Chinese Medicine
Vol. 3 No. 2 (2012) , Article ID: 18272 , 5 pages DOI:10.4236/cm.2012.32016
“Qi-Invigorating” Chinese Tonic Herbs (Shens) Stimulate Mitochondrial ATP Generation Capacity in H9c2 Cardiomyocytes in Situ and Rat Hearts ex Vivo
Division of Life Science, Hong Kong University of Science & Technology, Hong Kong, China
Email: *bcrko@ust.hk
Received December 20, 2011; revised February 1, 2012; accepted February 23, 2012
Keywords: Qi; Chinese Medicine; ATP; Mitochondria; Cardiomyocytes
ABSTRACT
In the study, using ethanol extracts of Renshen (Panax ginseng C A Meyer), Xiyangshen (Panax quinquefolius L.) and Dangshen (Codonopsis pilosulae), we investigated the effect of these “Qi-invigorating” Chinese tonic herbs on mitochondrial ATP generation capacity (ATP-GC) in H9c2 cardiomyocytes in situ and rat hearts ex vivo. All three types of “Shens” stimulated mitochondrial ATP-GC, with Renshen being most potent. While a parallel enhancement in mitochondrial ATP-GC was observed in Renshenand Xiyangshen-pretreated rats, Dangshen treatment did not produce detectable effect ex vivo. The discrepancy between in situ and ex vivo assays for Dangshen may be attributed by its limited oral-bioavailability to the heart. The tissue specific activity of Shens on mitochondrial ATP-GC may be explained by the “Meridian Theory” in traditional Chinese medicine.
1. Introduction
“Renshen”, a Chinese “pinyin” for Radix Ginseng (Panax ginseng C A Meyer), has a long history of use in Chinese medicine for promoting health. Early pharmacological investigations have demonstrated the adaptogenic effect of Renshen, which can enable the body to cope with various stress conditions [1]. Recent experimental studies have shown that Renshen or its ginsenosides produces anti-aging, anti-carcinogenic, anti-inflammatory and antioxidant actions [2-6]. According to the theory of Chinese medicine, Chinese tonic herbs can be classified into four functional categories, namely, “Yang-invigorating”, “Yin-nourishing”, “Qi-invigorating” and “Blood-enriching”, with various “Shens” being categorized in the “Qi” family. Due to the popularity and therapeutic values of “Qi-invigorating” herbs, the investigation of biological activities and the underlying mechanisms in relation to “Qi-invigoration” is of great pharmacological interest. In this regard, a recent study has demonstrated the relationship between “Qi-invigorating” action and energy level in skeletal muscle isolated from the thigh of “Qi-invigorating” herb-treated rats [7]. However, the pharmacological basis of “Qi-invigorating” action has yet to be established.
Previous experimental findings in our laboratory have demonstrated that all “Yang-invigorating” herbs are capable of enhancing mitochondrial ATP-GC in both cell and animal studies [8,9]. As a subcategory of “Yanginvigorating” herbs, “Qi-invigorating” herbs may also stimulate mitochondrial ATP generation capacity in various tissues. In the present study, using the ethanol extracts of Renshen, Xiyangshen (Panax quinquefolius L.) and Dangshen (Codonopsis pilosulae), we investigated the effect of “Qi-invigorating” Chinese tonic herbs on mitochondrial ATP-GC using in situ and ex vivo assay systems.
2. Materials and Methods
2.1. Herbal Materials
Renshen, Xiyangshen and Dangshen were purchased from a local herbal dealer (Lee Hoong Kee). The herbs were authenticated by the supplier and voucher specimens were deposited in the Division of Life Science, Hong Kong University of Science and Technology (HKUST). Individual herb was extracted by 95% ethanol under reflux for 2 h, as previously described [8], and obtained the ethanol extract. All the extracts were dried by evaporating the solvent under reduced pressure and the dried extracts at varied yields (see Table 1) were stored at 4˚C until use.
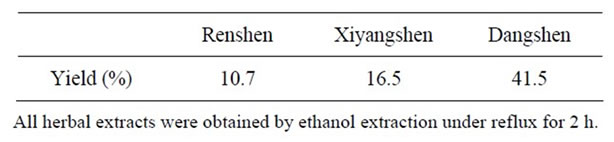
Table 1. Yields of herbal extractions.
