Health
Vol.5 No.7A2(2013), Article ID:34619,11 pages DOI:10.4236/health.2013.57A2004
A candidate identification questionnaire for postmenopausal osteoporosis patients switched from daily or weekly bisphosphonate to once-monthly ibandronate: An open, prospective, multicenter study—BONCURE study*
![]()
1Department of PMR, School of Medicine, Hacettepe University, Ankara, Turkey
2Department of PMR, Istanbul School of Medicine, Istanbul University, Istanbul, Turkey
3Department of PMR, School of Medicine, Uludag University, Bursa, Turkey
4Department of PMR, School of Medicine, Gazi University, Ankara, Turkey
5Department of PMR, School of Medicine, Selcuk University, Konya, Turkey
6Department of PMR, School of Medicine, Ege University, Izmir, Turkey
7Department of PMR, School of Medicine, Pamukkale University, Denizli, Turkey
8Klinicki Bolnicki Centar, Zagreb, Croatia
9Clinical Hospital Centre, Split, Croatia
10Clinic of Rheumatology, Clinical Centre, Skopje, Macedonia
11Clinic of Endocrinology, Diabetes and Metabolic Disorders, Skopje, Macedonia
12Clinic for Orthopaedic Surgery, Clinical Centre Vodnjansk, Skopje, Macedonia
13Institute of Rheumatology, Belgrade, Serbia
14Institute of Endocrinology CCS, Belgrade, Serbia
15Institut Za Prevenciju, Lecenje I Rehabilitaciju, Niska Banja, Serbia
16Clinical Center of Vojvodina, Novi Sad, Serbia
17Orthopaedic Department, University Hospital Center “Mother Teresa”, Tirana, Albania
18Department of Rheumatology, University Hospital Center “Mother Teresa”, Tirana, Albania
19Institute for Nuclear Medicine, Sarajevo, Bosnia and Herzegovina
20Institute of Physical Medicine & Rehabilitation, Sarajevo, Bosnia and Herzegovina
21University Hospital Center of Tuzla, Tuzla, Bosnia and Herzegovina
22Clinical Center, Banja Luka, Bosnia and Herzegovina
23Department of PMR, School of Medicine, Akdeniz University, Ankara, Turkey
24Department of PMR, School of Medicine, Marmara University, Istanbul, Turkey
25Department of PMR, School of Medicine, Celal Bayar University, Manisa, Turkey
26Department of PMR, School of Medicine, Adnan Menderes University, Aydin, Turkey
27Department of PMR, School of Medicine, Ankara University, Ankara, Turkey
28Department of PMR, School of Medicine, Dokuz Eylul University, Izmir, Turkey
29Department of PMR, Cerrahpasa School of Medicine, Istanbul University, Istanbul, Turkey
30Department of PMR, School of Medicine, Cukurova University, Adana, Turkey
31Department of PMR, School of Medicine, Karadeniz Technical University, Trabzon, Turkey
32Department of PMR, School of Medicine, Ataturk University, Erzurum, Turkey
33Department of PMR, School of Medicine, Gaziantep University, Gaziantep, Turkey
34Department of PMR, School of Medicine, Ondokuz Mayis University, Samsun, Turkey
35Department of PMR, School of Medicine, Erciyes University, Kayseri, Turkey
36Department of PMR, Dr. Josip Bencevic General Hospital, Slavonski Brod, Croatia
37Department of PMR, Polyclinic for Internal Medicine, Zagreb, Croatia
38Department of PMR, University Hospital Dubrava, Zagreb, Croatia; #Corresponding Author: ygkutsal@gmail.com
39Department of PMR, University Hospital “Sestre Milosrdnice”, Zagreb, Croatia
40Department of PMR, Clinical Hospital Center Rijeka, Rijeka, Croatia
41Roche Pharmaceuticals, Istanbul, Turkey
Copyright © 2013 Yesim Gokce Kutsal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 9 May 2013; revised 14 June 2013; accepted 2 July 2013
Keywords: Bisphosphonate; Compliance; Ibandronate; Postmenopausal Osteoporosis
ABSTRACT
A candidate identification questionnaire (CIQ) was tested to determine its predictive value for patient-reported satisfaction in patients switched from once-weekly or once-daily treatment with a bisphosphonate to once-monthly dosing. This was a prospective, open-label, multicenter international study in patients with postmenopausal osteoporosis who had been receiving once-daily or once-weekly alendronate or risendronate for at least 3 months. Patients completed a CIQ, then commenced 150 mg monthly ibandronate for 6 months. Patients completed the Osteoporosis Patient Satisfaction Questionnaire (OPSAT-QTM) at baseline for 6 months. Scores were converted to composite satisfaction scores (CSS, scale 0 - 100). Totally 677 patients completed a CIQ, 645 were enrolled in the treatment phase and comprised the intent-to-treat (ITT) population, and 630 completed the study. In the ITT population, 68.1% patients answered “yes” to one or more CIQ questions. OPSAT-Q scores increased for the convenience, quality of life and overall satisfaction domains (p < 0.001). Decreases in scores for the side effects domains were significant (p < 0.001) in the CIQ “yes” group, but not for the degree of bother (decrease in mean of 0.1 points, p = 0.50) or duration (no change, p = 0.84) of non-gastrointestinal side effects. Of 638 patients who completed the preference questionnaire, 93.0% of patients preferred the once-monthly dosing schedule and 563 patients (90.7%) found it more convenient. The most common adverse events were dyspepsia (1.9%), nausea (1.1%), and upper abdominal pain (0.9%). Patients are likely to prefer treatment with monthly ibandronate to a weekly or monthly bisphosphonate irrespective of their stated preference before switching treatment.
