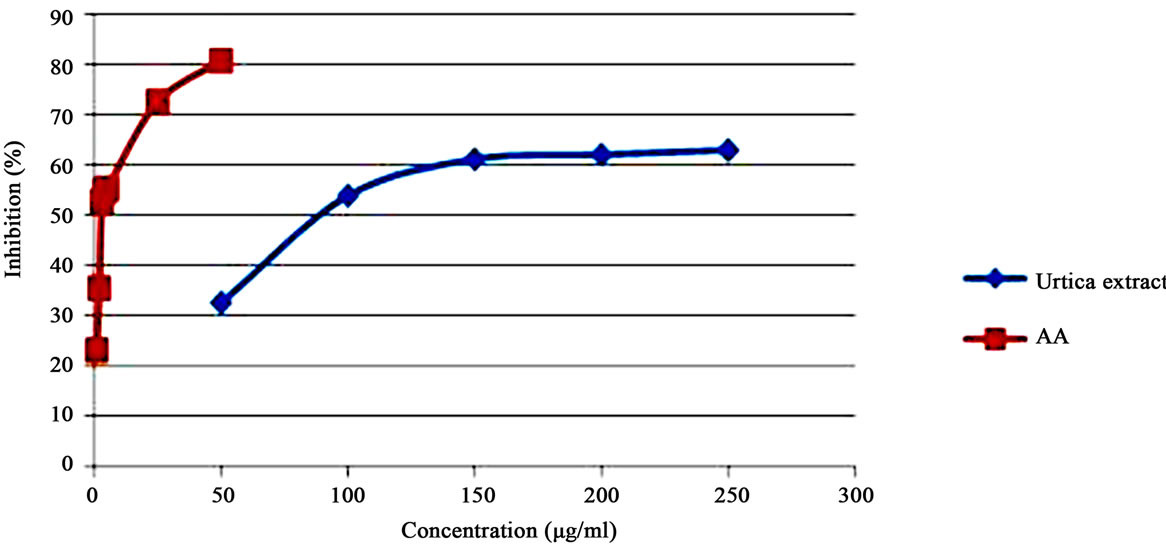
Figure 8. IC50 values of standard ascorbic acid and U. dioica.
densitogram and banding pattern obtained from extract shows ferulic acid 0.73%. During the analysis ferulic acid showed Rf 0.61 ± 0.01; and r2 of 0.98 (Figures 9-11).
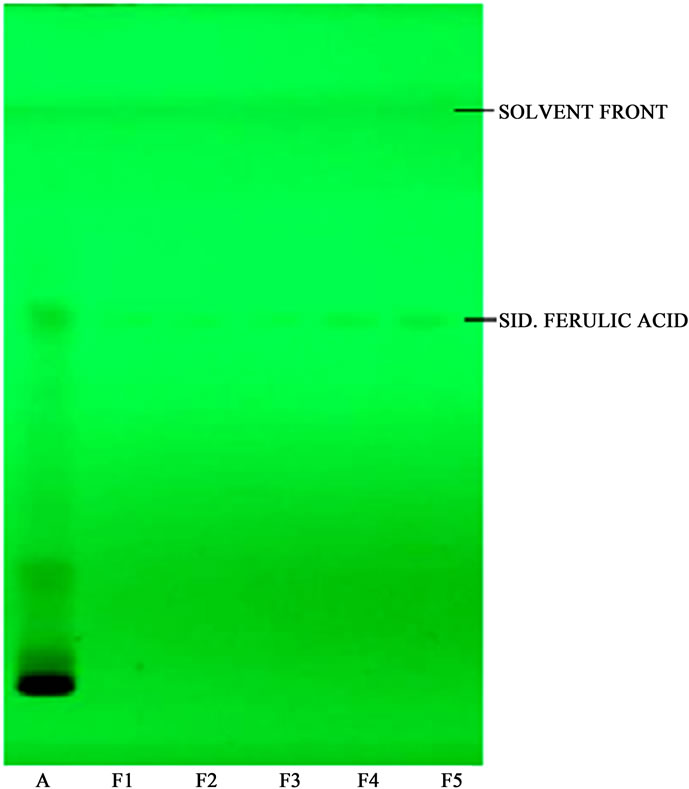
Figure 9. HPTLC profile of U. dioica hydro-alcoholic extract with standard ferulic acid A. Hydro-alcoholic extract of U. dioica; F1, F2, F3, F4 & F5 are different levels of ferulic acid.