2.2. Cell Culture
H9c2 cell, which is a subclone of the original clonal cell line derived from embryonic BD1X rat heart tissue, was purchased from American Tissue Culture Centre (ATCC). The cells were cultured as mono-layers in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco BRL Life Technologies, Grand Island, NY, US), supplemented with 10% (v/v) fetal bovine serum (FBS), 100 IU/mL of penicillin (Sigma, St. Louis, MO), 100 μg/mL of streptomycin and 17 mM NaHCO3. All cells were grown under an atmosphere of 5% (v/v) CO2 in air at 37˚C.
2.3. Measurement of ATP-GC in Situ
H9c2 cardiomyocytes were seeded at a density of 2.5 × 104 cells/well into 24-well microtiter plates. After the cell attachment, cells were incubated with herbal extracts for 4 h at 37˚C. Control group was given vehicle (DMSO) only. After the incubation, the ATP-GC assay was performed as previously described [8]. In brief, the removal of herbal extract-containing medium was followed by cell membrane permeabilization with digitonin (50 μg/ mL) in an incubation buffer (120 mM KCl, 5 mM KH2PO4, 2 mM EGTA, 10 mM HEPES, 0.1 mM MgCl2, 0.5% BSA, pH 7.4). Pyruvate (15 mM), malate (5 mM) and ADP (10 μM) were added for mitochondrial ATP generation at 37˚C, which was monitored at increasing time intervals ranging from 0 to 15 min. The reaction was terminated by the addition of 60 μL of perchloric acid (30%, w/v), and the reaction mixtures were then centrifuged at 540 × g for 10 min at 4˚C, and the supernatant were measured for ATP content by luciferase assay (ATPlite, PerkinElmer Inc., MA).
The mitochondrial ATP-GC of untreated control was estimated by computing the area under the curve of the graph (AUC1) plotting ATP generated (nmol/mg protein) against time (0, 7.5 and 15 min) and expressed in arbitrary unit. For herbal extract-treated samples, AUC1 values of increasing incubation times were normalized to a respective mean control value from untreated cells and expressed as percent control. The area under the curve (AUC2) of the graph plotting percent control values against incubation time (7.5 and 15 min) was computed and expressed in arbitrary unit. Data of herbal extracttreated groups were expressed as percent control. The two-step data processing aims at minimizing the interanimal and inter-assay variabilities under the present experimental conditions [10].
2.4. Animal Care
Female adult Sprague-Dawley rats (8-week old; 200 - 250 g) were maintained under a 12 h dark/light cycle at about 22˚C and allowed water and food ad libitum. Experimental protocols were approved by the Research Practice Committee at HKUST. Animals were randomly divided into groups, with five animals in each. In the experiment, rats were administered intragastrically with herbal extracts at daily doses of 0.5 to 3 g/kg for 3 consecutive days and were killed by decapitation 24 hours after the last dosing. Control animals were administered with the vehicle (water) only. Hearts were excised from control or herbal extract-pretreated rats, mitochondrial fractions were then isolated and subjected to the measurement of mitochondrial ATP generation capacity (ATP-GC) in vitro.
2.5. Preparation of Mitochondrial Fractions
Myocardial mitochondrial fractions were prepared from heart tissue homogenates by differential centrifugation at 4˚C. In brief, heart ventricular tissue samples were excised and rinsed with ice-cold heart sucrose buffer (320 mM sucrose, 1 mM EDTA, 50 mM Tris-base, pH 7.4). A 10% (w/v) heart homogenate was prepared by homogenizing the minced ventricular tissue with a teflon-glass homogenizer at 4000 rpm for 30 min. The homogenate was centrifuged at 600 × g for 10 min at 4˚C to remove nuclei and cell debris. The supernatant was then centrifuged at 9200 × g for 30 min at 4˚C to sediment the mitochondria. The pellets were resuspended in heart sucrose buffer and constituted the mitochondrial fractions.
2.6. Destruction of Contaminating ATP in Commercial ADP Preparation
The contaminating ATP was removed by an enzymatic procedure. To briefly describe, ATP was converted to ADP by reacting it with glucose at 25˚C during a 18 h incubation in the reaction mixture (34 mM commercial ADP, 100 mM Tris-HCl buffer (pH 8.1), 2 mM MgCl2, 3.4 mM glucose, 1 mM NADH, 5.6 U hexokinase/mL, and 6 U glucose-6-phosphate dehydrogenase/mL). To destroy the formed NADPH, the solution was acidified with 0.1 volume of 1 M HCl and allowed to stand at 25˚C for 10 min. The solution was then alkalinized by adding 0.017 volume of 6 M NaOH, and all enzymes were inactivated by heating the reaction mixture to 95˚C for 15 min [11].