1. INTRODUCTION
Bisphosphonates decrease the incidence of vertebral and nonvertebral fractures, increase bone mass, and normalize bone turnover to premenopausal levels in women with postmenopausal osteoporosis [1,2]. However, the usefulness of this therapy is compromised by complex dosing requirements. For the first thing in the morning, patients must take the bisphosphonate remain upright and fasting for at least 30 - 60 minutes before the first food or drink of the day (other than plain water), and must wait for up to one hour after dosing before taking other drugs, vitamins or supplements that might interfere with absorption of oral bisphosphonates. Furthermore, bisphosphonates have significant gastrointestinal adverse effects. Consequently, persistence with bisphosphonates is poor. In a study of postmenopausal women who began therapy with a daily oral bisphosphonate, 42% of patients discontinued after only 6 months of treatment [3]. Notably, the probability of continuing treatment decreased progressively over time (67%, 58%, 49% and 30% at 3, 6, 12 and 24 months, respectively). Some of the reasons cited for discontinuing treatment were gastrointestinal disorder (12.9%), physician’s advice (11.7%), complexity of dosing (4.3%) defined as “complicated way of taking the drug”, the “lack of acceptance of treatment” (3.7%), and cost of therapy (3.7%). Other studies report that up to one third of patients taking bisphosphonates do not comply with dosing instructions and schedules despite detailed information received from their physicians [4,5].
Ibandronate (BONVIVA®) can be given once monthly. At a dose of 150 mg, ibandronate has been shown to be effective in increasing bone mineral density, in suppressing the biochemical markers of bone resorption, and to be well tolerated [6,7]. In a recent study of patients who had experienced gastrointestinal tolerability issues with weekly bisphosphonates, only 20.2% of these women reported gastrointestinal symptoms with monthly ibandronate [8]. Patients also indicated significant improvements in satisfaction scores with monthly ibandronate compared with their previous weekly bisphosphonate [9].
It is known that “daily/weekly” and “monthly” bisphosphonate regimens have therapeutically equivalent efficacy [1,2]. However, the regimen with more convenient dosing for patients would enhance compliance and longterm persistence with therapy, thus provide better outcomes.
The present study evaluated a set of candidate identification questions that may predict patient-reported satisfaction with ibandronate 150 mg in those who had previously received once-weekly or once-daily alendronate or risendronate. It was anticipated, that the identification of a survey that is likely to find patients who are satisfied with a once-monthly medication regimen has the potential to be used as a tool to benefit patient compliance and persistence with oral bisphosphonate treatment.
2. MATERIALS AND METHODS
2.1. Study Design
BONCURE was a large, prospective, open-label, multicenter, 6-month study. The protocol, any modifications, and consent procedures were reviewed and approved by the Independent Ethics Committee of each study site. Written informed consent was obtained from each patient before any study-related procedures were performed.
The study was conducted in two sequential stages. In Part A, patients completed a candidate identification questionnaire (CIQ). In Part B, patients replaced their weekly bisphosphonate therapy with the study biphosponate treatment regimen (ibandronate 150 mg once-monthly) for 6 months.
2.2. Subjects
Patients could be included in Part A of the study if they were women who had been receiving once daily or once-weekly alendronate or risendronate for the treatment or prevention of postmenopausal osteoporosis for a minimum of 3 months. Eligible patients were ambulatory and were, in the opinion of the investigator, able to understand the questionnaires and willing to comply with the protocol requirements. Patients were able to read, understand, and sign, the written informed consent form. There were no exclusion criteria for Part A. Patients were enrolled in Part A of the study until 650 patients began Part B.
Patients who completed Part A of the study and who were willing to comply with the protocol requirements were eligible for enrolment into Part B. Patients were excluded from Part B if the investigator considered that they were unlikely to complete the 6-month study period due to significant medical condition, were hypersensitive to bisphosphonates (ibandronate and risendronate) and to any of their components, were unable to stand or sit upright for at least 60 minutes, or had any medical condition or requirement for concomitant medication that could influence the study results or represent a safety hazard for the patient.
2.3. Study Drug Administration
Patients took one 150 mg tablet of ibandronate (BONVIVA® Roche) monthly for a period of 6 months for a total of six planned doses. Study participants discontinued their current bisphosphonate treatment for 1 week ± 7 days before starting ibandronate treatment. Patients were instructed to take the study medication in the morning, after an overnight fast of at least 6 hours, to swallow the tablet whole with a full glass of plain water while sitting upright or standing, to remain upright for 1 hour after dosing and to wait at least 1 hour before consuming any food or drink other than water. Instructions were given not to chew or suck the tablet because of the potential for oropharyngeal ulceration. Patients were also instructed to take supplemental calcium and vitamin D tablets for the duration of the study but not to take these or any other medicines during the postdose fasting period. Patients were recommended to take calcium and vitamin D tablets in divided doses with a meal.
