Engineering
Vol.07 No.09(2015), Article ID:59957,19 pages
10.4236/eng.2015.79053
Thermodynamic Equilibrium of the Saturated Fluid with a Free Surface Area and the Internal Energy as a Function of the Phase-Specific Volumes and Vapor Pressure
Albrecht Elsner
Am Mühlbach 14, D-85748, Garching, Germany
Email: alimeli.elsner@gmail.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 July 2015; accepted 21 September 2015; published 25 September 2015
ABSTRACT
This study is concerned with describing the thermodynamic equilibrium of the saturated fluid with and without a free surface area A. Discussion of the role of A as system variable of the interface phase and an estimate of the ratio of the respective free energies of systems with and without A show that the system variables
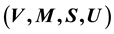 given by Gibbs suffice to describe the volumetric properties of the fluid. The well-known Gibbsian expressions for the internal energies of the two-phase fluid, namely
given by Gibbs suffice to describe the volumetric properties of the fluid. The well-known Gibbsian expressions for the internal energies of the two-phase fluid, namely
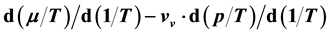 for the vapor and
for the vapor and
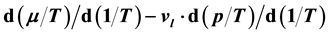 for the condensate (liquid or solid), only differ with respect to the phase-specific volumes
for the condensate (liquid or solid), only differ with respect to the phase-specific volumes
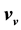 and
and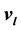 . The saturation temperature T, vapor presssure p, and chemical potential
. The saturation temperature T, vapor presssure p, and chemical potential
 are intensive parameters, each of which has the same value everywhere within the fluid, and hence are phase-independent quantities. If one succeeds in representing
are intensive parameters, each of which has the same value everywhere within the fluid, and hence are phase-independent quantities. If one succeeds in representing
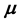 as a function of
as a function of
 and
and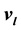 , then the internal energies can also be described by expressions that only differ from one another with respect to their dependence on
, then the internal energies can also be described by expressions that only differ from one another with respect to their dependence on
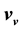 and
and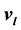 . Here it is shown that
. Here it is shown that
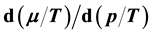 can be uniquely expressed by the volume function
can be uniquely expressed by the volume function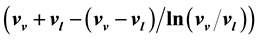 . Therefore, the internal energies can be represented explicitly as functions of the vapor pressure and volumes of the saturated vapor and condensate and are absolutely determined. The hitherto existing problem of applied thermodynamics, calculating the internal energy from the measurable quantities T, p,
. Therefore, the internal energies can be represented explicitly as functions of the vapor pressure and volumes of the saturated vapor and condensate and are absolutely determined. The hitherto existing problem of applied thermodynamics, calculating the internal energy from the measurable quantities T, p,
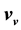 , and
, and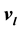 , is thus solved. The same method applies to the calculation of the entropy, chemical potential, and heat capacity.
, is thus solved. The same method applies to the calculation of the entropy, chemical potential, and heat capacity.
Keywords:
Fluid with Free Surface Area, Solution of Gibbs’s Internal Energy Equations, Chemical Potential Expression, Calculation of Entropy and Heat Capacity

1. Introduction
We are concerned here with electrically and magnetically neutral single-component matter under steady-state equilibrium conditions which are thermodynamically defined in the immediate vicinity of the critical point and below it. Between the critical gas temperature
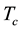 and the triple-point temperature
and the triple-point temperature









The critical point, from the experimental perspective, is the first occurrence or vanishing of a free surface A observed in V, which separates the volumes




The work in Section 2 is concerned with describing the thermodynamic equilibrium of the real gas. The stationary equilibrium of M in V is known from Gibbs to be expressed by the fundamental equation

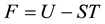
Section 3 firstly treats the equilibrium of the two-phase fluid without an internal free surface, i.e.










In Section 4, it is reminded that the entropy and energy functions S,






Finally, in Section 5, the central task of this work, viz. finding an explicit thermodynamic expression for













By means of the expression given for the volume function

2. Description of the Thermodynamic Equilibrium of the Real Gas
Thermodynamics uses intensive and extensive quantities to describe the equilibrium state of the gas mass M enclosed in the volume V. An intensive property of the gas is the same everywhere in the volume and is therefore independent of the mass. Intensive equilibrium state quantities are the temperature T, pressure p, and chemical potential

Variations in the extensive quantities S, V, and M lead to variations in the intensive quantities T, p, and


By means of Maxwell’s relations [2]







For the single-component gas the well-known Gibbs-Duhem relation between the intensive quantities in dif- ferential form reads [2] :

Explicit writing gives

Variations in S, V, and M can be performed independently of each other. For dV = 0 and dM = 0 one thus has




Using the Maxwell relations


Gibbs-Duhem form of the entropy:

With


and


In the following, the properties of the real gas are investigated under the condition of constant mass M in given volume V. The entropy thus has the form (6). The internal-energy expression immediately follows from
the fundamental equation, i.e.


