Materials Sciences and Applications
Vol.4 No.12(2013), Article ID:40533,6 pages DOI:10.4236/msa.2013.412099
Evaluation of the Antimicrobial Activity of Stryphnodendron barbatiman against Citrobacter freundii
![]()
1Grupo de Materiais Nanoestruturados, Universidade Federal de Mato Grosso, Barra do Garças, Brazil; 2Faculdades Padrão, Goiânia, Brazil.
Email: *ncsouza@ufmt.br
Copyright © 2013 Nara C. de Souza et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 17, 2013; revised October 21, 2013; accepted November 19, 2013
Keywords: Stryphnodendron barbatiman; Citrobacter freundii; Atomic Force Microscopy; Antimicrobial Activity
ABSTRACT
Medicinal plants have been presented as a valuable source of preservation of human health. In special, Stryphnodendron barbatiman has been employed due to its antimicrobial activity. This plant is rich in tannins and has been used in popular medicine for the treatment of gastrointestinal disorder, treatment of lesions, and also as anti-inflammatory microbicide. Citrobacter freundii is a member of the family Enterobacteriaceae and is one of the major causes of opportunistic infections. This microorganism is a bacterium (bacillus) aerobic gram-negative with a length in the range of 1 to 5 mm. C. freundii is commonly found in water, soil, food and occasionally in the gastrointestinal tract of animals and humans. In this paper, we have demonstrated the antibacterial activity of S. barbatiman by observing cellular death by using inhibition halo approach. Atomic force microscopy and FT-IR spectroscopy results suggested that interaction between the main active components of S. barbatiman with cellular wall of C. freundii gives rise to cellular wall damage, and then leads this microorganism to death.
1. Introduction
Bacterial infections are usually treated using antibacterial drug therapies. The problem with this approach is the resistance developed by bacteria to these types of drugs. Natural products have emerged as a suitable way to the discovery of effective compounds with low toxicity. Several plants are used in the popular medicine including the species Stryphnodendron barbatiman. This one has, as main active, ingredient the called tannins, which are recognized as healing and antimicrobial agent against bacteria [1]. In general, bacteria have thicknesses from 0.25 to 1.5 mm and length ranging from 1 to 10 mm. They have no defined nucleus and their genetic material is compacted and coiled in a region of the cytoplasm called nucleoid. Such microorganisms have a plasmatic membrane covered by a cellular wall composed of polysaccharides. This structure conveys high resistance to leukocytes or phagocytes, thus providing resistance to enzymatic or osmotic disruption [2,3]. A usual gram-negative bacterium found in nature is the Citrobacter freundii. These bacteria may be found in aqueous environments and also in organs of diseased animals. In clinical isolates, they are considered as opportunistic organisms [4,5]. C. freundii can cause nosocomial infections in urinary tract and blood [6]. In this paper, we have investigated the interaction and inactivation of bacteria C. freundii by S. barbatiman.
2. Materials and Methods
Bacteria used in this study were obtained from a scraping oropharyngeal sample and identified by an automated system Vitek/BioMérieux. S. barbatiman, popularly known as barbatimão, grows in Brazilian Cerrado and in Amazon region. Alcoholic extract was prepared using 5.0 g of fresh plant bark and dried with maceration in 96% ethanol (5.0 g/100.0 mL). Aqueous extract was obtained by cooking bark in purified water (5.0 g/100.0 mL). After filtering, the extract was stored in amber bottle. Suspension of the bacterium C. freundii was obtained from colony solubilisation in physiological solution (0.9% NaCl). For susceptibility test of C. freundii, the extracts of S. barbatiman were deposited in 10.0 mL droplets over the culture on the dish petri. Then, the plates were incubated at 36˚C for 24 h to monitor the formation of inhibition halo. Biofilms were prepared on glass substrates from the deposition of the suspension of C. freundii using the casting technique—which consisted in a deposition followed by solvent evaporation. Biofilms were left to stand for 20 min at 36˚C until complete solvent evaporation. Biofilms with extract of S. barbatiman were obtained by deposition of a combination of bacteria in suspension and extract after different contact times. Studies of the surface morphology of the biofilms were performed using an atomic force microscope (Nanosurf EasyScan II) in intermittent mode (512 × 512 pixels). Images were acquired in scanning windows of 5 mm × 5 mm. Roughnesses of biofilms were determined using the Nanosurf Instruments software and a spectrophotometer Perkin-Elmer Spectrum 100 FT-IR was used to obtain spectra of the extracts and bacteria.