Patients could elect to receive a monthly reminder to take their medication. Compliance was assessed by recording drug dispensed and returned.
It should be noted that there was no the cost for patients to switch from “daily/weekly drugs” to “monthly drugs”, all study drugs were provided free of charge by Roche, Turkey.
2.4. Measurements
All patients completed the CIQ at the first study visit. Patients were asked to answer either “yes” or “no” to the following 3 questions:
1) I would prefer a monthly oral dosing schedule to my current (daily or weekly) dosing schedule.
2) More than once per month, I have experienced stomach upset within 48 hours of taking my osteoporosis medication.
3) Over the past 3 months, I have missed taking 3 or more doses of my current (daily or weekly) osteoporosis medication.
The YES group consisted of all patients who answered YES to at least one of the questions on the CIQ; the NO group consisted of all patients who answered NO to all of the questions on the CIQ.
At baseline and the final visit (after 6 monthly doses of ibandronate treatment), patients completed an OPSAT-Q. The OPSAT-Q is a validated questionnaire designed to capture satisfaction with bisphosphonate treatment. It comprises four domains: convenience (questions 1 - 6), quality of life (questions 7 and 8), overall satisfaction (questions 9 and 10), and side effects (questions 11 - 16) [10]. Satisfaction with treatment was assessed using the OPSAT-Q composite satisfaction score (OPSAT-Q CSS), which was the average of the scores from the four domains of the OPSAT-Q converted to a 0 - 100-point scale.
At study end or on withdrawal, patients also completed a Preference Questionnaire, which has been validated by the MEDTAP Institute Inc. (Bethesda, MD) [11].
Safety was assessed by recording adverse events throughout the treatment period. Patients had a physical examination, and blood was taken for routine safety laboratory tests, at the baseline and final visits. Patients were followed up by telephone 15 days after the last visit in case of any safety concerns.
2.5. Statistical Analysis
Data from all of the patients enrolled in Part A were used to analyse the CIQ findings. Three analysis populations were defined to analyse the study endpoints collected from Part B. The intent-to-treat (ITT) population included all patients who received at least one dose of study medication. The per protocol (PP) population was the standard analysis population and excluded all patients in the ITT population who significantly violated the study protocol. The safety analysis population included all patients who received a dose of study medication and had at least one post-baseline safety measurement.
Patients with a positive change from their baseline CSS at Month 6 were considered to be satisfied with once-monthly dosing with ibandronate. The CochranMantel-Haenszel test was used to test the primary hypothesis: proportion of patients satisfied with oncemonthly daily dosing of ibandronate after 6 months of use between the YES and NO groups from the CIQ questionaire. The distribution of change in CSS (positive/no change/negative in CSS score) was compared between the CIQ-groups with Pearson Chi-square test. The change from baseline satisfaction score at Month 6 was analysed using a Wilcoxon test.