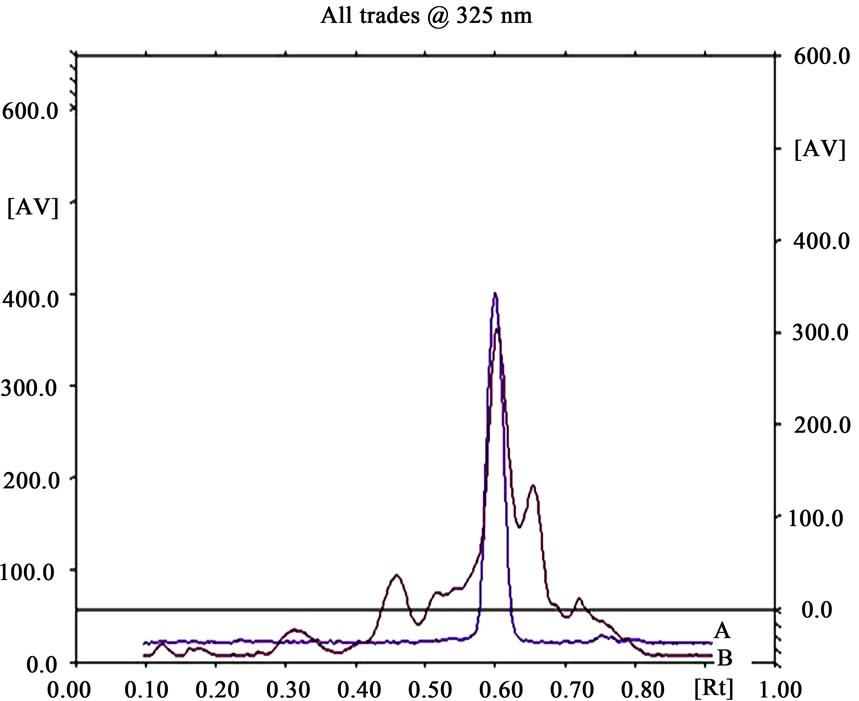
Figure 10. HPTLC chromatogram of extract (B) with standard ferulic acid (A).
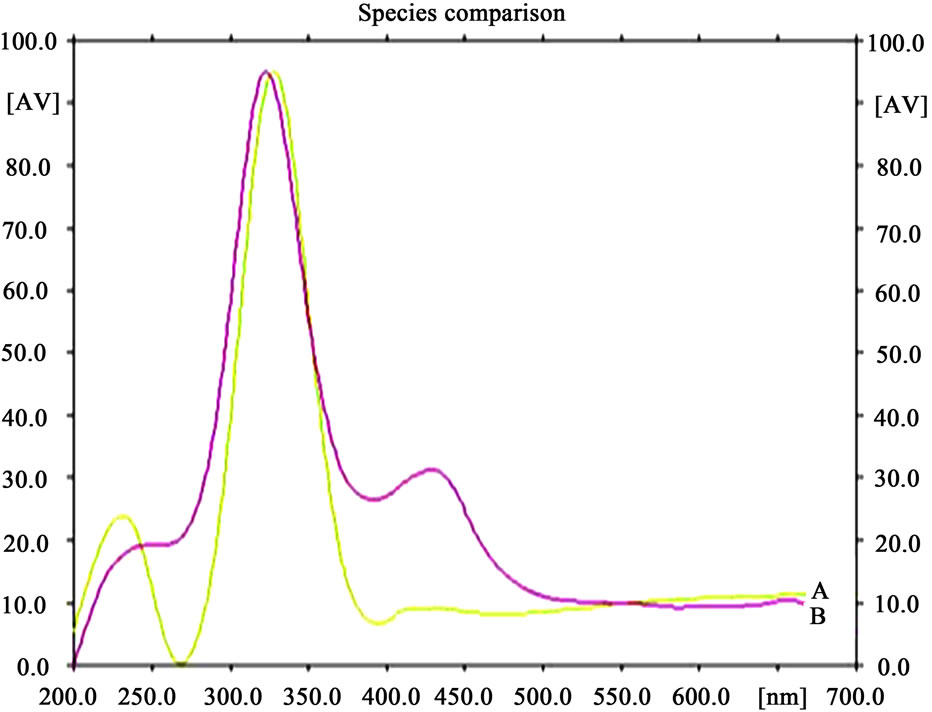
Figure 11. HPTLC spectra of U. dioica extract (B) with standard ferulic acid (A).