2.7. Measurement of ATP-GC
Aliquots (50 μL) of mitochondrial fractions (1 mg protein/mL) were mixed with 50 μL of substrate solution (3 mM pyruvate and 3 mM malate) and 25 μL of pretreated ADP (30 mM) solution, and the reaction mixtures were incubated for increasing period of time (0, 10 and 20 min) at 37˚C to allow mitochondrial ATP generation. The ATP content was then measured as described before.
2.8. Protein Assay
Protein concentration was determined by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA), using bovine serum albumin as standard.
2.9. Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM), unless otherwise specified. Data were analyzed by one-way analysis of variance (one-way ANOVA) and inter-group difference was detected by Least Significant Difference (LSD) when p < 0.05.
3. Results
Figure 1 shows that pretreatment with Renshen at concentrations ranging from 50 to 300 μg/mL significantly increased the mitochondrial ATP-GC in a concentration dependent manner in H9c2 cardiomyocytes, with the extent of stimulation being 42% at 300 μg/mL. Xiyangshen and Dangshen extracts significantly stimulated mitochondrial ATP-GC by 26% and 25%, respectively, at the concentration of 300 μg/mL (Figures 2 and 3).
As shown in Figure 4, pretreatment with Renshen extract at daily doses of 0.5 and 1 g/kg significantly enhanced the ATP-GC in mitochondria isolated from rat hearts in a dose-dependent manner, with the extent of
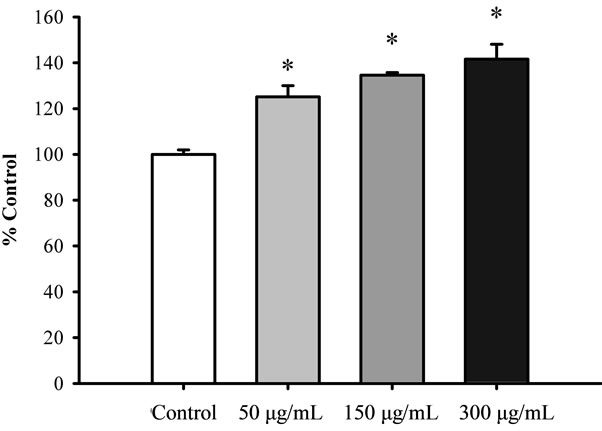
Figure 1. The effect of Renshen extract on mitochondrial ATP-GC in H9c2 cardiomyocytes. Cells were pre-incubated with Renshen extract at concentrations of 50 to 300 μg/mL for 4 h. Upon the removal of herbal extract-containing medium, the cells were subjected to the measurement of ATPGC in situ. Data were expressed in percent control with respect to the untreated control. Values given are means ± SEM, with n = 3. *Significantly different from the control group.
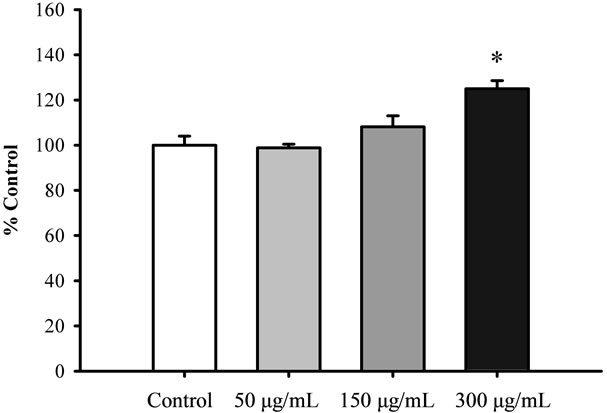
Figure 2. The effect of Xiyangshen extract on mitochondrial ATP-GC in H9c2 cardiomyocytes. Cells were pre-incubated with Xiyangshen extract at concentrations of 50 to 300 μg/ mL for 4 h. Mitochondrial ATP-GC was measured as described in Figure 1. Data were expressed in percent control with respect to the untreated control. Values given are means ± SEM, with n = 3. *Significantly different from the control group.
