Natural Science
Vol.5 No.3(2013), Article ID:29023,8 pages DOI:10.4236/ns.2013.53047
Removal of chromium from water effluent by adsorption onto Vetiveria zizanioides and Anabaena species
![]()
1Department of Biotechnology and Biosciences, Lovely Professional University, Phagwara, India; *Corresponding Author: manojjnu@gmail.com
2Aarupadai Veedu Institute of Technology, Tamilnadu, India
3Institute of Nuclear Medicine & Allied Sciences, Defence Research and Development Organisation, New Delhi, India
4Department of Microbiology, South Campus, University of Delhi, New Delhi-110021, India
Received 8 August 2012; revised 17 September 2012; accepted 2 October 2012
Keywords: Bioadsorption; Vetiveria; Anabaena; Adsorption Isotherm; Chromium
ABSTRACT
Bioadsorption phenomenon is more or less like a chemical reaction and several parameters are bound to affect the process. The pH, amount of adsorbent and agitation time influence the biosorptive potentiality. Hence, the present study on adsorption of Cr(VI) by activated Vetivera roots and Blue green algae Anabaena supports that it is an effective low cost adsorbent for the removal of Cr(VI) from plating effluent. Langmuir and Freundlich adsorption isotherm correlate the equilibrium adsorption data. In batch experiments both Vetiveria and Anabaena species were found to be cost effective biosorbent for the efficient removal of Cr(VI) from the effluent and comparatively Anabaena species was found to adsorb maximum Cr(VI) (88.86%) at a low contact time of 60 min. The data obtained from the experiments and modeling would prove useful in designing and fabricating an efficient treatment plant for Cr(VI) rich effluent.
1. INTRODUCTION
The treatment of waste water containing metals is a challenging problem; Due to rise in rigorous environmental policies, scientists all around the world are desired to develop precise techniques to control the amount of heavy metal in waste water and drinking water. Chromium in particular has received a great deal of attention. The increased environmental burden of Cr(VI) may come from various industrial sources like those from electroplating, leather tanning, textiles and metal finishing industries. The presence of heavy metals in wastewater is not only of great environmental concern but also strongly declining microbial activity, as a result adversely affecting biological wastewater treatment processes.
Earlier the method of ion exchange has been widely employed to remove heavy metals in water bodies [1,2], but the cost is high. There are also reports available on the removal of heavy metals in effluent by complexation of dry biomass [3,4]. Unfortunately, these methods were not employed on large scale. Many researchers have reported the methods of biosorption on chemical modified solid surface [5], it takes some time for the adsorption of heavy metals in water bodies, especially, at ppm level.
Vetiveria zizanioides, due to its unique morphological and physiological characteristics, and tolerance to high levels of heavy metal and adverse conditions, has also been successfully used in the field of environmental protection [6,7]. It is excellent for the removal of heavy metals from contaminated soil [8,9] and rehabilitating landfills [10]. Even though it is not an aquatic plant, vetiver can be established and survive under hydroponic conditions [11]. It can purify eutrophic water [12], garbage leachates [13] and wastewater from pig farms [14]. Therefore, vetiver has high potential to be used for industrial wastewater treatment [12].
Among the most promising types of biosorbents studied is the algal biomass [15,16]. This is due to the presence of various functional groups such as carboxyl, amino, sulphate and hydroxyl groups, which can act as binding sites for metals [17]. Anabaena Bory ex Bornet & Flahault (Cyanobacteria), a blue green alga, is among a number of cyanobacteria that present gas vacuoles (aerotope) and form dense populations on the surface of lakes and reservoirs, the so-called blooms. Anabaena being structurally similar might also possesses the capabilities of heavy metal removal from effluents as this alga contain lipopolysaccaride envelop, that is proven to be helpful in ionic exchange properties/intracellular accumulation/adsorption onto cell surface.
The present study characterizes waste water from chrome plating effluent, and finally to optimize conditions pertaining to removal and recovery of Cr(VI) by these selected bioadsorbents, Vetiveria and Anabaena species.
2. MATERIALS AND METHODS
To envisage the bioadsorptive potentiality of Anabaena and Vetiveria, various methods pertaining to physicochemical analysis of chrome plating effluent, preparation of bioadsorbents, optimization and adsorption isotherm studies were performed.
2.1. Physiochemical Analysis
Effluents from chrome plating industry were collected in plastic containers as per standard methods [18]. The industrial effluent was analyzed as per standard methods [19]. Bio chemical Oxygen Demand (BOD) measured by the methods described in APHA [20] procedure. The sample was diluted sufficiently so that the demand of oxygen does not exceed the amount of available oxygen. Dilution extract for dilution of sample was prepared by adding one 1 ml each of phosphate buffer, magnesium sulphate solution, calcium chloride solution and ferric chloride in one liter of distilled water, then after diluted sample was filled in BOD bottles. For subsequent dilution of sample, two BOD bottles were prepared. Initial Dissolved Oxygen (DO) was noted from the first one and second one was incubated at 20˚C for five days. DO of diluted sample was recorded and water blank was diluted before incubation and kept for 5 days of incubation. BOD was calculated in mg/l from the difference between initial and final DO by the following formula.
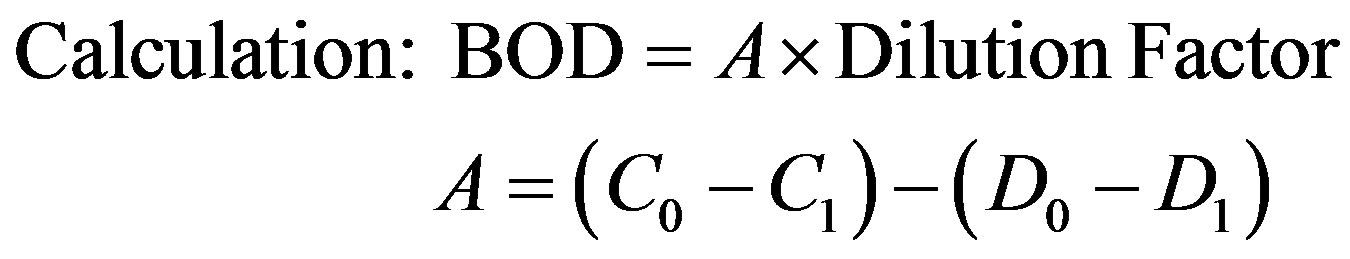
where, D0 = DO in dilution water; D1= DO in dilution water, after incubation; C0 = DO in sample; C1 = DO in sample after incubation; D0 − D1= oxygen depletion in dilution water; C0 − C1 = oxygen depletion due to biomass in diluted original sample; Dilution factor = 102.
2.2. Total Dissolved Solids
Total Dissolved Solids (TDS) was examined by taking 20 ml of the sample, after filtering through a dry whatman filter paper. The filtrate was taken in a pre-weighed porcelain crucible and evaporated to dryness in a hot air oven. The evaporated sample was further dried for an hour at 105˚C in a hot air oven. The crucible was cooled in desiccators and weighed. TDS was calculated with standard formula:
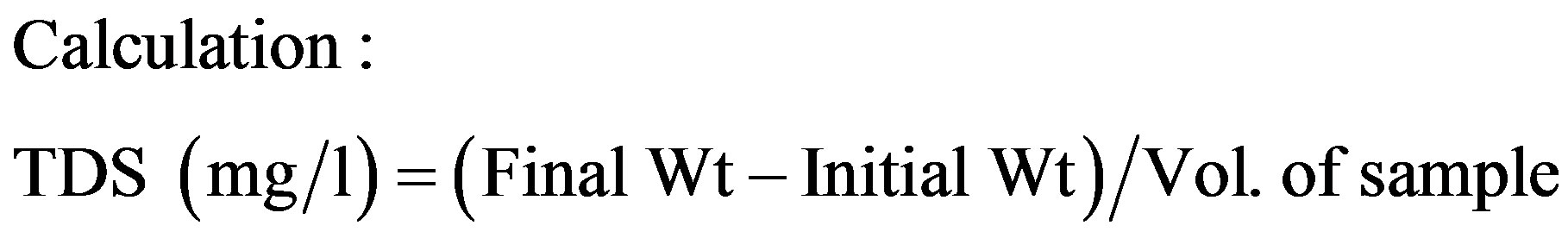
2.3. Total Suspended Solids
Total Suspended Solids (TSS) was calculated by collecting 20 ml of the sample filtered through a preweighed whatman filer paper and then after drying it in a hot air oven for an hour. Filter paper was cooled in desiccators and weighed. From the readings, TSS was calculated by using the formulae
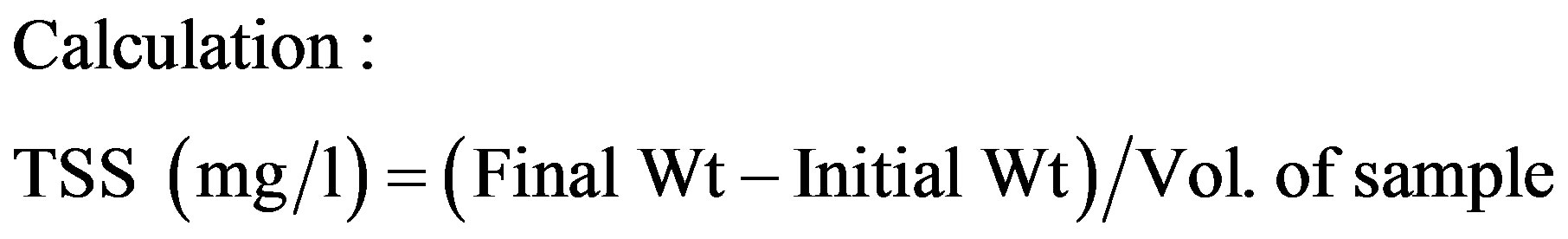
Chemical Oxygen Demand (COD) was measured by taking 0.4 g of Ag2SO4 in the reflux flask along with 20 ml of the pharmaceutical sludge, diluted with 20 ml distilled water. The content was shaken and 10 ml of 0.25 N K2 Cr2O7 solution was added, to it some glass beads are drooped and 30 ml of Ag2SO4∙H2SO4 reagent was added with continuously swirling (if the color changes to green add more K2Cr2O7 and Ag2SO4∙H2SO4 reagent). The content was mixed thoroughly; connected to condenser and slowly heated for 2 hours and cooled.
The condenser was washed with distilled water so that the washings fall into the flask and diluted to 150 ml with distilled water. The un-reacted K2Cr2O7 was titrated with N/10 Ferrous Ammonium Sulphate (FAS) solution using ferroin as indicator till the color changes from blue green to wine red. A blank experiment is carried out with distilled water instead of sample.
Calculation:
COD of water sample 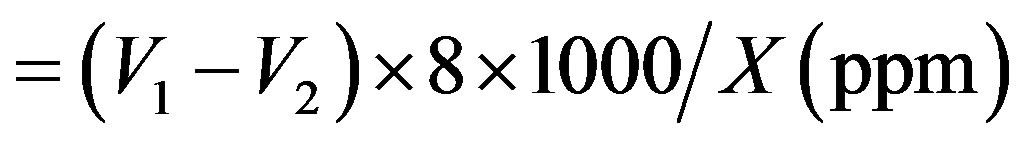
WhereV1 = Vol. of FAS consumed by blank;
V2 = Vol. of FAS consumed by sample;
N = Normality of FAS solution;
X = volume of sample taken.