4. Discussion
Standardization is an important tool for herbal drugs in order to establish their identity, purity, safety and quality. In order to standardize U. dioica, various macroscopic, microscopic, physic-chemical and preliminary phytochemical and HPTLC was performed along with its in-vitro antioxidant screening. Microscopic method is one of the cheapest and simplest methods to start with establishing the correct identification of the drug. Morphological and microscopical studies of the leaf will enable to identify U. dioica aerial parts. These parameters help in the evaluation of purity of drugs. The information obtained from preliminary phytochemical screening was useful in finding the quality of the drug. These simple but reliable standards will be useful to a lay person in using the drug as a natural remedy. It has been recognized that phenolics show antioxidant activity and their effects on human nutrition and health are considerable. The mechanisms of action of phenolics are through scavenging or chelating process [26]. Phenolic compounds are a class of antioxidant agents which act as free radical terminators [27]. DPPH stable free radical method is an easy, rapid and sensitive way to survey the antioxidant activity of a specific compound or plant extracts [28]. Figure 8 shows the amount of extract needed for 50% inhibition (IC50). IC50 of extract shows positive antioxidant activity of the plant. These studies can also help the manufacturers for identification and selection of the raw material for drug production.
5. Conclusion
The pharmacognostical parameters reported here can be considered as distinctive enough to identify and decide the authenticity of U. dioica in herbal industry. Hydroalcoholic extract of U. dioica showed positive in-vitro antioxidant activity. Ferulic acid, a potential antioxidant present in this species, has been studied through HPTLC. The presence of ferulic acid has not yet been reported and quantified in this species. Presence of phenolic compound suggests that the antioxidant activity may be due to the ferulic acid content. Identification of all chemical constituents in U. dioica extract that are responsible for antioxidant activity requires further investigation, the crude extracts merits further experiments in-vivo. Present study showed new natural antioxidant that can replace the synthetic ones to be used in foods and cosmetics. Thus, the effective source of U. dioica could be employed in medicinal preparations to combat myriad diseases associated with oxidative stress and related disorders.
6. Acknowledgements
The Authors are thankful to Director, NBRI for his encouragement and support during the course of the work.
REFERENCES
- L. T. Ozen and H. Korkmaz, “Modulatory Effect of Urtica dioica,” Phytomedicine, Vol. 10, No. 5, 2003, pp. 405- 415.
- H. Wetherilt, “Evaluation of Urtica Species as Potential Sources of Important Nutrients,” Developments in Food Science, Vol. 29, 1992, pp. 15-25.
- G. Granier, L. Bezanger-Beauquense and G. Debraux, “Ressources Medicinales de la Flore Francaise,” Vigot Freres, Paris, Vol. 2, 1961, pp. 962-964.