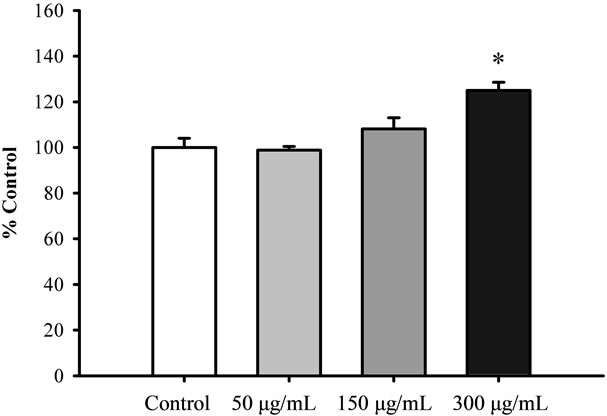
Figure 3. The effect of Dangshen extract on mitochondrial ATP-GC in H9c2 cardiomyocytes. Cells were pre-incubated with Dangshen extract at concentrations of 50 to 300 μg/mL for 4 h. Mitochondrial ATP-GC was measured as described previously. Data were expressed in percent control with respect to the untreated control. Values given are means ± SEM, with n = 3. *Significantly different from the control group.
stimulation being 9.3% and 22.9%, respectively. Figure 5 shows that pretreatment with Xiyangshen extract at daily doses of 2 and 3 g/kg significantly increased mitochondrial ATP-GC, with the extent of stimulation being 13% and leveled at the high dose. However, Dangshen pretreatment at daily doses up to 3 g/kg did not produce any detectable effect on ATP generation in mitochondria isolated from rat hearts (Figure 6).
4. Discussion
In the present study, we demonstrated that the ethanol
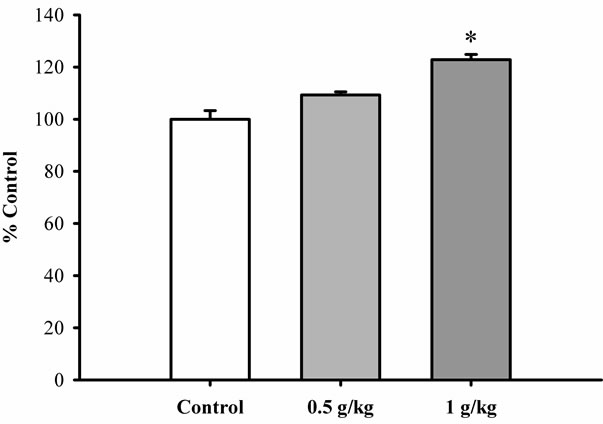
Figure 4. The effect of Renshen extract on ATP-GC in mitochondria isolated from rat hearts. Rats were orally administered with Renshen extract at daily doses of 0.5 and 1 g/kg for 3 consecutive days. Heart mitochondrial fractions were isolated and subjected to the measurement of ATP-GC ex vivo. Data were expressed in percent control with respect to the untreated control animals. Values given are means ± SEM, with n = 5. *Significantly different from the control group.
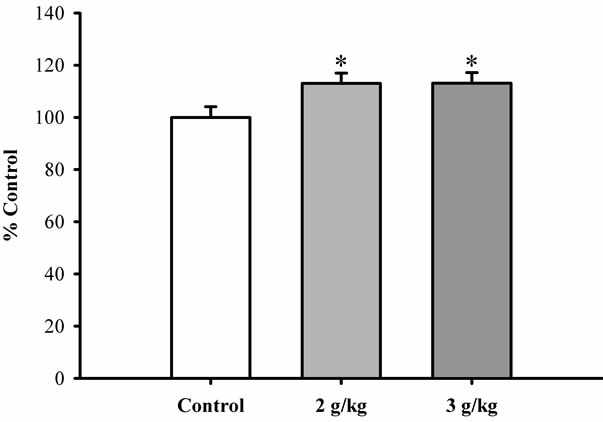
Figure 5. The effect of Xiyangshen extract on ATP-GC in mitochondria isolated from rat hearts. Rats were orally administered with Xiyangshen extract at daily doses of 2 and 3 g/kg for 3 consecutive days. ATP-GC of heart mitochondria was measured as described in Figure 4. Data were expressed in percent control with respect to the untreated control animals. Values given are means ± SEM, with n = 5. *Significantly different from the control group.