2.4. Estimation of Chloride
Chloride estimation was done as per the method described in APHA [19]. Chloride solution was titrated against silver nitrate taking potassium chromate as indicator. The end point was noted through the formation of reddish brown precipitate. The volume of silver nitrate consumed was noted.
Amount of chloride present in 1 liter of solution was calculated by:
Normality × 35.5 × 1000 mg/l
2.5. Estimation of Sulphate
20 ml of sample was initially filtered to remove the suspended solids and was further taken for analysis. To this, 4 ml of 10% Barium chloride solution and 2 ml of dilute Sulphuric acid was added; which results into a white precipitate of Barium sulphate.
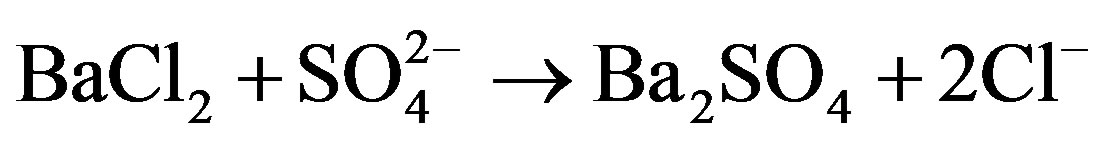
The solution was filtered through Whatman filter paper, whose initial weight was priorly known. Thenafter it was filtered, the filter paper was completely dried in hot air oven and final weight was noted.
Amount of sulphate = Final Wt. – Initial Wt./20 mg/l
2.6. Estimation of Chromium
The chromium present in the given sample was estimated spectrophotometrically by Diphenyl carbazide method [21]. Different concentrations of chromium standard solutions and sample solutions were taken in test tubes. To each test tube, 1ml each of 0.1 N concentrated Hydrochloric acid and Phosphoric acid was added, and allowed to rest for 5 minutes. Then after 0.05 ml of Diphenyl carbazide was added and tubes are allowed for further 15 minutes rest, to develop colour.
The absorbance was observed spectrophotometrically at 540 nm. A graph between absorbance and concentration of solution was plotted. Accordingly the amount of chromium present in the samples is estimated. The other parameters like pH and Dissolved Oxygen were measured by using pH meter and DO meter respectively.
2.7. Preparation of Bio Adsorbent
2.7.1. Vetiveria
The V. zizanioides root was washed well with distilled water and treated with 0.1 N NaOH solution for a period of 10 hrs, for delignification. It was further treated with 0.1 N shulphuric acid for about 4 - 5 hrs to remove the alkalinity, washed with distilled water till the washing water became colorless. The treated adsorbent material was dried in sun light and stored in desiccators.
2.7.2. Anabaena
Anabaena sps. were cultured in Allen-Arnon medium (gm/l), with the composition: Potassium nitrate, 2.02 g; Di potassium hydrogen phosphate tri hydrate, 0.456 g; Magnesium sulphate hepta hydrate, 0.24 g; Sodium chloride, 0.234 g; Calcium chloride, 0.074 g; Fogg’s micronutrient solution, 1 ml; Fe-EDTA complex, 0.8 ml; Distilled Water, 1000 ml. The wet biomass of Anabaena was utilized directly for biosorption experiment.
2.8. Optimization of Biosorption Parameters
To study the adsorption capacity of the Anabaena and Vetiveria, batch experiments were conducted at room temperature. The effect of pH ranging from 2 - 5; contact time ranging between 30 - 150 minutes at 180 rpm; adsorbent dosage ranges from 0.5 - 3 g. The suspension from 100 ml of effluent was filtered through Whatman filter paper no. 41 and the filtrate was analyzed for residual Cr(VI) concentration. The elution studies were performed with 0.1 N HCl, 0.1 N H2SO4, 0.1 N HNO3 and 0.1 M EDTA.
2.9. Adsorption Isotherms
Langmuir and Freundlich adsorption isotherm models were applied for Cr(VI) adsorption as per the method of Seidel-Morgenstern [22]. The Langmuir equation correlates the amount of adsorbate adsorbed with the equilibrium aqueous concentration can be given as
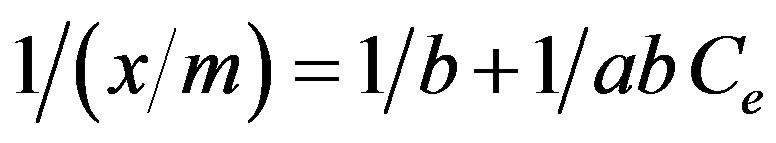
where “x” is the amount of Cr(VI) adsorbed in mg/100 ml, “m” is the weight of Cr(VI) adsorbent (g), Ce is the residual concentration of Cr(VI) at equilibrium in mg/100 ml. Langmuir constants “a” and “b” are the measures of maximum adsorption capacity and energy of adsorption given in Table 1.
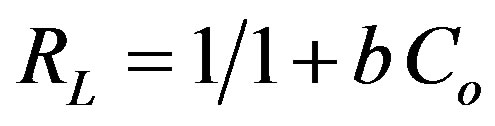
where Co is the initial concentration of the adsorbate and “b” the Langmuir constant.
The value of RL < 1, obtained in this study indicates the applicability of Langmuir adsorption isotherm.
The linear form of Freundlich equation is represented as follows [21].
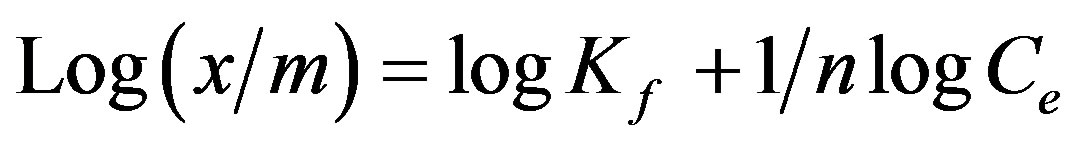
where “x” is the amount of Cr(VI) adsorbed in mg/100 ml, “m” is the weight of adsorbent (g), Ce is the residual concentration of Cr(VI) at equilibrium in mg/100 ml. Kf and 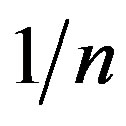 are Freundlich constants related to the adsorption capacity and adsorption intensity, respectively. The values of adsorption intensity
are Freundlich constants related to the adsorption capacity and adsorption intensity, respectively. The values of adsorption intensity 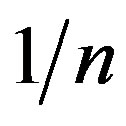 << 1 reveal the application of this adsorption isotherm.
<< 1 reveal the application of this adsorption isotherm.
3. RESULTS
3.1. Physicochemical Analysis of Chrome Plating Effluent
The effluent was slightly acidic in nature with the value of pH 4.1 - 5.5 and orange in colour. The COD and
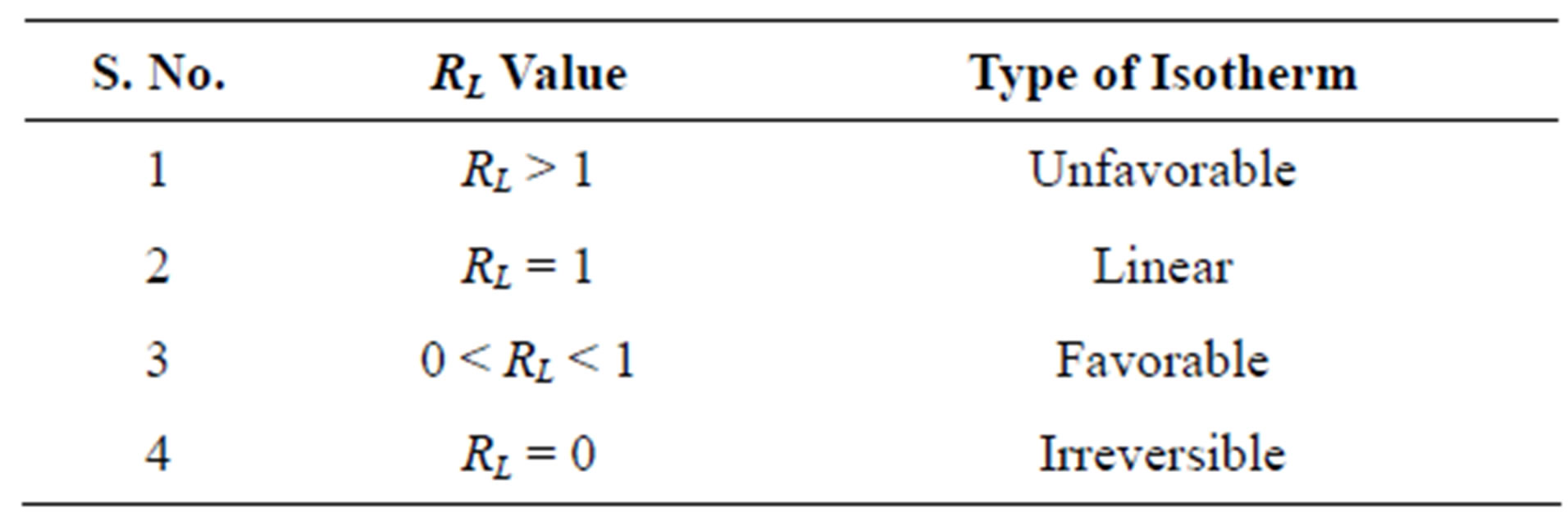
Table 1. Relation between RL value and the type of isotherm.
BOD were too high, lies between 248 - 431 mg/l and 170 - 262 mg/l respectively. TSS and TDS were present in the concentration of 3.8 - 5.4 and 21.0 - 23.5 mg/l respectively. Concentration of DO, SO4 and Cl2 varied from 6.8 - 7.6, 0.53 - 1.01 and 0.40 - 0.80 mg/l respectively and the chromium content was 1.05 - 4.32 mg/l, which is beyond the limit value as analyzed by Tamilnadu Pollution Control Board Norms.
3.2. Optimization of Biosorptive Parameters
3.2.1. Vetiveria zizanioides
1) Effect of pH pH is an important controlling factor in adsorption process and thus the role of hydrogen ion concentration was studied in the present investigation, covering range of 2 - 8. It was observed that, higher pH (4 - 8) has no effects on metal adsorption (Figure 1). At pH 3.5 chromium removal was increased 55% and at pH 2.5 metal sorption was maximum around 97%.