- A. Ziyyat, A. Legssyer, H. Mekhfi, A. Dassouli, M. Serhrouhni and W. Benjelloun, “Phytotherapy of Hypertension and Diabetes in Oriental Morocco,” Journal of Ethnopharmacology, Vol. 58, No. 1, 1997, pp. 45-54. doi:10.1016/S0378-8741(97)00077-9
- K. Reihemann, B. Behnke and K. Schulze-Osthoff, “Plant Extract from Stinging Nettle (Urtica dioica), an Antirheumatic Remedy, Inhibit the Pro-Inflammatory Transcription Factor,” FEBS Letters, Vol. 442, No. 1, 1999, pp. 89-94. doi:10.1016/S0014-5793(98)01622-6
- T. Krzeski, M. Kazon, A. Brokowski and J. Kuczera, “Combined Extracts Urtica dioica and Pygeum african in the Treatment of Hyperplasia: Double-Blind Comparison of Two Doses,” Clinical Therapeutics, Vol. 6, No. 15, 1993, pp. 1011-1020.
- J. J. Lichius and C. Muth, “The Inhibiting Effect of Urtica dioica Root Extracts on Experimentally Induced Prostatic Hyperplasia in the Mouse,” Planta Medica, Vol. 63, No. 4, 1997, pp. 307-310. doi:10.1055/s-2006-957688
- A. Tahri, Y. Sabah, A. Legssyer, M. Aziz, H. Mekhfi, M. Bnnouham and A. Ziyyat, “Acute Diuteric, Natriuretic and Hypotensive Effects of a Continuous Perfusion of Aqueous Extract of Urtica dioica in the Rat,” Journal of Ethnopharmacology, Vol. 73, No. 1-2, 2000, pp. 95-100. doi:10.1016/S0378-8741(00)00270-1
- M. Delcourt, W. J. Peumans, M. C. Wanger and P. TruffaBachi, “V Beta Specific Deletion of Mature Thymocytes Induced by the Plant Superantigen Urtica dioica Agglutinin,” Cellular Immunology, Vol. 168, No. 2, 1996, pp. 158- 164. doi:10.1006/cimm.1996.0062
- P. Musette, A. Galelli and H. Harbre, “Urtica dioica Agglutinin, a V Beta 8.3-Specific Superantigen, Prevents the Develop Mentor the Systemic Lupus Erythematosus like Pathology of MRL Ipr/Ipr Mice,” European Journal of Immunology, Vol. 26, No. 8, 1996, pp. 1707-1711. doi:10.1002/eji.1830260807
- A. A. Basaran, I. Ceritoglu, U. Undeger and N. Basaran, “Immunomodulatory Activities of Some Turkish Medicinal Plants,” Phytotherapy Research, Vol. 11, No. 8, 1997, pp. 609-611. doi:10.1002/(SICI)1099-1573(199712)11:8<609::AID-PTR165>3.0.CO;2-0
- P. Rovira, M. Buckle, J. P. Abastado, W. J. Peumans and P. Truffa-Bachi, “Major Histocompatibility Class I Molecules Present Urtica dioica Agglutinin, a Superrantiden of Vegetalorigin to Lymphocytes,” European Journal of Immunology, Vol. 29, No. 5, 1999, pp. 1571-1580. doi:10.1002/(SICI)1521-4141(199905)29:05<1571::AID-IMMU1571>3.0.CO;2-X
- S. Chrubasik and E. Eisenberg, “Treatment of Rheumatic Pain with Medicine in Europe. Part 2,” Urtica dioica L. Pain Clinic, Vol. 11, No. 3, 1999, pp. 179-185.