extracts of all three types of “Shens” (namely, Renshen, Xiyangshen and Dangshen) increased mitochondrial ATP-GC in H9c2 cardiomyocytes, with Renshen being most potent among the tested “Shens”. This finding, which is consistent with the observation in “Yang-invigorating” herbs [9], suggested that “Qi-invigorating” herbs can also stimulate mitochondrial ATP-GC in H9c2 cardiomyocytes. The stimulation of ATP-GC by Renshen and Xiyangshen in the cell-based in situ assay was associated with a parallel enhancement of ATP-GC in mito-
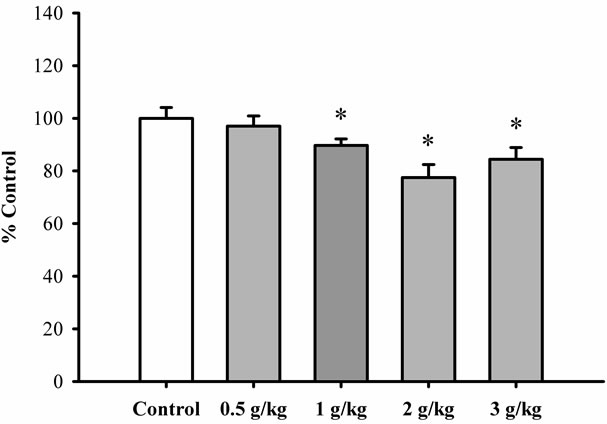
Figure 6. The effect of Dangshen extract on ATP-GC in mitochondria isolated from rat hearts. Rats were orally administered with Dangshen extract at daily doses ranging from 0.5 to 3 g/kg for 3 consecutive days. ATP-GC of heart mitochondria was measured as described previously. Data were expressed in percent control with respect to the untreated control animals. Values given are means ± SEM, with n = 5. *Significantly different from the control group.
chondria isolated from Renshenor Xiyangshen-pretreated rats, with the extent of stimulation by Renshen being larger than that of Xiyangshen at the same crude herb equivalent dose. However, Dangshen treatment did not cause any detectable change in ATP-GC in rat heart mitochondria. The discrepant observation between in situ and ex vivo assays for Dangshen on mitochondrial ATPGC may be related to its limited oral bioavailability to the heart.
Dangshen has been used as a milder and more economical substitute for Renshen, and sometimes they are interchangeably used in many Chinese herbal formulations. However, a pharmacopoeia published in the Qing Dynasty differentiated Dangshen from the Renshen family, in which Dangshen belongs to the family of Campanulaceae while Renshen and Xiyangshen are grouped under the family of Aralaceae [12]. Although Dangshen shares similar biological activities with Renhsen and Xiyangshen, the tissue specificity varies in these two groups of herbs. It has been demonstrated that majority of the metabolites of Renshen and Xiyangshen could be found in the liver, heart and small intestine [1], while the action of Dangshen seemed to be limited to digestive, immune and respiratory systems [13-15]. Consistent with this, Dangshen, as observed in the present study, produced stimulatory effect on ATP-GC in H9c2 cardiomyocytes in situ but not in rat hearts ex vivo. In the context of Chinese medicine, this finding may be explained by the “Meridian Theory” (Jingluo), in which “Meridian” is the path that “Qi” (life energy) flows. According to the literature on materia medica, Renshen and Xiyangshen enter the “Meridians” of heart, spleen and lung after oral administration and then bring about the beneficial effects to the respective organs. Dangshen only enters the “Meridians” of spleen and lung, but not heart, which may be attributed to its inability to stimulate the ATP-GC in rat heart mitochondria.
In conclusion, the results obtained from the present study showed that the three tested “Qi-invigorating” Shens in Chinese medicine stimulated the ATP-GC in situ in the cell-based assay system. Interestingly, the “Qiinvigorating” Shens herbs may produce the beneficial action in a tissue specific manner. Further investigations should examine whether “Yang-invigorating” and “Qiinvigorating” Chinese tonic herbs can stimulate mitochondrial ATP-GC ex vivo in a tissue specific manner according to the “Meridian Theory” in Chinese medicine.