2) Effect of Agitation Time The uptake of Cr(VI) from effluent by activated Vetiveria root increased from 22% to 93%, when agitation time was varied from 30 to 120 min and attained equilibrium after 150 min at 30˚C (Figure 2). The increase in adsorption of Cr(VI) with increase in agitation time may
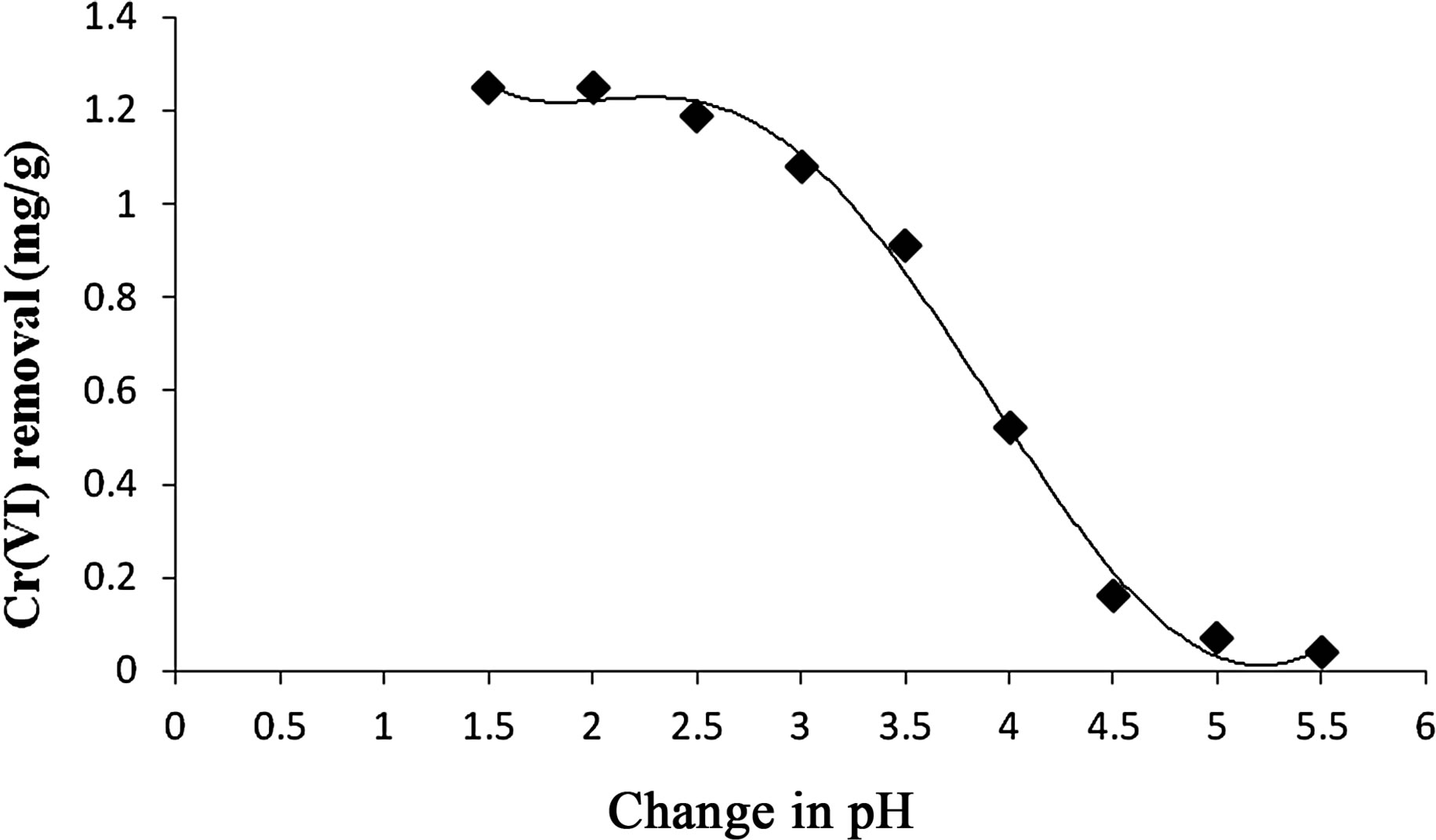
Figure 1. Effect of pH on Cr(VI) removal by Vetiveria zizanioides.
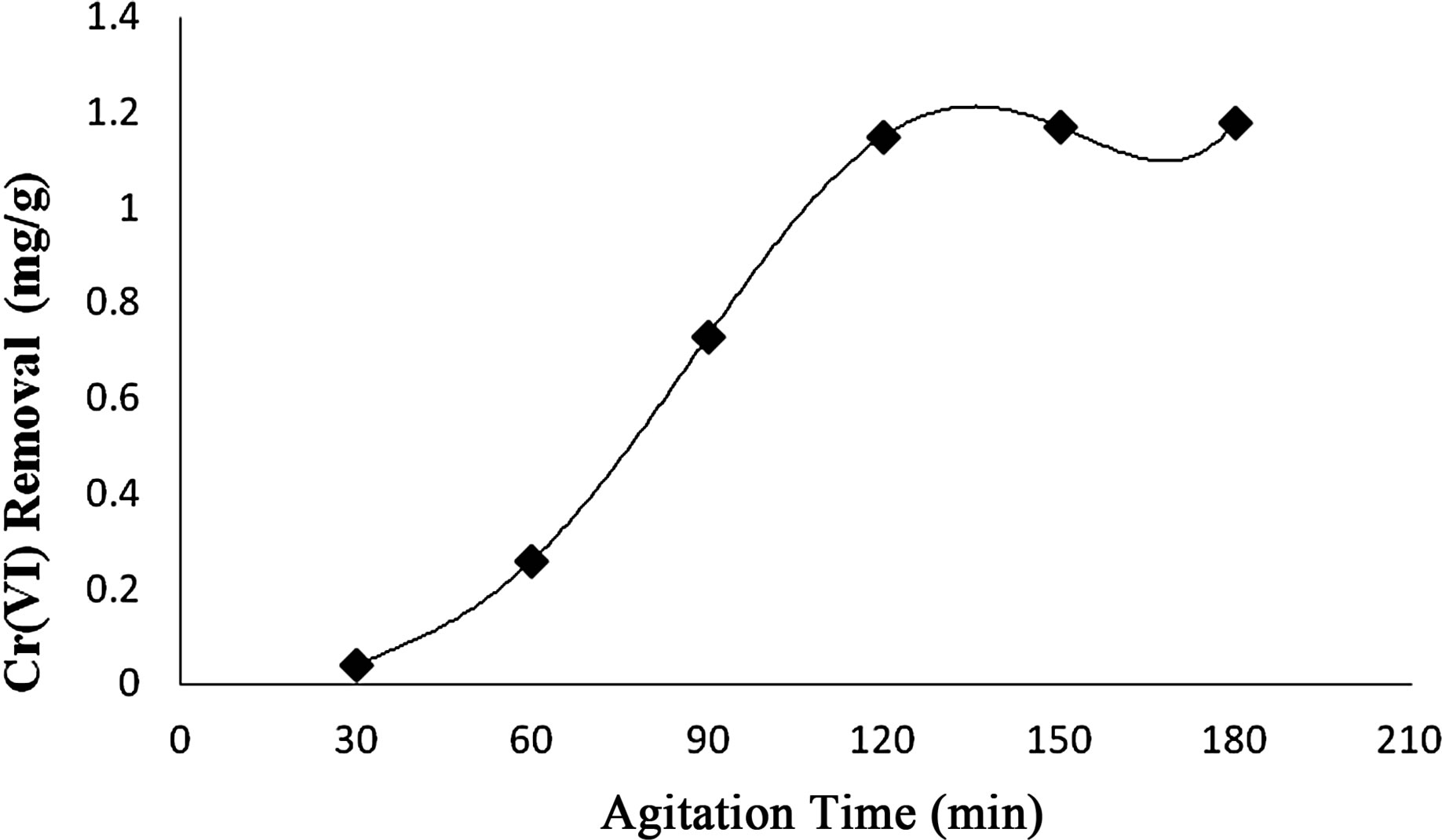
Figure 2. Effect of agitation time on Cr(VI) removal by Vetiveria zizanioides.
be attributed to the increased intra-particle diffusion that occurred at longer shaking period.
3) Effect of Adsorbent Dosage Increase in adsorbent dosage increased the adsorption of Cr(VI) species, batch adsorption studies were carried out at pH 2.5 and 30˚C. The adsorbent dosage was varied from 0.5 g to 3.0 g of plant biomass and the removal of Cr(VI) was found to be increased from 30% - 80%. This is to be expected because for a fixed effluent concentration increasing total adsorbent doses, provides a greater surface area or adsorption site, thus the adsorption increases with adsorbent dosage as depicted in Figure 3.
4) Elution Studies Because of the economic success and reuse of the biomass, elution studies have an importance for the bioadsorption process. The efficiency of elution and preservation of bioadsorption capacity of bioadsorbent are important factors to choose the elution agents. Based on the optimized conditions viz., pH 2.5, contact time 120 min and adsorbent dosage of 1 g for Vetiveria, various eluting agents such as 0.1 N HCl, HNO3, H2SO4 and 0.1 M EDTA were utilized to study the removal and recovery capacity. It was observed that 0.1 N H2SO4 eluted maximum 86.3% of Cr(VI) from the aqueous solution (Figures 4 and 5).
5) Adsorption Isotherm Based on Freundlich and Langmuir formulae it was found that adsorption dosage of 1 g of Vetiveria was sufficient to adsorb maximum Cr(VI) i.e. 86.3% as depicted in Figures 6 and 7.
3.2.2. Anabaena
1) Effect of pH The uptake of the metallic cations by Anabaena trimed down with increase in pH. Hence, the change of pH affects the adsorption process through dissociation of func-
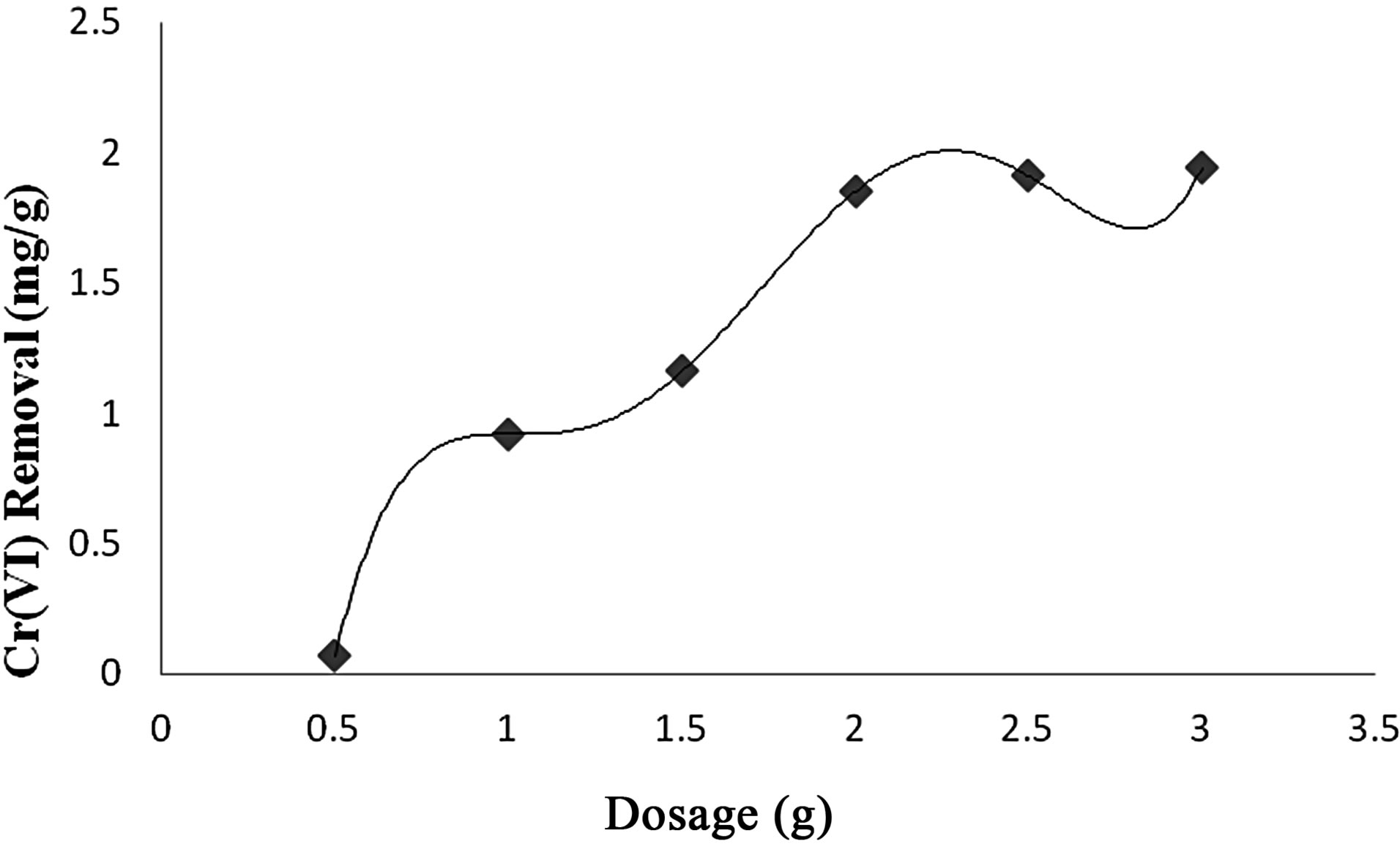
Figure 3. Effect of adsorbent dosage on Cr(VI) removal by Vetiveria zizanioides.