According to relations (6) and (7), the entropy and internal energy of the gas mass are defined by the temperature derivatives of the intensive quantities



3. Two-Phase Equilibrium without and with a Free Surface Area
For every real gas there is a certain temperature











The relation





The decomposition of mass M into



Taking



It is seen, because of

The proof of











Then equation


temperature:



If one investigates the temperature dependence of the volumes











Evaluation of relations (10) and (11) is given by means of published volume data for argon in Figure 1.
Let us now turn to the problem of thermodynamic treatment of the physics of the free interface surface A. As stated, the existence of A affords the possibility of distinguishing in V between two fluid phases of different mass densities

















Figure 1. Mass ratio




the interface mass



Independently of prehistory, it holds for the saturated fluid that if the condition for forming a free surface between the liquid and vapor phases is given, then there is an interface particle layer, which represents a new equilibrium state described by a minimum internal energy U and simultaneously a maximum entropy S. Hence formation of the free interface surface A lowers the free energy of the fluid, F. This situation is formally taken into account by introducing the phase “interface” in keeping with the additive property of a variable X in addition to the phases “vapor” and “condensate” [6] :

The energy term








Then the enlarged Gibbs relations (6) and (7) read [6]

Figure 2 shows the temperature dependence of


In order to put the interface quantity







terface density

tension and interface quantities,

which, however, remains numerically indeterminable owing to the hypothetical length




The relative energy contribution of an interface quantity to the respective system quantity depends on the ratio of the numbers of interacting particles in the interface volume



4. The Thermodynamic Zero of Thermodynamic Functions
According to the Gibbsian energy Equation (7), the mass- and phase-specific internal energies of the saturated
Figure 2. Analysis of free-surface quantities. From fitted published surface tension data







fluid are interrelated as follows:

Since








at absolute temperature zero it holds that








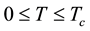


Relations (16) say that the internal vapor energy


In keeping with W. Gibbs [1] , it is hypothetically asserted in the literature (e.g. [11] -[13] ) that the temperature dependence of u,


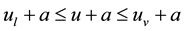


Moreover, some thermodynamic relations are mentioned in relation to the thermodynamic value 0:

The chemical potential functions are given in explicit form as energy functions:

Setting


The critical value is finite for


5. The Unsolved Problem in Applied Thermodynamics
Endeavors to publish data of the energy and entropy functions







Solution of the problem is not trivial, as the following solution ansatzes for the volume function


Temperatures T < Tc, because this ansatz leads to a value of the condensate at absolute zero of




In order to find a solution for

As the equations show, what is needed is a thermodynamic expression for




Van der Waals showed that the volumes








At the critical point one gets



one gets













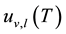

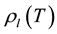

Taking the difference





Appropriate as variable of the phase functions









A phase function


which can also be expressed by the ratio

that


and




monotonically as z and, at the same time, the function

the function



What is now needed is a solution of the functional equation for


The general solution is of the form

Proof: Suppose




and
Of the mathematical solutions possible the following (with



This equation yields the physically relevant solution. It is noted that the solution ρ according to Equation (28) can be represented as a convergent Taylor series. Figure 3 shows the functions


Before tackling the important investigation of the uniqueness of this solution, one should consider the method of solution that uses the variable

Admittedly, the latter equation does not yield a direct solution






With



Figure 3.




and one calculates




According to Equations (20) and (22) one obtains

Inserting the solution for


Let us now turn to the uniqueness of the solution (28). It is immediately seen that the functions





It is claimed that every solution



It follows that
Expanding


If



Then



6. Explicit Expression for
The expression proposed [15] [16] for the volume function


It is symmetric in the variables and linear in both


shows the temperature dependence of



which are set by relations (31).
Figure 4. Volume function





Expression (33) is the only one thermodynamically possible and it alone satisfies all known thermodynamic conditions (see Appendix).
The description of the two-phase state of the saturated fluid by the expression (33) admits further formu- lations of the two-phase equilibrium.
Relations (15) and (33) yield the result of the ambitious task of representing the phase-specific internal en- ergies in terms of phase-specific volumes and vapor pressure, i.e. measurable quantities:

The positive term


Rearranging this to

thermodynamic equations valid for

Equation (36) are valid for temperatures






which is in agreement with Equation (34). Figure 5 shows some energy functions of saturated argon.
As mentioned by relation (35), the term

Figure 5. Energies of saturated argon: 1






The term






it holds that

The function



value 1 for



tonically to 0. The physical meaning of









The procedure in Ref. [16] is the exact opposite of that described here: There Equation (41) serves as starting point to derive relation (40) and find their solution (38). The energy ratio



It is found that



The ratios of the phase-specific internal energies to the evaporation energy are likewise universal and for temperatures


Finally, the integral


yields, of course, the temperature value of the fluid internal energy,

The entropy value





follows that

According to Equations (10), (34), and (45), one has
Figure 6. Heat capacity



Thermodynamic equations for

7. Results and Discussion
In treating the thermodynamic equilibrium of a fluid mass M in a volume V, a distinction is made between an equilibrium state with a free surface area