REFERENCES
- D. D. Kitts and C. Hu, “Efficacy and Safety of Ginseng,” Public Health Nutrition, Vol. 3, No. 4a, 2000, pp. 473- 485. doi:10.1017/S1368980000000550
- J.-M. Lu, Q. Z. Yao and C. Y. Chen, “Ginseng Compounds: An Update on Their Molecular Mechanisms and Medical Applications,” Current Vascular Pharmacology, Vol. 7, No. 3, 2009, pp. 293-302. doi:10.2174/157016109788340767
- L.-W. Qi, C.-Z. Wang and C.-S. Yuan, “American Ginseng: Potential Structure-Function Relationship in Cancer Chemoprevention,” Biochemical Pharmacology, Vol. 80, No. 7, 2010, pp. 947-954. doi:10.1016/j.bcp.2010.06.023
- T. K. Yun, “Panax Ginseng—A Non-Organ-Specific Cancer Preventive?” Lancet Oncology, Vol. 2, No. 1, 2001, pp. 49-55. doi:10.1016/S1470-2045(00)00196-0
- S. F. Chu and J. T. Zhang, “New Achievements in Ginseng Research and Its Future Prospects,” Chinese Journal of Integrative Medicine, Vol. 15, No. 6, 2009, pp. 403- 408. doi:10.1007/s11655-009-0403-6
- W. C. S. Cho, W.-S. Chung, S. K. W. Lee, A. W. N. Leung, C. H. K. Cheng and K. K. M. Yue, “Ginsenoside Re of Panax Ginseng Possesses Significant Antioxidant and Antihyperlipidemic Efficacies in Streptozotocin-Induced Diabetic Rats,” European Journal of Pharmacology, Vol. 550, No. 1-3, 2006, pp. 173-179. doi:10.1016/j.ejphar.2006.08.056
- X. T. Li, J. J. Zhang and W. W. Chen, “The Effects of TCM Qi-Invigorating, Qi Regulating Drugs and Polysaccharides on the Energy Charge of Rat Skeletal Muscle Cells,” Journal of Beijing University of Traditional Chinese Medicine, Vol. 23, No. 5, 2000, pp. 36-38.
- H. Y. Leung and K. M. Ko, “Herba Cistanche Extract Enhances Mitochondrial ATP Generation in Rat Hearts and H9c2 Cells,” Pharmaceutical Biology, Vol. 46, No. 6, 2008, pp. 418-424. doi:10.1080/13880200802055883
- H. S. Wong, H. Y. Leung and K. M. Ko, “Yang-Invigorating Chinese Tonic Herbs Enhance Mitochondrial ATP Generation in H9c2 Cardiomyocytes,” Chinese Medicine, Vol. 2, No. 1, 2011, pp. 1-5. doi:10.4236/cm.2011.21001
- H. Y. Leung, P. Y. Chiu, M. K. T. Poon and K. M. Ko, “A Yang-Invigorating Chinese Herbal Formula Enhances Mitochondrial Functional Ability and Antioxidant Capacity in Various Tissues of Male and Female Rats,” Rejuvenation Research, Vol. 8, No. 4, 2005, pp. 238-247. doi:10.1089/rej.2005.8.238
- P. Ronner, E. Friel, K. Czerniawski and S. Frankle, “Luminometric Assays of ATP, Phosphocreatine, and Creatine for Estimation of Free ADP and Free AMP,” Analytical Biochemistry, Vol. 275, No. 2, 1999, pp. 208-216. doi:10.1006/abio.1999.4317
- Z. C. Lin, J. Y. Zhang, Y. R. Ke, Z. C. Chen and Z. L. Guo, “A Pentsaological Research on Dangshen (Codonopsis SPP., Campanulaceae),” Journal of Chinese Medicine, Vol. 18, No. 1-2, 2007, pp. 51-64. (in Chinese)
- H. J. Jiao, “The Pharmacological Basis and Clinical Applications of Dangshen,” Clinical Medicine, Vol. 25, No. 4, 2005. (in Chinese)
- Y. X. Sun and J. C. Liu, “Structural Characterization of A Water-Soluble Polysaccharide from the Roots of Codonopsis Pilosula and Its Immunity Activity,” International Journal of Biological Macromolecules, Vol. 43, No. 3, 2008, pp. 279-282. doi:10.1016/j.ijbiomac.2008.06.009
- Y. Shen, “Protective Effect of Spleen Invigorating Chinese Medicinal Herbs for Gastric Mucosa,” World Chinese Journal of Digestology, Vol. 4, No. 7, 1999, pp. 357-358.
NOTES
*Corresponding author.