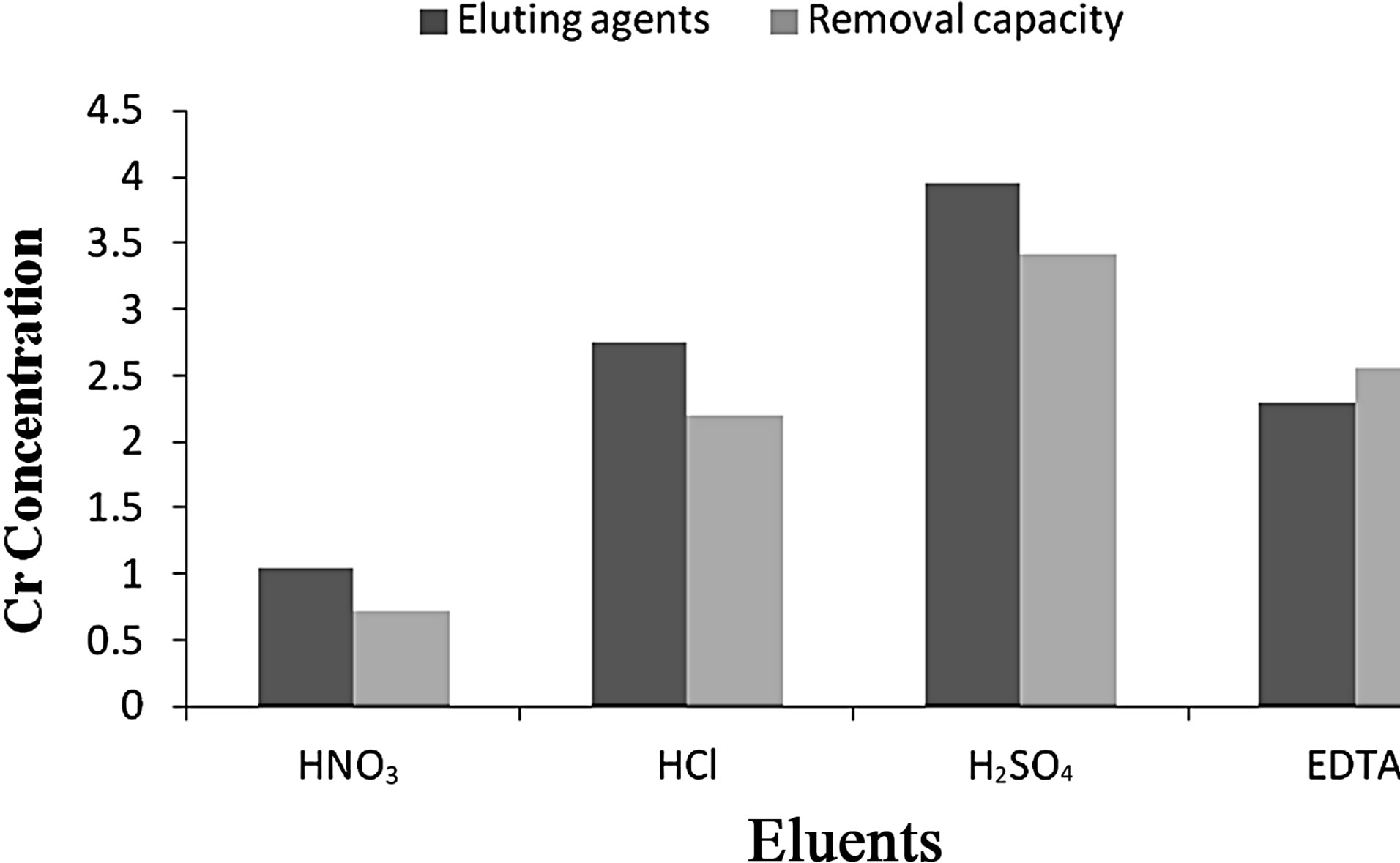
Figure 4. Effect of eluting agents on Cr(VI) removal by Vetiveria zizanioides.
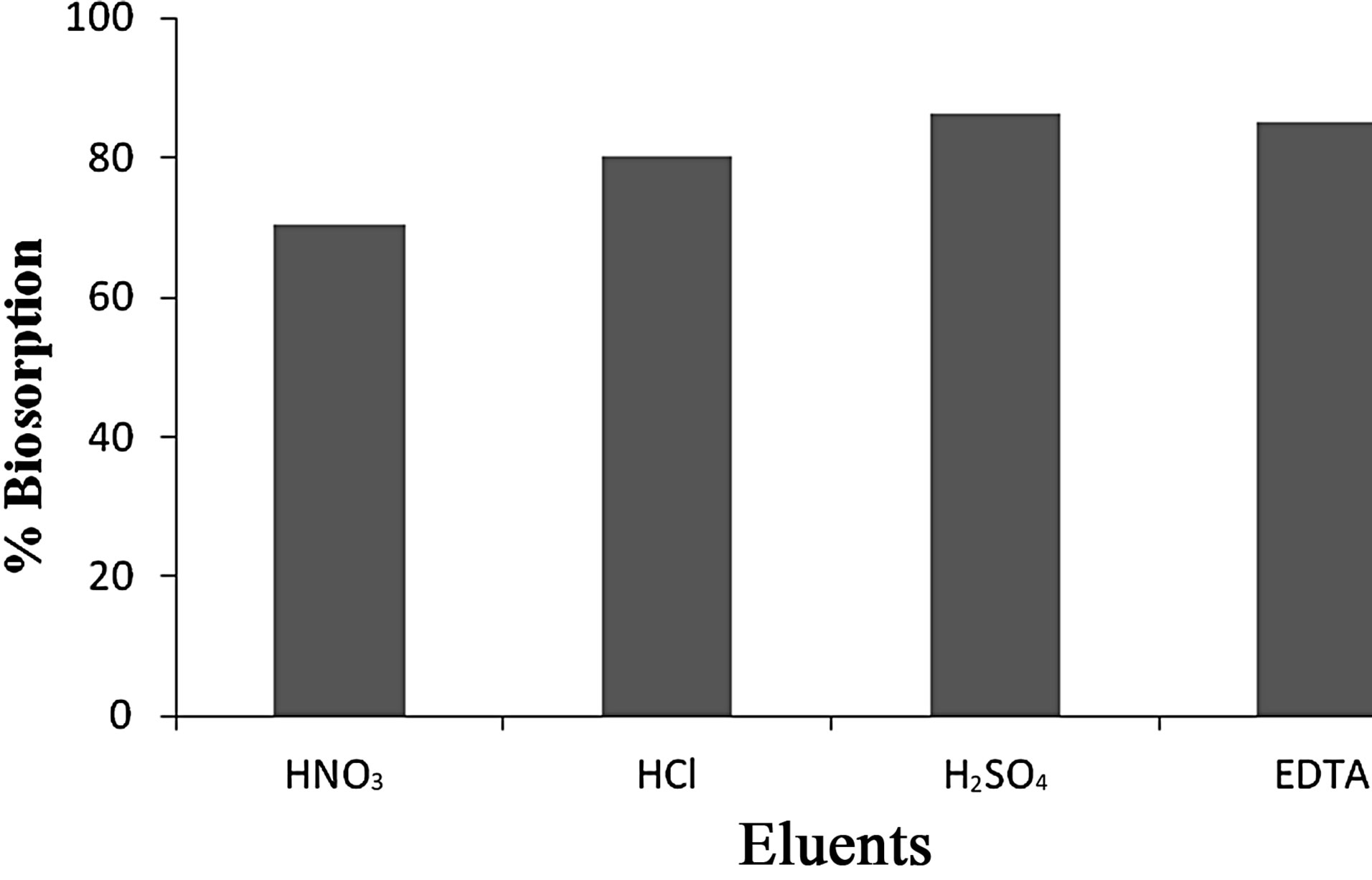
Figure 5. Percentage removal/recovery capacity of Cr(VI) by Vetiveria zizanioides.
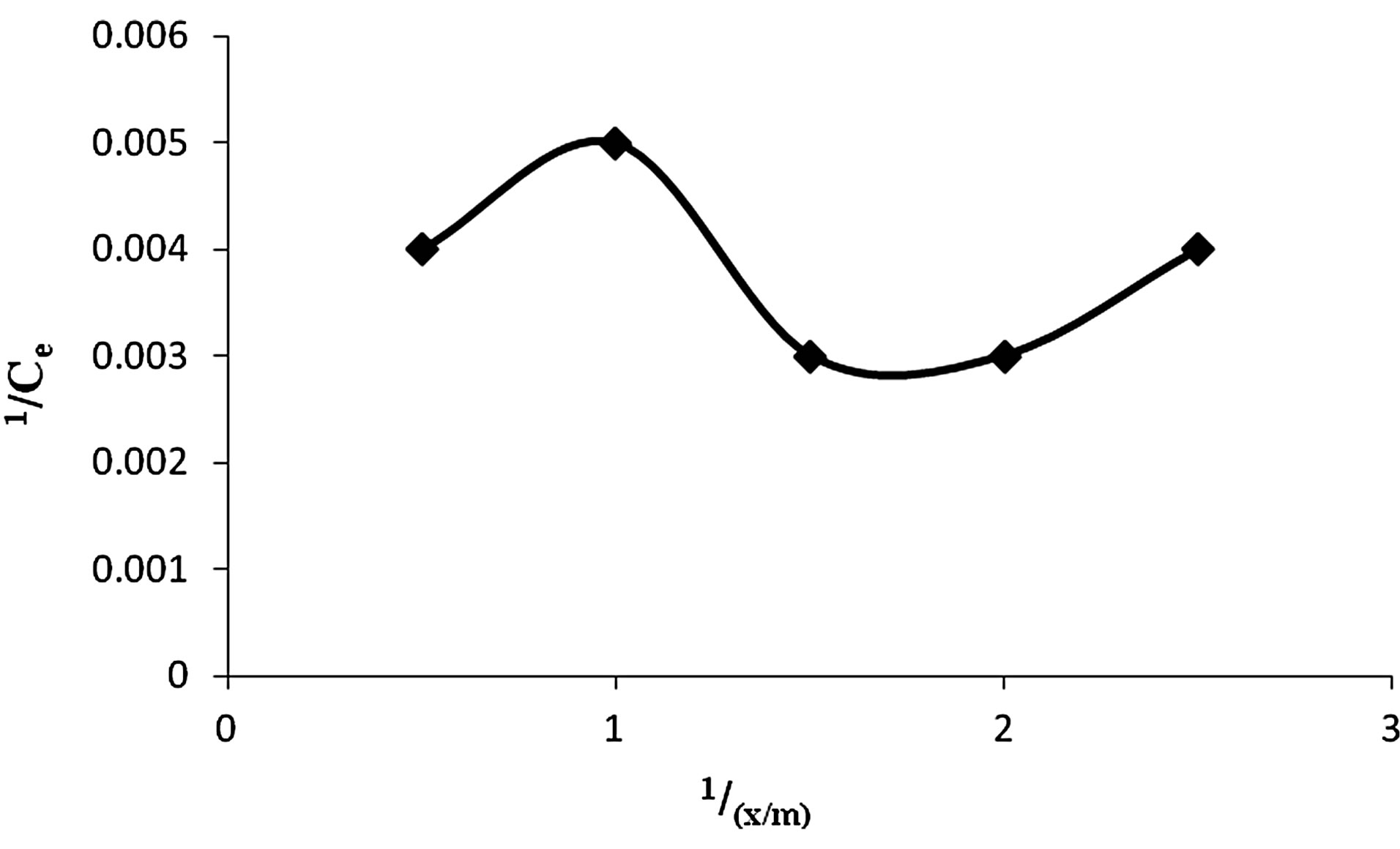
Figure 6. Langmuir adsorption isotherm for adsorption of Cr(VI) by Vetiveria zizanioides.