Table 1. Chemical potential relations [3] .
quantities of the fluid are the temperature

potential



When a free surface






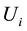










relation to its value in V can be completely ignored if volumetric considerations are to the fore, as in this work.
Here it is investigated whether there is a definitely specifiable functional relation between phase-specific energies









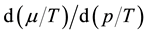






Acknowledgements
The author would like to thank Max Planck Institute of Plasma Physics (IPP), Garching, for providing computing facilities. He is also grateful to A. Kechriniotis and H. Tasso for helpful discussions concerning the unique solution of the phase function

Cite this paper
AlbrechtElsner, (2015) Thermodynamic Equilibrium of the Saturated Fluid with a Free Surface Area and the Internal Energy as a Function of the Phase-Specific Volumes and Vapor Pressure. Engineering,07,577-596. doi: 10.4236/eng.2015.79053
References
- 1. Gibbs, J.W. (1973) A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces. Transactions of the Connecticut Academy, 2, 382-404.
- 2. Callen, H.B. (1960) Thermodynamics. John Wiley & Sons, New York.
- 3. Elsner, A. (2012) Applied Thermodynamics of the Real Gas with Respect to the Thermodynamic Zeros of the Entropy and Internal Energy. Physica B: Condensed Matter, 407, 1055-1067.
http://dx.doi.org/10.1016/j.physb.2011.12.118 - 4. Tegeler, C., Span, R. and Wagner, W. (1999) Thermodynamic Properties of Saturated Argon. Journal of Chemical and Physical Reference Data, 28, 779-850.
- 5. Landau, L.D., Lifshitz, E.M. and Pitaevskii, L.P. (1980) Statistical Physics: Course of Theoretical Physics. Volume 5, Pergamon Press, Oxford.
- 6. Elsner, A. (2014) Thermodynamic Fit Functions of the Two-Phase Fluid and Critical Exponents. Engineering, 6, 789- 826.
http://dx.doi.org/10.4236/eng.2014.612076 - 7. Stierstadt, K. (1989) Physik der Materie. VCH Verlagsgesellschaft, Weinheim, 235.
- 8. Stansfield, D. (1958) The Surface Tensions of Liquid Argon and Nitrogen. Proceedings of the Physical Society, 72, 854-866.
http://dx.doi.org/10.1088/0370-1328/72/5/321 - 9. Sprow, F.B. and Prausnitz, J.M. (1966) Surface Tensions of Simple Liquids. Transactions of the Faraday Society, 62, 1097-1104.
http://dx.doi.org/10.1039/tf9666201097 - 10. Dörfler, H.D. (1994) Chapter 2: Grenzflächen und Kolloidchemie. VCH Verlagsgesellschaft, Weinheim.
- 11. Hahne, E. (1993) Technische Thermodynamik. Addison-Wesley Publishing Company, Boston, 66.
- 12. Hudson, J.B. (1996) Thermodynamics of Materials. John Wiley & Sons, New York, 22, 145, 185.
- 13. Lauth, J.G. and Kowalczyk, J. (2015) Thermodynamik. Springer Verlag, Berlin, 67, 68, 115, 135, 138-142, 199-211, 365.
http://dx.doi.org/10.1007/978-3-662-46229-4 - 14. Huang, K. (1987) Statistical Mechanics. John Wiley & Sons, New York.
- 15. Elsner, A. (1987) Temperature Dependence of Absolute Fluid-State Parameters. Cryogenics, 27, 189-201.
http://dx.doi.org/10.1016/0011-2275(87)90018-X - 16. Elsner, A. (1988) Thermodynamic Representation of the State of a Saturated Fluid. Physics Letters A, 130, 225-233.
http://dx.doi.org/10.1016/0375-9601(88)90598-1 - 17. Elsner, A. (1993) Specific Heats of Saturated Water Vapor and Liquid. Physical Review E, 48, 1839-1843.
http://dx.doi.org/10.1103/PhysRevE.48.1839
Appendix: Test Functions for the Volume Function
The correctness of Equation (33) is now demonstrated in a few test cases. With



From


which shows the symmetry of the volume function in respect of its variables


At this point it is appropriate to comment on the argument of the logarithm in expression (33). As can be seen, the relations of the last line can be transformed to the relations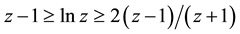









A particularly critical test for the correctness of Equation (33) is afforded by the internal relations (16) and (34). As it holds that
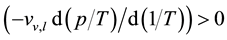


that have to be investigated. It is in fact found that




According to relation (20) it is postulated that the sum


It should be emphasized that the energy sum


The fluid energy


It is negative and greater than



The difference between the energy sum



This difference is not negative if

see also Figure 7. Thermodynamically correct density data confirm the validity of condition (54). For example, the formulae for




region. They lead to



which means that condition (54) is satisfied and that the expression



The next example investigated is the ratio



Finally, the ratio of the phase-specific internal energies to the evaporation energy is considered, for which the theory yields according to relation (21)

Chemical potential relations: with the identity
as starting point and by means of the expression for the volume function

Figure 7. Functions


Equation (33),







and (see Figure 8)

Figure 8. Functions






