3. Results and Discussion
It was observed that C. freundii is sensitive to extracts of S. barbatiman (aqueous or alcoholic) showing more stable results in inhibition halos for aqueous extracts of S. barbatiman (Figure 1).
Although the halo after 24 h for alcoholic extract is slightly larger than that of the aqueous extract, after 48 h is possible to observe the appearance of some colony in the region of the halo for alcoholic extract. This find was expected, since other studies have shown that S. barbatiman has antimicrobial activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans, Candida krusei, Enterococcus faecalis, Klebsiella pneumoniae, Kocuria rhizophila, Neisseria gonorrhoeae, and Shigella flexneri [7]. This effect can be attributed to high tannin content, which is able to inhibit bacterial adhesion and enzymes [7].
Figure 2 shows AFM images for S. barbatiman, C. freundii and C. freundii dispersed in S. Barbatiman aqueous extract at different times of contact. We observe that the pure bacteria exhibit an expected rod-shaped symmetry, whereas for C. freundii dispersed into S. barbatiman are noted bacteria with irregular forms. The height profiles of the images (Figure 3) reveal a dependence of bacteria symmetry on the contact time. This change in the form of bacteria can be associated to cellular damages to bacteria wall, which can lead the bacteria to death [8]. Cellular damages can result from interaction between tannins of S. barbatiman and the components of the cellular walls of the bacteria.
As shown in Figure 4, we observed that the surface roughness increased with increasing contact time. These
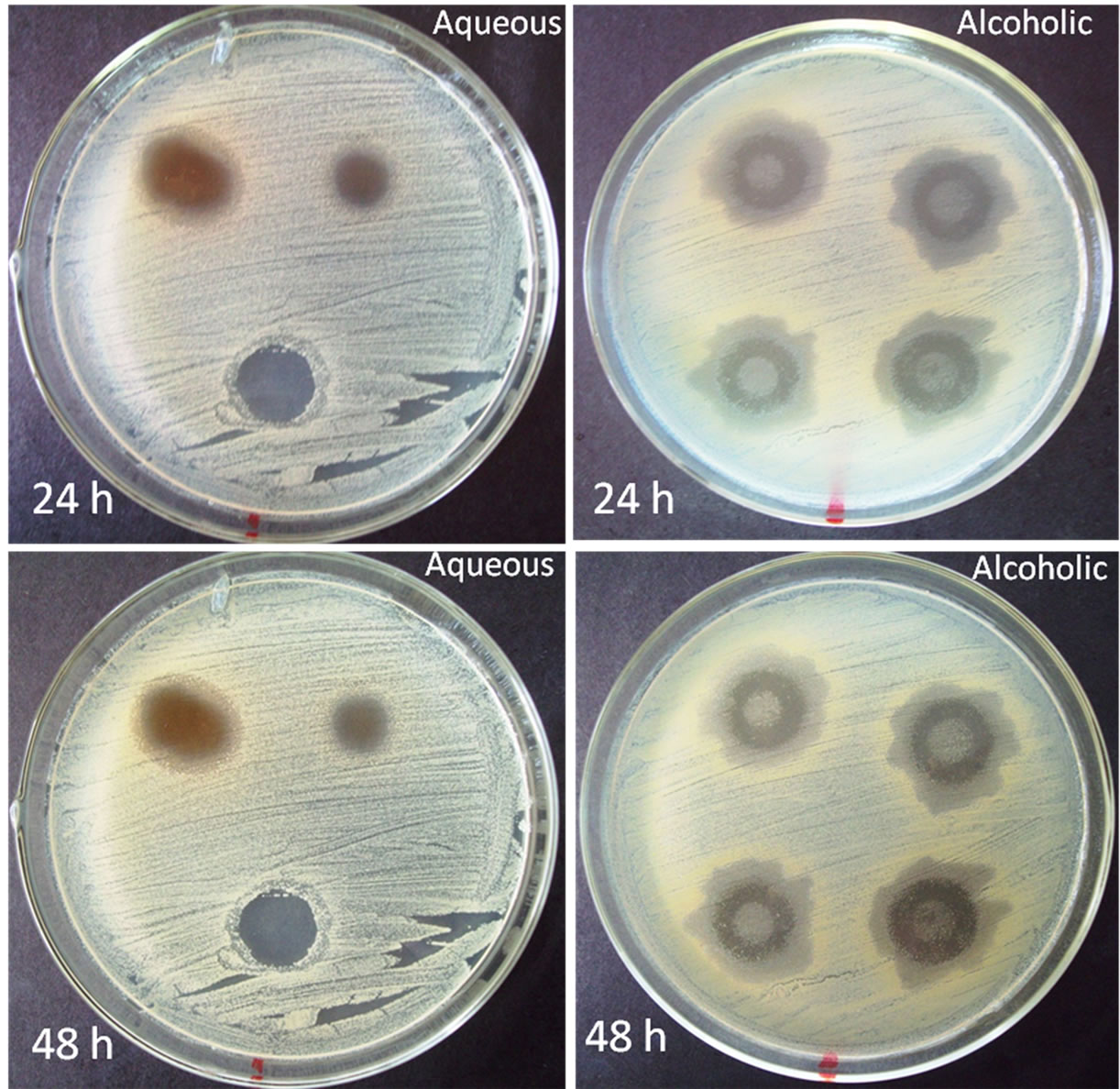
Figure 1. S. barbatiman inhibition halo for different solutions.
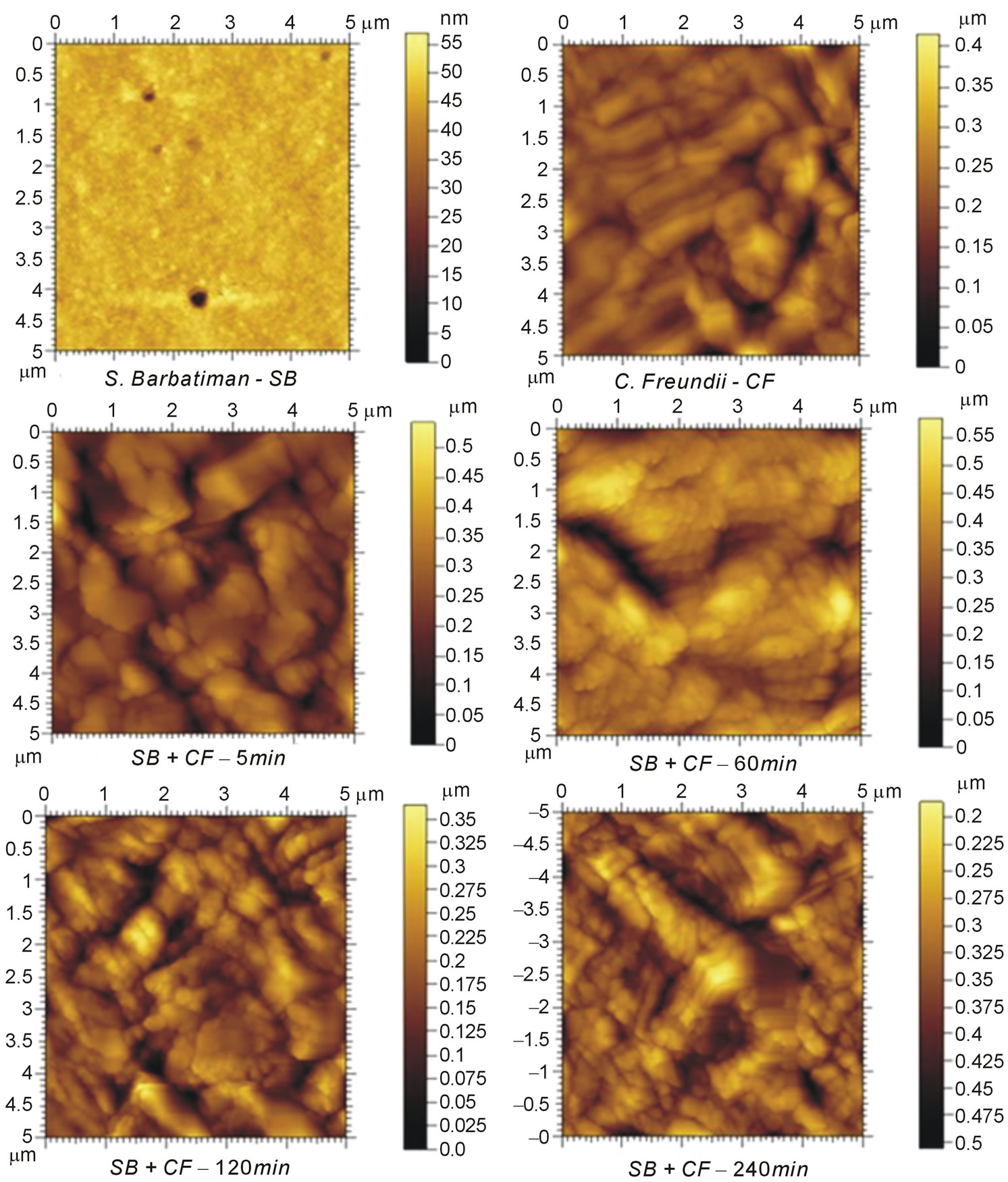
Figure 2. AFM images of S. barbatiman and C. freundii casting films and C. freundii dispersed in S. barbatiman at different times of contact. The scanning window was of 5 μm × 5 μm.
results support the hypothesis that cellular wall damage of C. freundii can be caused by chemical compounds like tannins, which may be found in S. barbatiman and are involved in antimicrobial activity.
FT-IR spectroscopy has been used to investigate the interaction between S. barbatiman and C. freundii, which could lead to death of these bacteria [9]. The main absorption bands of S. barbatiman (Figure 5) can be associated to axial deformation of aromatic rings C=C of tannins. The bactericidal effectiveness of S. barbatiman can be observed in spectral changes shown in Figure 5, which are associated with the ester groups of lipids, fatty acids, proteins and nucleic acids in the region between 1800 and 1300 cm−1 [9].
The most intense band of C. freundii includes the functional C=O group of lipid at 1741 cm−1. The amide I band at ~1630 cm−1 is associated with the C = O stretching of amide group of proteins. The region between 1300 and 900 cm−1 is characterized by the characteristic vibration of groups of nucleic acids, cell membrane and cell wall components [9-11]. The major band of this region includes stretching of the P = O groups (phosphodiester) at 1219 cm−1 in the backbone of nucleic acids and polysaccharide vibration (1200 - 900 cm−1) of the cell wall peptidoglycans and lipopolysaccharides.
The spectrum of C. freundii dispersed in S. barbatiman extract indicated significant variations in the region between 1300 and 1200 cm−1. This suggested a denaturation of nucleic acid and/or damages to the cellular walls [9]. The S. barbatiman may has affected the bacterial structure as evidenced by the vibration difference of the corresponding P = O groups.