tional groups on the adsorbent and adsorbate. Physical parameter like pH is an important controlling factor in adsorption process and thus the role of hydrogen ion concentration was studied in the present investigation, covering range of pH 2 - 5.5. The effect of pH on the adsorption of Cr(VI) is shown in Figure 8. It was observed that the higher pH 4 - 8, has no effects on metal adsorption. At lower pH, large number of H+ ions neutralizes the negatively charged Anabaena or converts a
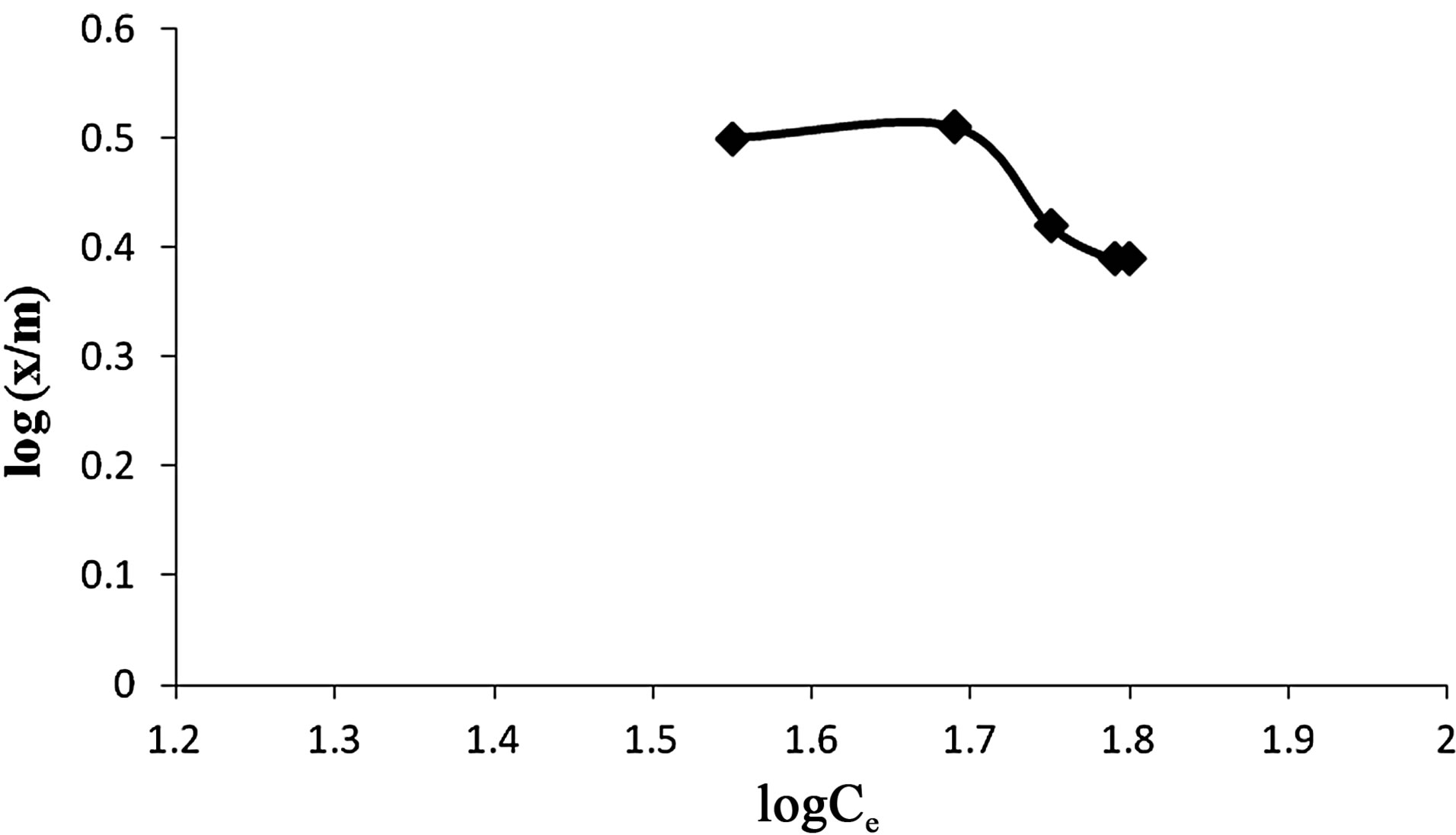
Figure 7. Freunlich adsorption isotherm for adsorption of Cr(VI) by Vetiveria zizanioides.
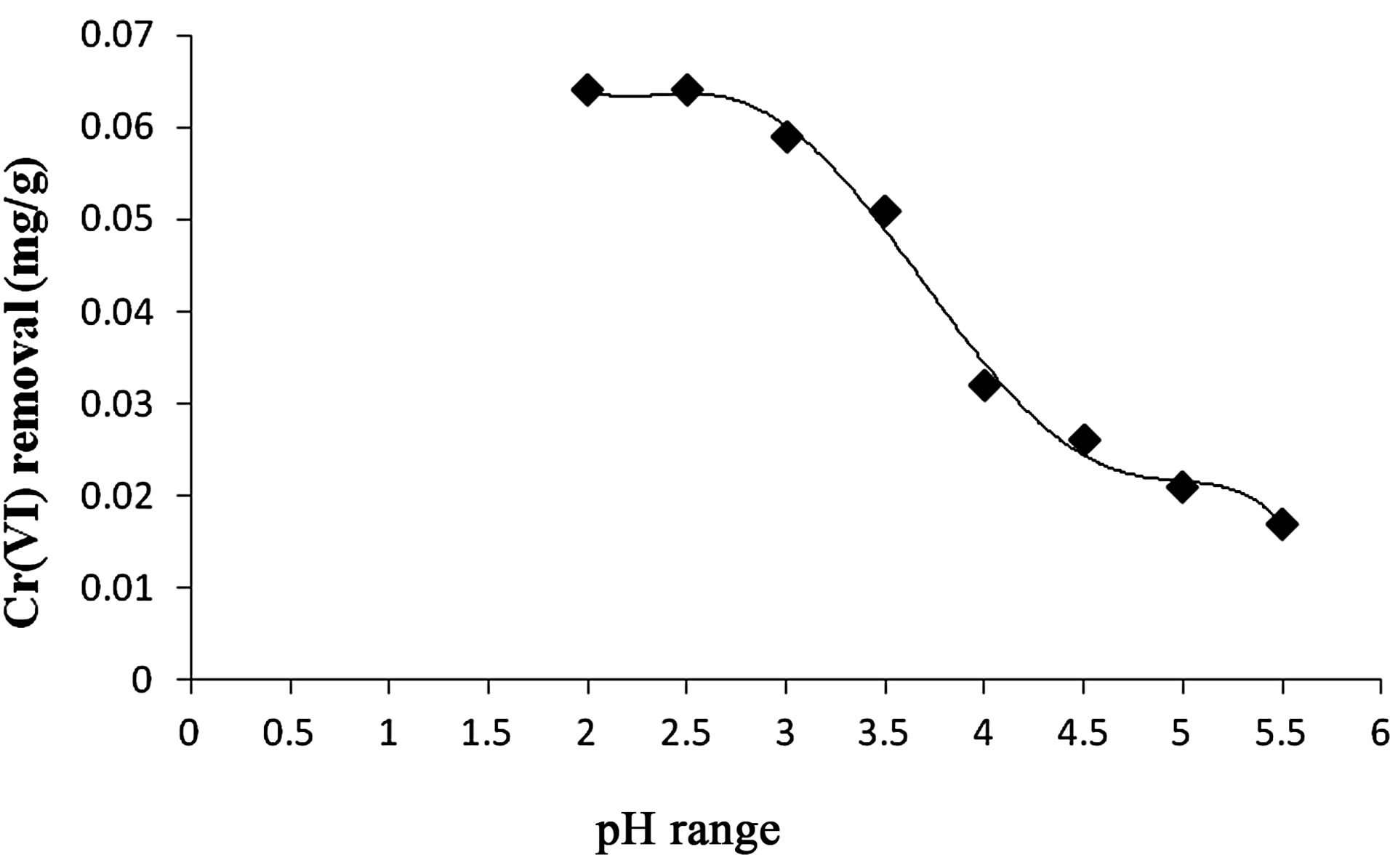
Figure 8. Effect of pH on Cr(VI) removal by Anabaena.
neutral group to a positively charged group and thus, enhances the adsorption of Cr(VI) species. At pH 2.5 in 60 min of agitation time, equilibrium was attained.
2) Effect of Agitation Time The uptake of Cr(VI) from effluent by Anabaena was found to increased, when the agitation time varied around 30 to 120 min and equilibrium was attained after 150 min at 30˚C (Figure 9).
3) Effect of Adsorption Dosage At pH 2.5 batch adsorption studies were carried out at 30˚C and adsorption of Cr(VI) species was found to be improved with increase in adsorbent dosage. The adsorbent dosage was varied from 0.5 g to 3.0 g of wet biomass of Anabaena (Figure 10).
4) Elution Studies Depending on the optimized conditions viz., pH 2.5, contact time 60 min and adsorbent dosage of 1 g for Anabaena various eluting agents such as 0.1 N HCl, HNO3, H2SO4 and 0.1 M EDTA were utilized to study the removal and recovery capacity. From the experiments it was observed that 0.1 M EDTA eluted maximum of 88.86% of Cr(VI) from the aqueous solution as seen in Figures 11 and 12.