3. RESULTS
3.1. Study Population
A total of 677 patients were enrolled in Part A of the study and completed a CIQ. The patients were enrolled at 43 centers in Albania, Bosnia-Herzegovina, Croatia, Macedonia, Serbia and Turkey. Patient disposition is summarized in Figure 1. Thirty-two enrolled patients did not participate in Part B. Therefore, 645 patients were enrolled in Part B, took one or more doses of study medication and were included in the ITT population, of
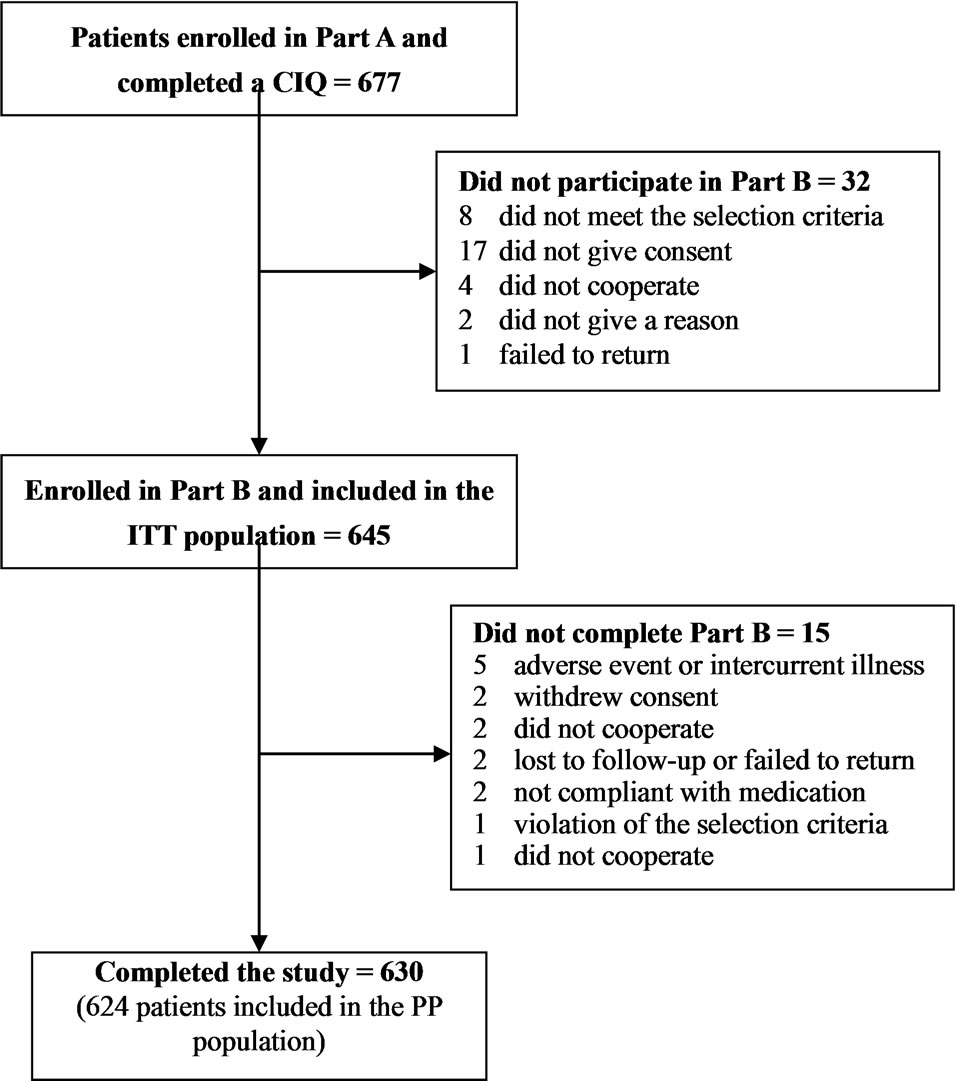
Figure 1. Patient disposition.
which 630 patients (97.7%) completed the study. The most common reason for not completing the study was adverse event or intercurrent illness (5 patients). Fiftythree enrolled patients were excluded from the PP population. Of these, 34 (64.2%) patients were also excluded from the ITT population. Therefore, the PP population included 624 patients, 618 (99.0%) of whom completed the study.
Patient demography is summarized in Table 1. All of the patients who provided information were female, postmenopausal and all except 1 patient was Caucasian. Mean age was 62.7 years (range 37 to 87 years). Mean time since diagnosis of osteoporosis was 4.59 years and ranged from newly diagnosed to over 27 years since diagnosis. Most patients (449, 67.0%) were educated to High School level or higher, 543 patients (81.0%) were retired and 649 patients (96.9%) were living at home and were independent.
A total of 349 (52.4%) patients had a history of previous diseases and 51 (7.6%) patients had concurrent diseases (Table 2). The most common previous or concomitant diseases were vascular hypertensive disorders (181 events) followed by osteoarthropathies (42 events) and hyperlipidaemias (41 events). One-hundred and two patients (15.2%) had a history of osteoporosis-related fracture.
3.2. Candidate Identification Questionnaire
Of the 677 patients who completed the CIQ, 461 patients (68.1%) answered YES to one or more of the ques-
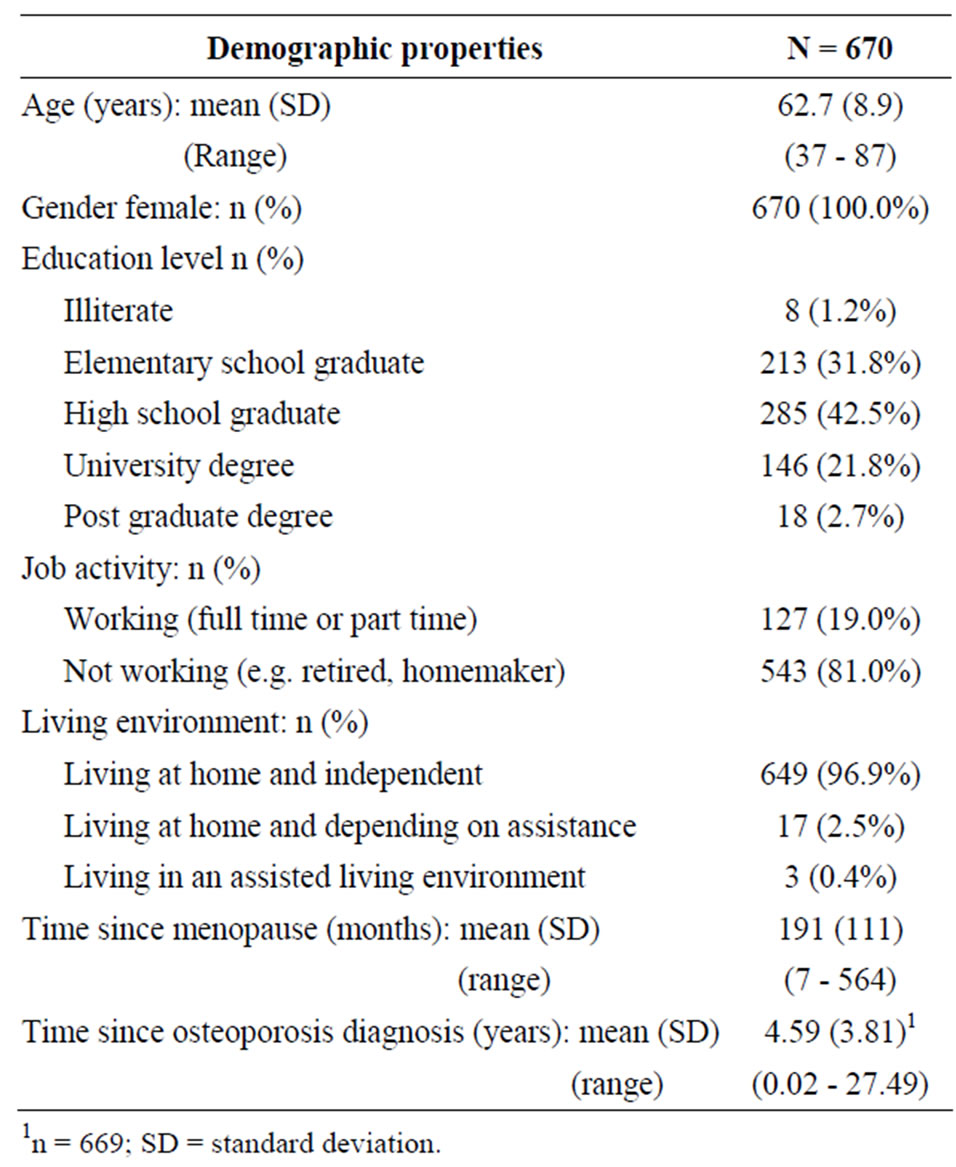
Table 1. Patient demography.

Table 2. Previous and concurrent.
tions (439/645 patients in the ITT population and 425/ 624 patients in the PP population). Four-hundred and fifty-seven patients (67.5%) would prefer a monthly oral dosing schedule to their current (daily or weekly) dosing schedule, 121 patients (17.9%) experienced stomach upset within 48 hours of taking their osteoporosis medication more than once per month, and 110 patients (16.2%) missed taking 3 or more doses of their daily or weekly osteoporosis medication in the previous 3 months.
3.3. Satisfaction with Treatment (OPSAT-Q)