- C. Randall, K. Mccthan, H. Randall and F. Dobbs, “Nettle Sting of Urtica dioica for Joint Pain: An Exploratory Study of This Complementary Therapy,” Complementary Therapies in Medicine, Vol. 7, No. 3, 1999, pp. 126-131. doi:10.1016/S0965-2299(99)80119-8
- E. Sezika, E. Yeşiladaa, G. Hondab, Y. Takaishic, Y. Takedad and T. Tanaka, “Traditional Medicine in Turkey X. Folk Medicine in Central Anatolia,” Journal of Ethnopharmacology, Vol. 75, No. 2-3, 2001, pp. 95-115. doi:10.1016/S0378-8741(00)00399-8
- E. Sezik, E. Yesilada, M. Tabata, G. Honda, Y. Takaishi, T. Fujita, T. Tanaka and Y. Takeda, “Traditional Medicine in Turkey VIII. Folk Medicine in East Anatolia,” Economic Botany, Vol. 51, No. 3, 1997, pp. 195-211. doi:10.1007/BF02862090
- E. Yesilada, G. Honda, E. Sezik, M. Tabata, K. Goto and Y. Lkeshiro, “Traditional Medicine in Turkey IV. Folk Medicine in Mediterranean Subdivision,” Journal of Ethnopharmacology, Vol. 39, No. 1, 1993, pp. 31-38. doi:10.1016/0378-8741(93)90048-A
- A. A. Basaran, I. Ceritoglu and U. Undeger, “Immunomodulatory Activities of Some Turkish Medicinal Plants,” Phytotherapy Research, Vol. 11, No. 8, 1997, pp. 609-611. doi:10.1002/(SICI)1099-1573(199712)11:8<609::AID-PTR165>3.0.CO;2-0
- A. A. Lebedev, E. A. Batakov and V. A. Kurkin, “The Antioxidative Activity of a Complex Hepatoprotective Preparation,” Silybokhol Rastitel’nye Resursy, Vol. 37, 2001, pp. 69-75.
- D. A. Johansen, “Plant Microtechnique,” MC Graw Hill Book Co. Inc., New York and London, 1940, pp. 15-93.
- K. Peach and M. V. Tracy, “Modern Methods of Plant Analysis, Vol. III and IV,” Springer Heidelberg, Berlin 1955, pp. 258-261.
- Anonymous, “Indian Pharmacopoeia, Government of India, Ministry of Health and Family Welfare,” Controller of Publications, New Delhi, 2007, p. 191.
- Anonymous, “Official Methods of Analysis (AOAC), Association of Official Chemists,” 4th Edition, Washington DC, 1984, pp. 187-188.
- Anonymous, “Ayurvedic Pharmacopeoeia of India, Part I, Vol. I. Department of Health, Ministry of Health and Family Welfare,” Government of India, New Delhi, 2004, pp. 152-165.
- S. A. Al-Nooman, O. Ela-Segaey and A. Ab-Allah, “Experimental Study of Antioxidant and Hepatoprotective Effects of Clove and Cardamom in Ethanol Induced Hepatotoxicity,” Tanta Medical Sciences Journal, Vol. 2, No. 1, 2007, pp. 27-36.
- M. Kessler, G. Ubeaud and L. Jung, “Antiand Pro-Oxidant Activity of Rutin and Quercetin Derivatives,” Journal of Pharmacy and Pharmacology, Vol. 55, No. 1, 2003, pp. 131-142. doi:10.1211/002235702559
- F. Shahidi and W. P. K. J. P. D. Anasundara, “Phenolic Antioxidants,” Critical Reviews in Food Science and Nutrition, Vol. 32, No. 1, 1992, pp. 67-103. doi:10.1080/10408399209527581
- Koleva II, T. A. Van Beek, J. P. H. Linssen, A. de Groot and L. N. Evstatieva, “Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods,” Phytochemical Analysis, Vol. 13, No. 1, 2002, pp. 8-17. doi:10.1002/pca.611
Abbreviations
HPTLC: High Performance Thin Layer Chromatography;
DPPH: 2,2-Diphenyl-1-picrylhydrazyl;
AA: Ascorbic Acid.
NOTES
*Authors’ contribution: VK: Botanical studies; PK: Botanical studies; AG: Physicochemical studies; SV: HPTLC studies; SS: Collection of species, compilation & preparation of MSS; AKSR: Authentication of material & overall supervision.