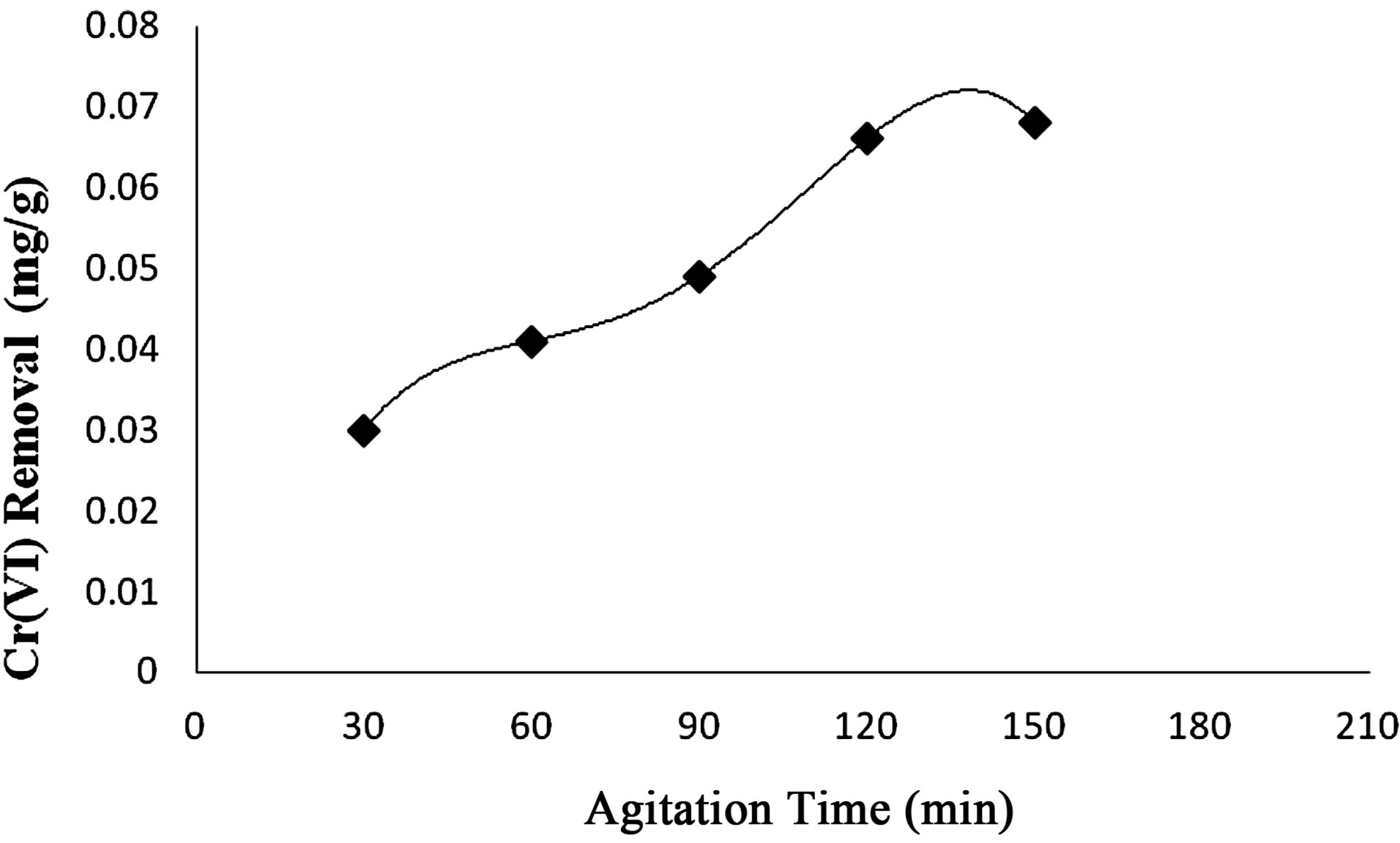
Figure 9. Effect of agitation time by Anabaena.
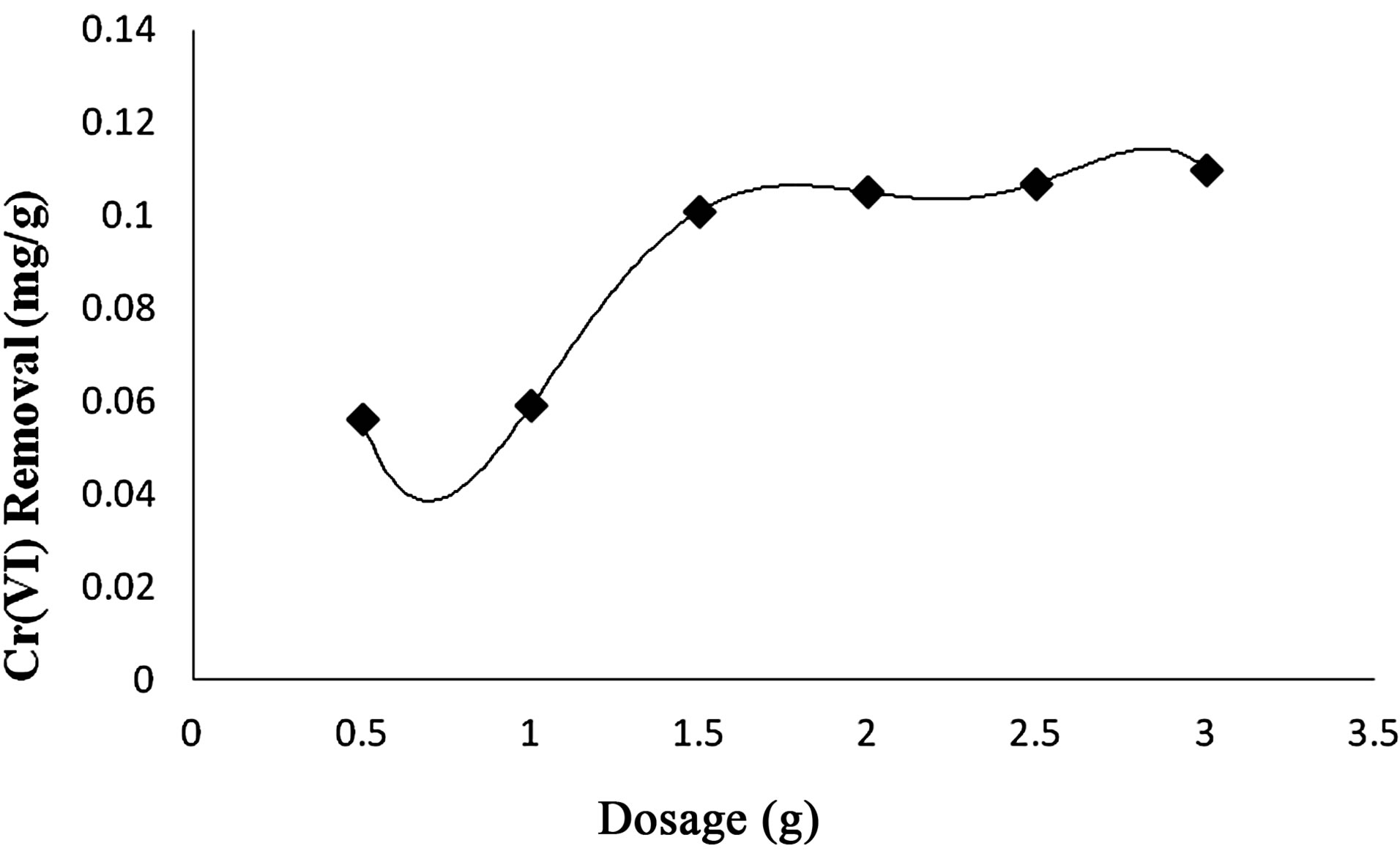
Figure 10. Effect of adsorbent dosage on Cr(VI) Removal by Anabaena.
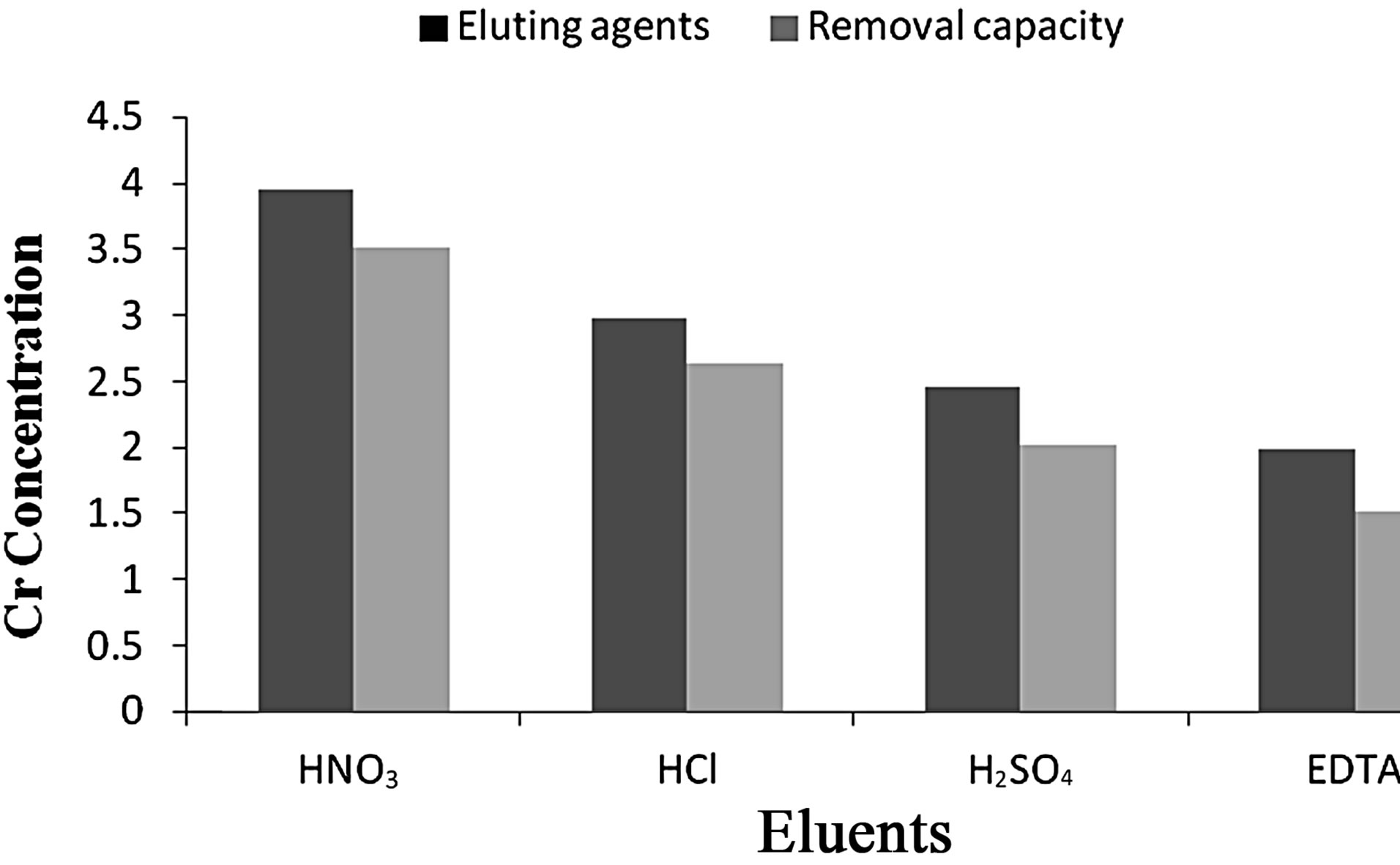
Figure 11. Effect of eluting agents on Cr(VI) removal by Anabaena.