Most patients had a positive change from baseline in CSS at Month 6 irrespective of whether they gave positive or negative responses to the CIQ. Summary statistics for the responses to each question on the OPSAT-Q, for all patients and by response to the CIQ, are provided in Table 3. There were statistically significant increases at Month 6 compared with baseline in mean satisfaction scores for all patients for the convenience, quality of life and overall satisfaction domains. Mean scores were between 4 (neither satisfied nor dissatisfied) and 5 (somewhat satisfied) at baseline and increased to above 6 (satisfied) at Month 6. Although patients in the CIQ “no” group had higher mean satisfaction scores compared with patients in the CIQ “yes” group at baseline, increases in mean satisfaction scores were statistically significant (p < 0.001) in both groups and for all patients.
Decreases in mean OPSAT-Q scores were observed for the domain of bothered by side effects. Mean scores were low at baseline; between 1 (not at all bothered) and 2 (slightly bothered). For the specific questions relating to heartburn and other gastrointestinal side effects, mean OPSAT-Q scores were between 0.2 and 0.5 points lower at Month 6 compared with baseline and the change was statistically significant (p < 0.01). Patients in the CIQ “no” group had lower mean baseline scores than patients in the CIQ “yes” group. Similarly, the number of days with heartburn or other gastrointestinal side effects were statistically significantly lower at Month 6 compared with baseline in both groups and in the all patients population (p ≤ 0.01). Other side effects and the number of days with side effects was significantly (p < 0.001) decreased between baseline and Month 6 for the all patients population and for the CIQ “yes” group. However, in the CIQ “no” group, the mean score for bothered by side effects was low at baseline (1.3 points) and the decrease to 1.2 points at Month 6 was not significant (p = 0.50) and the number of days with side effects was unchanged (score of 1.2 at baseline and Month 6, p = 0.84).
Dichotomized CSS scores (positive change vs. a composite of “no change” and “negative change”) indicate that patients in the CIQ “yes” group were more likely to have a positive change in OPSAT-Q compared with pa-


Table 3. OPSAT-Q scores at baseline and after 6 months of oral ibandronate treatment (ITT Population).
tients in the CIQ “no” group (Table 4). Eighty-eight point two percent of patients in the ITT population who answered “yes” to CIQ Question 1 and 80.0% of patients who answered “no” to this question had a positive response in the CSS. The difference between the groups was statistically significant (p = 0.0066). Similarly, 93.0% of patients who answered “yes” to CIQ Question 2 compared with 83.8% of patients who answered “no” to this question had a positive response in the CSS and the difference was statistically significant (p = 0.011). For CIQ

Table 4. Change in total CSS by CIQ Question (ITT Population).
Question 3, the proportion of patients with positive responses in CSS were 94.8% and 83.8% in the “yes” and “no” groups, respectively (p = 0.0044).
3.4. Patient Preference
A total of 638 patients completed the preference questionnaire (Table 5). Of these, 593 patients (93.0%) preferred the once-monthly dosing schedule and 563 patients (90.7%) found it more convenient.
Of the patients who preferred the once-monthly dosing schedule, a higher proportion of patients in the CIQ “yes” group compared with the CIQ “no” group agreed that the schedule fitted better into their lifestyle (77.1% and 22.9%, respectively) and agreed that it would be easier to follow the once-monthly dosing schedule for a long time (68.6% and 31.4%, respectively) but there was less difference between the CIQ groups in the questions about side-effects. Patients who preferred their previous daily or weekly schedule were more likely to agree with the statements in the questionnaire if they were in the CIQ “no” group than if they were in the CIQ “yes” group, although there were only 30 patients who preferred their previous schedule.
3.5. Compliance
Patients were generally compliant with their medication, i.e. ≥80% tablets taken. Only 9 patients (1.4%) overall were not compliant: 4 (1.9%) patients in the CIQ “yes” group and 5 (1.1%) patients in the CIQ “no” group. Most patients in the ITT population (99.4%) chose to have a monthly reminder to take ibandronate.
3.6. Safety
Of the 640 patients in the safety population, 62 (9.7%) experienced one or more adverse events. The most common adverse events were dyspepsia (1.9% of patients), nausea (1.1% of patients) and upper abdominal pain (0.9% of patients).
Six patients experienced serious adverse events. A life-threatening episode of upper abdominal pain and severe episodes of pyrexia and bone pain were considered possibly related to ibandronate. Postoperative hernia and acute renal failure (in the same patient with upper abdominal pain), pulmonary embolism, pericarditis, iridocyclitis and hiatus hernia were unrelated. Four of these patients were withdrawn from treatment because of these adverse events. A fifth patient was withdrawn from treatment because of a respiratory tract infection that was unrelated to ibandronate.
4. DISCUSSION
Poor treatment compliance, persistence and adherence to prescribed medication in chronic diseases have been shown in numerous studies and bisphosponates are not an exception [12,13]. Research outcomes indicate an apparently rapid decline in persistence during the first

Table 5. Patient preference questionnaire (ITT Population).
three months of treatment and decline further at a much slower rate [14] specifically for daily as well as weekly bisphosphonate treatment schedules. Some improvements in persistence were recorded with once monthly bisphosphonate treatment as in the BOOSTER study [15] in which patients were with significantly higher satisfaction scores for OPSAT-Q domains with monthly treatment over weekly option and in overall expressed preference for monthly ibandronate treatment. However, this improvement has not been confirmed in any studies as well [16,17]. An additional comment was made by Silverman, Schousboe and Gold [14] in a recent review article which noted that “complex dosing schedules that interfere with daily activities may result in non-persistence and non-compliance and poor compliance may be unintentional.” Various publications refer to unintentional poor compliance which may have resulted in referred controversial outcomes while drawing a general conclusion on treatment compliance as different testing methods were used to predict compliance and persistence. Such prediction of compliance becomes important in treatments where cumulative drug availability is required, as in osteoporosis treatment.
The purpose of the BONCURE study was to determine whether a Candidate Identification Questionnaire (CIQ) could be used to predict patient satisfaction with a monthly bisphosphonate dosing regimen before any treatment is initiated and its relative correlation with patient preference for monthly treatment in patients with postmenopausal osteoporosis. Dosing simplification is an important strategy in assisting patients to comply with long-term therapy [18,19], and the ability to predict patient satisfaction based on a set of screening questions could be of significant value in identifying patients who are most likely to benefit from a monthly treatment regimen.
The CIQ implemented in this study comprised three questions aimed at identifying patients who might have a preference for, or who might benefit from a monthly dosing regimen rather than a daily or weekly schedule. The questions were targeted at the patient’s preference for a monthly schedule, gastrointestinal side effects from taking bisphosphonate medication during their past treatment period and compliance. Patients who gave a positive response to any of the questions were included in the CIQ “yes” group (68.1%).
Patient responses to the CIQ indicated that the majority of patients (65.4%) would prefer monthly dosing to daily or weekly regimens, in parallel to experiencing any gastrointestinal symptoms after taking their usual treatment and missing 3 or more doses in the previous 3 months. These findings suggest a tendency for monthly treatment regimen with bisphosphonates as patients’ overall treatment compliance may be bothersome due to gastrointestinal symptoms.
For an additional prediction of overall satisfaction in this study, OPSAT-Q was implemented at two different time points for convenience of treatment, quality of life and overall satisfaction in the complete set of bisphosphonate treated patients. The analysis of OPSAT-Q outcomes were significantly higher at Month 6 compared with baseline, including patients who gave negative answers to all three screening questions at baseline (CIQ “no” group). Mean scores for the individual questions in the OPSAT-Q tended to support the CIQ outcomes for the “CIQ yes” group, which indicated less satisfaction with the convenience of the previous treatment, quality of life and overall satisfaction and with bothersome side effects when compared with patients in the “CIQ no” group.