5) Adsorption Isotherm Based on Freundlich and Langmuir formulae it was found that adsorption dosage of 0.5 g of wet biomass of Anabaena was sufficient to adsorb maximum Cr(VI) i.e. 88.86% (Figures 13 and 14).
4. DISCUSSION
The key factors for preparation for bioadsorbent depend on its morphological properties such as particle size and shape, binding surface area, and overall effluent re-
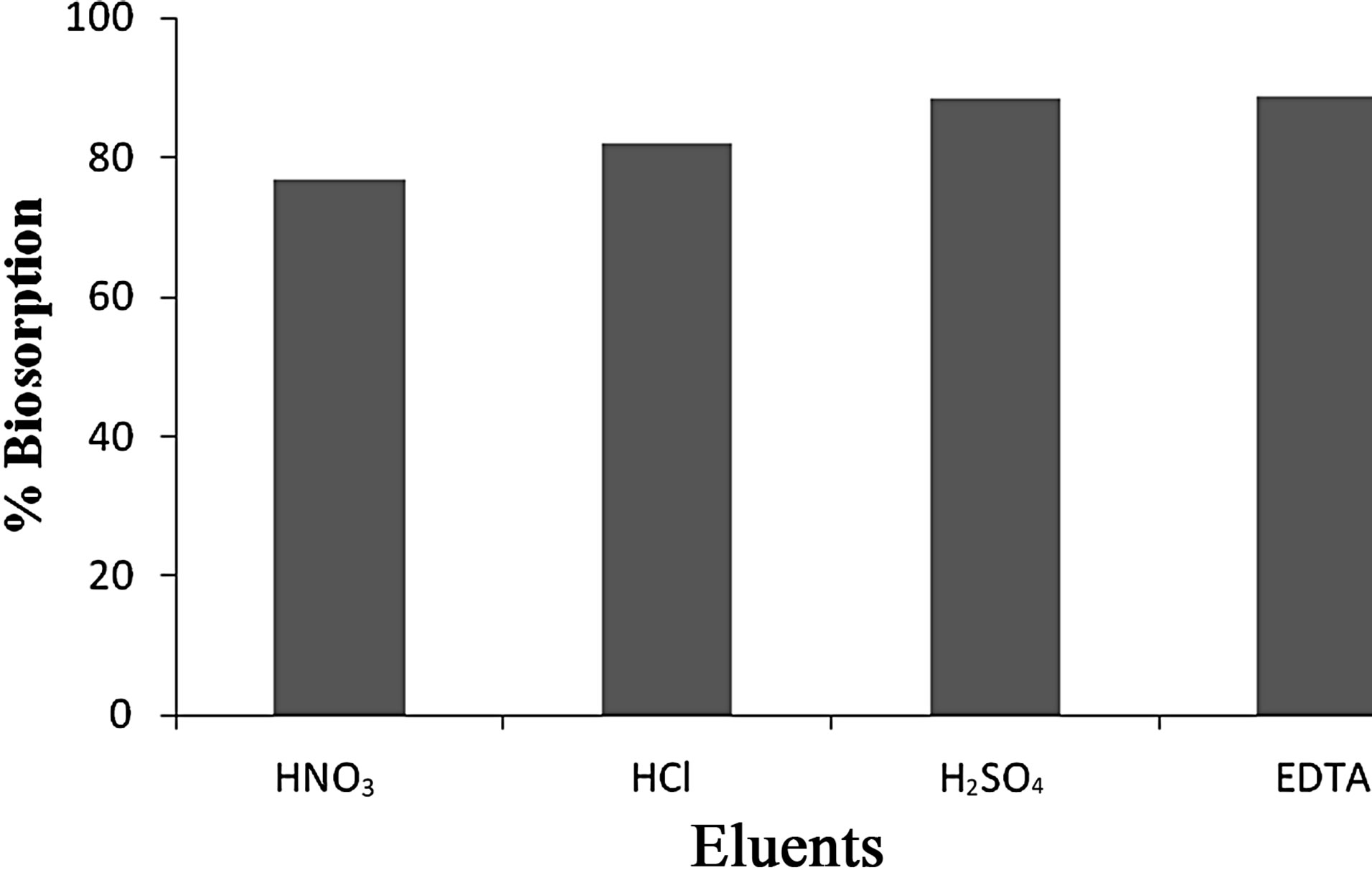
Figure 12. Percentage removal/recovery capacity of Cr(VI) by Anabaena.
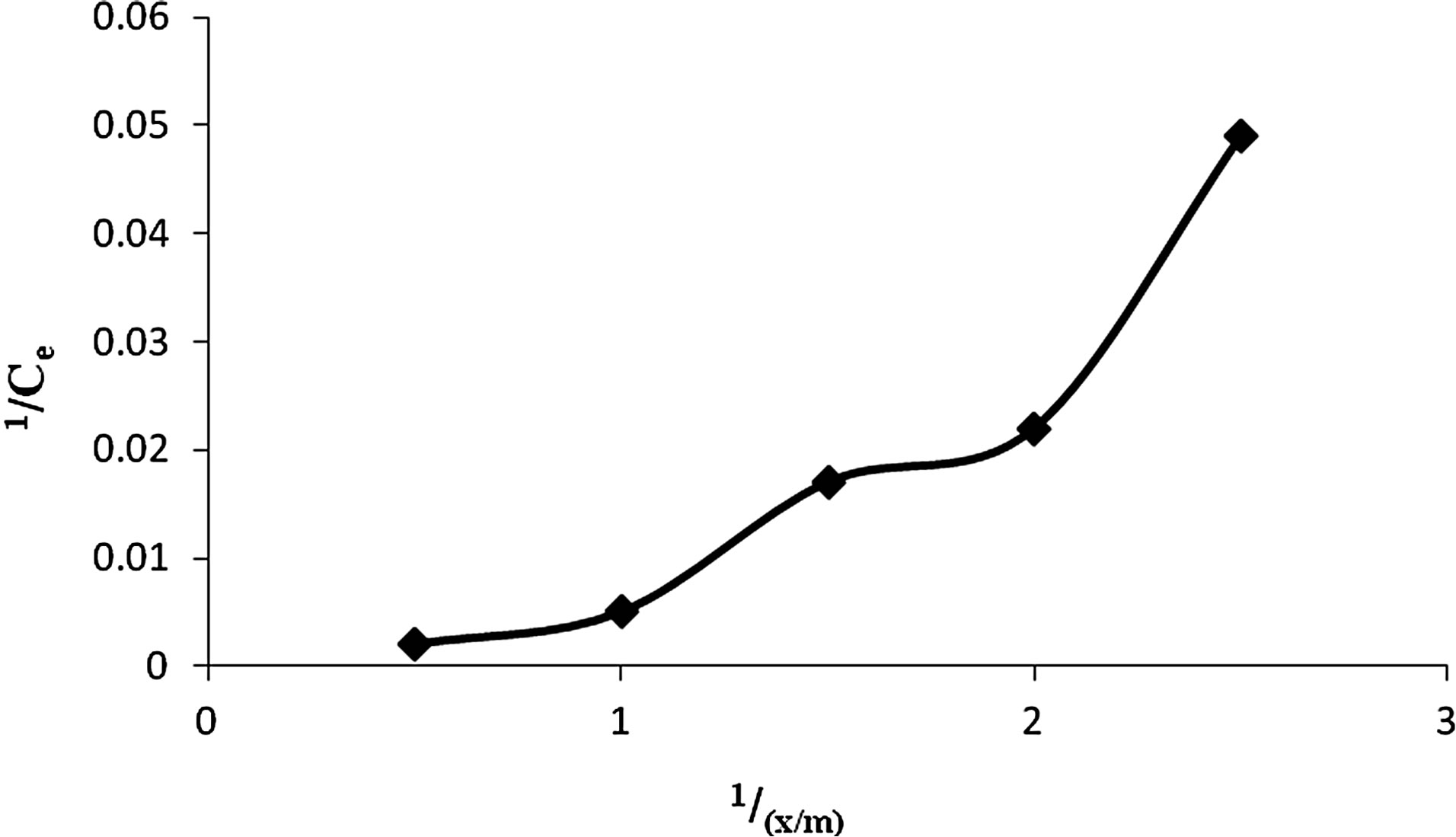
Figure 13. Langmuir adsorption isotherm for adsorption of Cr(VI) by Anabaena.
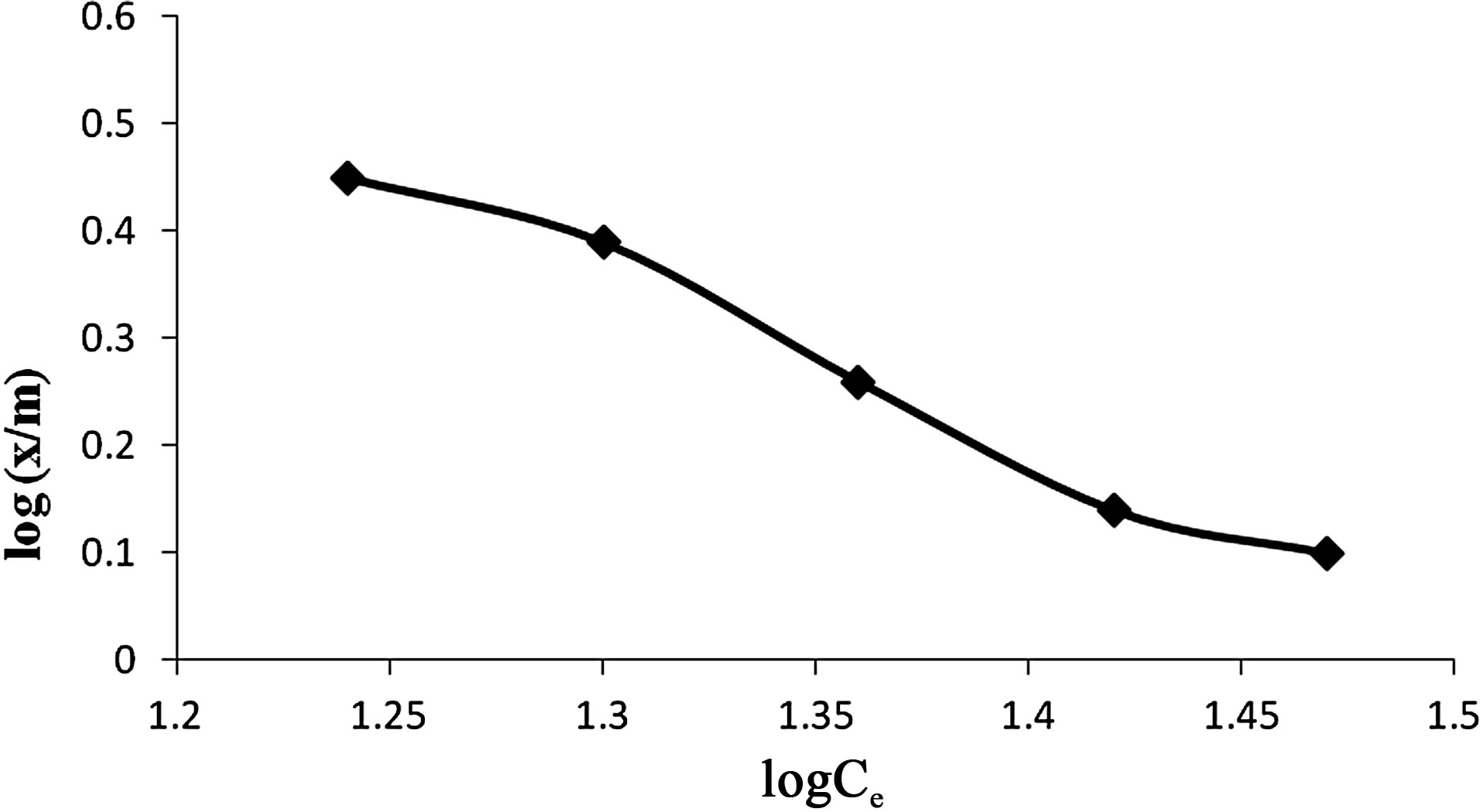
Figure 14. Freunlich adsorption isotherm for adsorption of Cr(VI) by Anabaena.
moval capacity. The two selected bioadsorbents were conditioned/optimized based on morphological properties, binding surface area, and overall Cr(VI) removal capacity. The eco-friendly approach on lower cost was found superior in all the parameters [23-25].
The pH of the metal solution is the most influential factor as it affects surface properties of the adsorbent and metal speciation. The uptake of the metallic cations by adsorbent is reduced at pH below 3 and above 8; lowest at its isoelectric pH and highest at alkaline pH range as observed by Pagnanelli et al. [26]. Hence, the change of pH affects the adsorption process through dissociation of functional groups on the adsorbent and adsorbate. Similar results were obtained by many researchers [27,28].
Variation in adsorbent dosage increased the removal of Cr(VI) upto 80%. This is to be expected because for a fixed effluent concentration increasing total adsorbent doses, provides a greater surface area or adsorption site. This kind of observation was reported by Stephen et al. [29] with the removal of Pb using lignite. Water lettuce (Pistia stratiotes L.), an aquatic plant, removed arsenate [30]. Young plants were harvested from a pollution-free pond and hydroponically cultured, effectively absorbed arsenic in a range from 0.25 to 5.0 mg/L. From 22.8% to 82.0% of the As was removed for a biomass loading of 20 g/L at pH 7.0 after 144 h. The sorption capacity was 1.43 mg/g of biomass. Aspergillus niger, coated with iron oxide removed 95% of As(V) and 75% of As(III)) at a pH of 6. No strong relationship was reported between the surface charge of the biomass and arsenic removal [31].
Esmaeili et al. [32] employed activated carbon prepared from Gracilaria and obtained more than 90% removal of Cu from waste water. With advantages of high metal biosorption and desorption capacities, the biomass of Azolla is a promising application as a cost-effective biosorbent material for the removal of copper and nickel ions from wastewater [32]. The Comparison of various literature studies of Cu2+ removal by biosorption was considered by Ahmady-Asbchin [33].
The low elution efficiency of HNO3 solution can be attributed to the greater affinities of divalent cations for the negative charged sites on the bioadsorbent than monovalent cations. HCl and EDTA showed the maximum efficiency for the desorption process. This result obtained with EDTA can be attributed to the strong complexing ability to Cr(VI). HCl was selected as an effective desorbing agent due to the similar result with EDTA, and low cost of HCl [34].
The mathematical calculations with reference to Langmuir and Freundlich adsorption isotherm, SeidelMorgenstern [22] revealed the suitable dosages that were sufficient for maximum adsorption of Cr(VI) and that were employed in our study.
5. CONCLUSION
The selected bioadsorbents, Vetiveria and Anabaena have proved to be high capacitate, economically viable and low cost adsorbent for Cr(VI) removal. Adsorption of Cr(VI) on these two selected bioadsorbents shows high association with Langmuir and Freundlich isotherm model. This present study can conclude that both Vetiveria and Anabaena are favourable alternate of chromium removal from water effluents.
REFERENCES
- Dorfner, K. (1972) Ion exchangers: Properties and applications. Ann Arbor Science, Newton, 177.
- Pajunen, P. (1995) Hard chrome bath purification and recovery using ion exchange. Metal Finishing, 93, 40-45. doi:10.1016/S0026-0576(05)80048-0
- Schiewer, S. and Volesky, B. (1997) Ionic strength and electrostatic effects in biosorption of protons. Environmental Science and Technology, 31, 1863-1871. doi:10.1021/es960434n
- Fourest, E. and Volesky, B. (1996) Contribution of sulphonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environmental Science and Technology, 30, 277-282. doi:10.1021/es950315s
- Barba, D., Beolchini, F. and Vegliò, F. (2001) A simulation study on biosorption of heavy metals by confined biomass in UF/MF membrane reactors. Environmental Science and Technology, 35, 3048.
- Truong, P. and Hart, B. (2001) Vetiver system for wastewater treatment. Technical Bulletin No. 2001/21, Pacific Rim Vetiver Network, Office of the Royal Development Projects Board, Bangkok.
- Ash, R. and Truong, P. (2004) The use of vetiver grass for sewerage treatment. Proceedings of Sewage Management Queensland EPA Conference: Risk Assessment and Triple Bottom Line, Cairns, 5-7 April 2004.
- Roongtanakiat, N. and Chairoj, P. (2001a) Uptake potential of some heavy metals by vetiver grass. Kasetsart Journal (Natural Science), 35, 46-50.
- Roongtanakiat, N. and Chairoj, P. (2001b) Vetiver grass for the remediation of soil contaminated with heavy metals. Kasetsart Journal (Natural Science), 35, 433-440.
- Roongtanakiat, N. (2009) Vetiver phytoremediation for heavy metal decontamination. PRVN Technical Bulletins No. 2009/1. ORDPB, Pacific Rim Vetiver Network, Office of the Royal Development Projects Board, Bangkok.
- Hart, B., Cody, R. and Truong, P. (2003) Efficacy of vetiver grass in the hydroponic treatment of post septic tank effluent. Proceedings of the 3rd International Conference on Vetiver and Exhibition, Guangzhou, 6-9 October 2003.
- Roongtanakiat, N., Tangruangkiat, S. and Meesat, R. (2007) Utilization of vetiver grass (Vetiveria zizanioides) for removal of heavy metals from industrial wastewaters. Science Asia, 33, 397-403. doi:10.2306/scienceasia1513-1874.2007.33.397
- Percy, I. and Truong, P. (2005) Landfill leachate disposal with irrigated vetiver grass. Proceedings of 2005 National Conference on Landfill, Brisbane.
- Kong, X.H., Lin, W.W. and Wang, B.Q. (2003) Study on vetiver’s purification for wastewater from pig farm. Proceedings of the 3rd International Conference on Vetiver and Exhibition, Guangzhou, 6-9 October 2003.
- Figueira, M.M., Volesky, B., Ciminelli, V.S.T. and Roddick, F.A. (2000) Biosorption of metals in brown seaweed biomass. Water Research, 34, 196-204. doi:10.1016/S0043-1354(99)00120-7
- Kumar, J.I.N., Oommen, C. and Kumar, R.N. (2009) Biosorption of heavy metals from aqueous solution by green marine macroalgae from Okha Port, Gulf of Kutch, India. American-Eurasian Journal of Agricultural & Environmental Sciences, 6, 317-323.
- Khorramabadi, G.S. and Soltani, R.D.C. (2008) Evaluation of the marine algae Gracilaria salicornia and Sargassum sp. for the biosorption of Cr(VI) from aqueous solutions. Journal of Applied Sciences, 8, 2163-2167. doi:10.3923/jas.2008.2163.2167
- Huang, C.P. and Wu, M.H. (1977) The removal of chromium(VI) from dilute aqueous solution by activated carbon. Water Research, 11, 673-679. doi:10.1016/0043-1354(77)90106-3
- APHA (American Public Health Association) (2005) Standard methods for the examination of water and wastewater. 21st Edition, American Public Health Association, Washington DC.
- APHA (American Public Health Association) (1985) American health association standards for examination of waste waters. 18th Edition, American Public Health Association, New York.
- Mabbett, A.N., Lloyd, J.R. and Macaskie, L.E. (2002) Effect of complexing agents on reduction of Cr(VI) by Desulfovibrio vulgaris ATCC 29579. Biotechnology and Bioengineering, 79, 389-397. doi:10.1002/bit.10361
- Seidel-Morgenstern, A. (2004) Experimental determination of single solute and competitive adsorption isotherms. Journal of Chromatography A, 1037, 255-272. doi:10.1016/j.chroma.2003.11.108
- Gao, H., Liu, Y., Zeng, G., Xu, W., Li, T. and Xia, W. (2008) Characterization of Cr (VI) removal from aqueous solutions by a surplus agricultural waste-Rice straw. Journal of Hazardous Materials, 150, 446-452. doi:10.1016/j.jhazmat.2007.04.126
- Basha, S., Murthy, Z.V.P. and Jha, B. (2008) Biosorption of hexavalent chromium by chemically modified seaweed, Cystoseira indica. Chemical Engineering Journal, 137, 480-488. doi:10.1016/j.cej.2007.04.038
- Al-Asheh, S., Banat, F., Al-Omari, R. and Duvnjak, Z. (2000) Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemosphere, 41, 659-665. doi:10.1016/S0045-6535(99)00497-X
- Pagnanelli, F., Esposito, A., Toro, L. and Veglio, F. (2003) Metal speciation and pH effect on Pb, Cu, Zn and Cd biosorption onto Sphaerotilus natans: Langmuir-type empirical model. Water Research, 37, 627-633. doi:10.1016/S0043-1354(02)00358-5
- Moussavi, G. and Barikbin, B. (2010) Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chemical Engineering Journal, 162, 893-900. doi:10.1016/j.cej.2010.06.032
- Sahranavard, M., Ahmadpour, A. and Doosti, M.R. (2011) Biosorption of hexavalent chromium ions from aqueous solutions using almond green hull as a low-cost biosorbent. European Journal of Scientific Research, 58, 392- 400.
- Stephen, B., Inbaraj, B. and Sulochana, N. (2002) Basic dye adsorption on a low cost carbonaceous sorbent-kinetic and equilibrium studies. Indian Journal of Chemical Technology, 9, 201-208.
- Basu, A., Kumar, S. and Mukherjee, S. (2003) Arsenic reduction from aqueous environment by water lettuce (Pistia stratiotes L.). Indian Journal of Environmental Health, 45, 143-150.
- Pokhrel, D. and Viraraghavan, T. (2006) Arsenic removal from an aqueous solution by a modified fungal biomass. Water Research, 40, 549-552. doi:10.1016/j.watres.2005.11.040
- Esmaeili, A., Ghasemi, S. and Rustaiyan, A. (2008) Evaluation of the activated carbon prepared from the algae Gracilaria for the biosorption of Cu(II) from aqueous solutions. African Journal of Biotechnology, 7, 2034-2037.
- Ahmady-Asbchin, S., Omran, A.N. and Jafari, N. (2012) Potential of Azolla filiculoides in the removal of Ni and Cu from wastewaters. African Journal of Biotechnology, 11, 16158-16164.
- Alpat, S., Alpat, S.K., Cadirci, B.H., Ozbayrak, O. and Yasa, I. (2010) Effects of biosorption parameter: Kinetics, isotherm and thermodynamics for Ni(II) biosorption from aqueous solution by Circinella sp. Electronic Journal of Biotechnology, 13, 5.