Nonetheless, although significant improvements in satisfaction scores occurred in both groups for all 10 questions in these domains, changes in mean OPSAT-Q scores from baseline to Month 6 for the side effects domain were not statistically significant in the CIQ “no” group for non-gastrointestinal side effects. In overall, our findings were not consistent with the hypothesis that the CIQ might be predictive of patient satisfaction as patient satisfaction was found to be higher in both groups after 6 months of ibandronate treatment than at baseline irrespective of patient satisfaction, gastrointestinal side effects or compliance with their previous daily or weekly bisphosphonate.
The findings of the mean scores for the individual OPSAT-Q questions in this study were further supported by the dichotomized Composite Satisfaction Scores (CSS). Although patients in the CIQ “yes” group were significantly more likely to have a positive change in preference score compared with patients in the CIQ “no” group, both groups had positive changes in CSS, irrespective of how they responded to the individual questions in the CIQ. At completion or withdrawal for any reason, patients were asked to complete a preference questionnaire and most patients (93.0%) indicated that they preferred the monthly dosing schedule. The most common reasons for the preference were that it fitted better into the patients’ lifestyles (77.1%) and would be easier to follow the schedule for a long period (53.0%). In essence, monthly dosing was found to be more convenient than daily or weekly dosing. However, almost all patients requested a reminder when their next dose was due. This suggests that compliance with a monthly dosing regimen could be regarded as “excellent” provided a process is put in place to remind patients when to take their next dosage.
The patient preference results of BONCURE study are supported by the findings of a 6-month study in which 1678 patients were switched from weekly oral bisphosphonates to monthly oral ibandronate 150 mg. In this study, OPSAT-Q composite satisfaction scores improved by 9 points from a high baseline value of 80.1 points [9]. In a crossover study of 3 months treatment with oncemonthly ibandronate 150 mg compared with once-weekly alendronate 70 mg, 71.4% patients preferred once-monthly ibandronate and 28.6% preferred once-weekly alendronate after switching treatment options twice. As in BONCURE study, the reason for preference of a monthly regimen was “the ease of following a treatment regimen for a long period” (61% of patients) [20]. Furthermore, 17% of patients who preferred once-monthly ibandronate considered that “it is easier to tolerate side effects” compared with 4.3% of patients who preferred alendronate.
The main implication of these results is that predicting patient preference might not be necessary to improve patient satisfaction. In a study by Clowes et al., compliance and persistence in osteoporosis differed in groups who had no monitoring during treatment or nurse monitoring and nurse and bone monitoring. The results of this randomized study indicated that both monitored groups had better persistence ratios when compared with nonmonitored patients [21]. Interestingly in some studies, it was shown that giving patients a positive feedback regarding their proceedings of treatment had comparatively better persistence and compliance [22].
However, this study was planned for testing a brief questionnaire for the prediction of preference and compliance for a presumably long-term treatment in a medical condition which does not have any symptoms and signs until a bone fracture occur. Thus, practicality of a brief questionnaire was tested in BONCURE study together with a preference questionnaire which predicted successful outcomes and preferences but without any positive results for the predictive properties of an initial questionnaire.
The potential limitations of this study include the open-label design, which clearly enables selection bias. It could be argued that most patients who agreed to participate in the study were expecting to prefer the new treatment. Despite this proposed limitation, the first question on the CIQ which asked whether the patient would prefer monthly dosing to their current daily or weekly dosing was answered “no” in almost one third of the study population but were nonetheless willing to participate in the study and to take ibandronate monthly for 6 months.
As a conclusion, patients are likely to prefer treatment with monthly ibandronate in preference to a weekly or monthly bisphosphonate irrespective of their stated preference before switching treatment. The CIQ is predictive of the relative improvement in patient satisfaction but it is not necessary to identify patients who might benefit from this change in their treatment.
5. ACKNOWLEDGEMENTS
This trial was fully sponsored by F. Hoffmann-La Roche Ltd., Istanbul. Every financial aspect of the study has been provided by Roche; including investigator fees and study drugs. Study drugs were provided free of charge to patients by Roche.
The authors acknowledge the assistance of Tiina Hakonen MSc of Encorium Ltd, Espoo, Finland, for the statistical analysis and Wendy Kingdom PhD of Encorium Ltd., for assistance in the preparation of this manuscript.
REFERENCES
- Papapoulos, S.E. (2001) Bisphosphonates in the management of postmenopausal osteoporosis. In: Marcus, R., Feldman, D. and Kelsey, J., Eds., Osteoporosis, 2nd Edition, Academic Press, New York, 631-650. doi:10.1016/B978-012470862-4/50073-8
- Schnitzer, T., Bone, H.G., Crepaldi, G., Adami, S., McClung, M., et al. (2002) Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group, Aging, Milano, 12, 1-12.
- Lombas, C., Hakim, C. and Zanchetta, J.R. (2000) Compliance with alendronate treatment in an osteoporosis clinic. Journal of Bone and Mineral Research, 15, M406.
- Finigan, J., Bainbridge, P.R. and Eastell, R. (2001) Adherence to osteoporosis therapies. Osteoporosis International, 12, 110.
- Hamilton, B., McCoy, H. and Taggart, H. (2001) Tolerability and compliance with risendronate in clinical practice. Osteoporosis International, 12, 111.
- Reginster, J.Y., Adami, S., Lakatos, P., Greenwald, M., Stepan, J.J., Silverman, S.L., Christiansen, C., Rowell, L., Mairon, N., Bonvoisin, B., Drezner, M.K., Emkey, R., Felsenberg, D., Cooper, C., Delmas, P.D. and Miller, P.D. (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Annals of the Rheumatic Diseases, 65, 654-661. doi:10.1136/ard.2005.044958
- Stakkestad, J.A., Lakatos, P., Lorenc, R., Sedarati, F., Neate, C. and Reginster, J.Y. (2008) Monthly oral ibandronate is effective and well tolerated after 3 years: The MOBILE long-term extension. Clinical Rheumatology, 27, 955-960. doi:10.1007/s10067-007-0824-6
- Binkley, N., Martens, M.G., Silverman, S.L., Derman, R.J., Greenwald, M., Kohles, J.D. and Bachmann, G.A. (2009) Improved GI tolerability with monthly ibandronate in women previously using weekly bisphosphonates. Southern Medical Journal, 102, 486-492. doi:10.1097/SMJ.0b013e31819ed0dd
- Bonnick, S.L., Silverman, S., Tanner, S.B., Martens, M., Bachmann, G., Kohles, J.D. and Civitelli R. (2009) Patient satisfaction in postmenopausal women treated with a weekly bisphosphonate transitioned to once-monthly ibandronate. Journal of Womens Health, 18, 935-943. doi:10.1089/jwh.2008.1064
- Flood, E.M., Beusterien, K.M., Green, H., Shikiar, R., Baran, R.W., Amonkar, MM. and Cella, D. (2006) Psychometric evaluation of the Osteoporosis Patient Treatment Satisfaction Questionnaire (OPSAT-Q), a novel measure to assess satisfaction with bisphosphonate treatment in postmenopausal women. Health and Quality of Life Outcomes, 4, 42. doi:10.1186/1477-7525-4-42
- Simon, J.A., Lewiecki, E.M., Smith, M.E., Petruschke, R.A., Wang, L. and Palmisano, J.J. (2002) Patient preference for once-weekly alendronate 70 mg versus oncedaily alendronate 10 mg: A multicenter, randomized, openlabel, crossover study. Clinical Therapeutics, 24, 1871- 1886. doi:10.1016/S0149-2918(02)80085-6
- Cotte, F.E., Fardelione, P., Mercier, F., Gaudin, A.F. and Roux C. (2009) Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporosis International, 29, 125-139.
- Gold, D.T., Safi, W. and Trish, H. (2006) Patient preference and adherence: Comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Current Medical Research and Opinion, 22, 2382-2391. doi:10.1185/030079906X154042
- Silverman, S.L., Schousboe, J.T. and Gold, D.T. (2011) Oral bisphosphonate compliance and persistence: A matter of choice? Osteoporosis International, 22, 21-26. doi:10.1007/s00198-010-1274-6
- Vlak, T., Kastelan, D., Lozo, P., Aljinović, J., Gradišer, M., et al. (2011) Monthly or weekly bisphosphonate? Evaluation of satisfaction in patients with postmenopausal osteoporosis using OPSAT-Q questionnaire during the BOOSTER study in Croatia. Clinical Rheumatology, 30, 1549-1554. doi:10.1007/s10067-011-1858-3
- Cramer, J.A., Gold, D.T., Silverman, S.L. and Leviecki, E.M. (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporosis International, 18, 1023-1031. doi:10.1007/s00198-006-0322-8
- Vrijens, B., Vincze, G., Krisanto, P., Urquhart, J. and Burnier, M. (2008) Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. British Medical Journal, 336, 1114- 1117. doi:10.1136/bmj.39553.670231.25
- Claxton, A.J., Cramer, J. and Pierce, C. (2001) A systematic review of the associations between dose regimens and medication compliance. Clinical Therapeutics, 23, 1296-1310. doi:10.1016/S0149-2918(01)80109-0
- Sclar, D.A. (1991) Improving medication compliance: A review of selected issues. Clinical Therapeutics, 13, 436- 440.
- Emkey, R., Koltun, W., Beusterien, K., Seidman, L., Kivitz, A., Devas, V. and Masanauskaite, D. (2005) Patient preference for once-monthly ibandronate versus onceweekly alendronate in a randomized, open-label, crossover trial: The Boniva Alendronate Trial in Osteoporosis (BALTO). Current Medical Research and Opinion, 21, 1895-1903. doi:10.1185/030079905X74862
- Cloves, J.A., Peel, N.F. and Estell, R. (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: A randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism, 89, 1117-1123. doi:10.1210/jc.2003-030501
- Delmas, P.D., Vrijens, B., Estell, R., Roux, C., Pois, H.A., Ringe, J.D., Grauser, A., Cahall, D. and Watts, N.B. (Improvement Measurement of Persistence on Actonell Treatment Investigators) (2007) Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. The Journal of Clinical Endocrinology & Metabolism, 92, 1296-1305. doi:10.1210/jc.2006-1526
NOTES
*The study was supported and funded by F. Hoffmann-La Roche Ltd.
Fatih Ozdener and Hakan Oncel are currently are employees of Roche, Istanbul.
All other authors have no conflict of interest.
†Deceased.