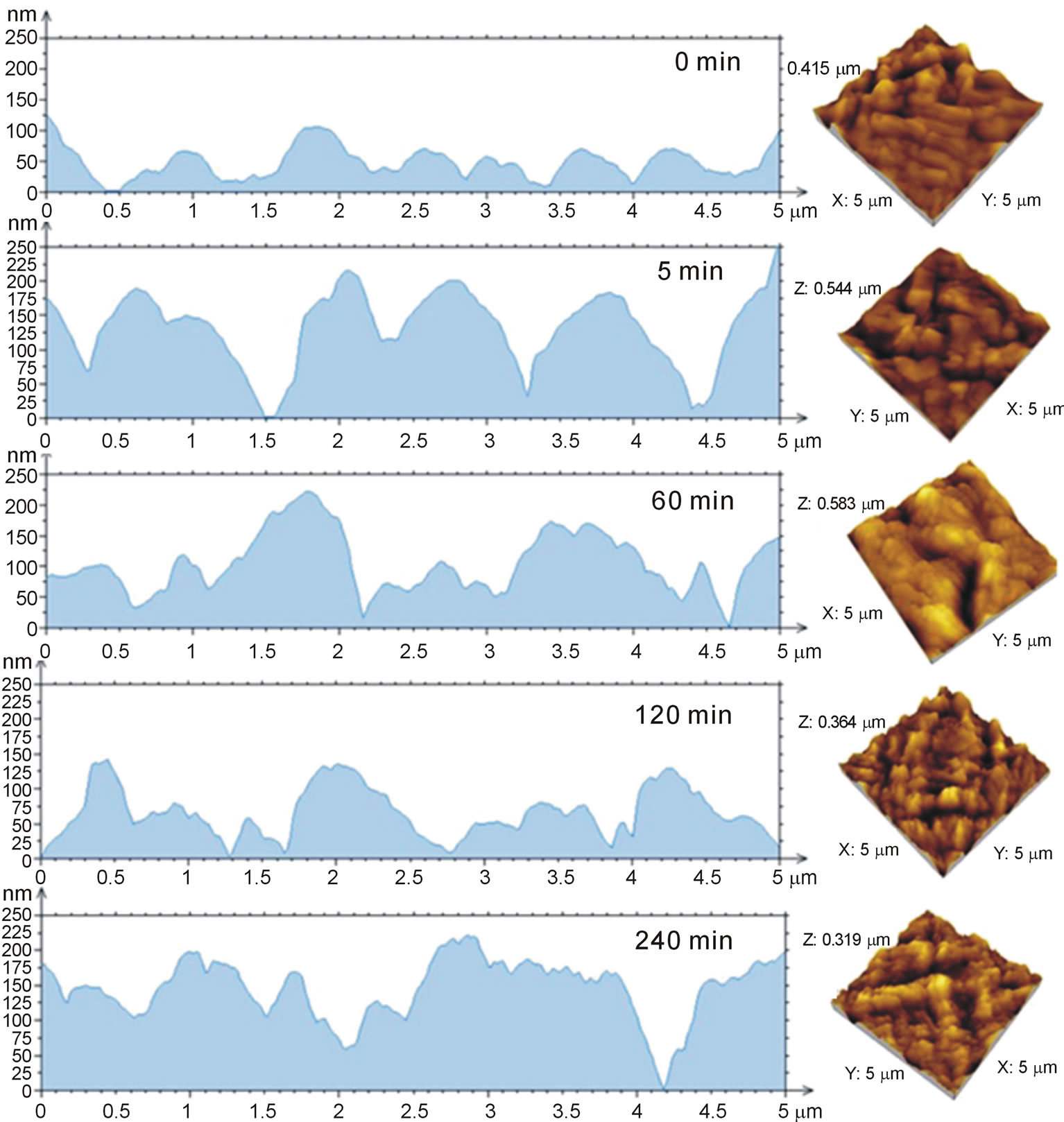
Figure 3. Height profiles of biofilms of C. freundii at different contact time with S. barbatiman.
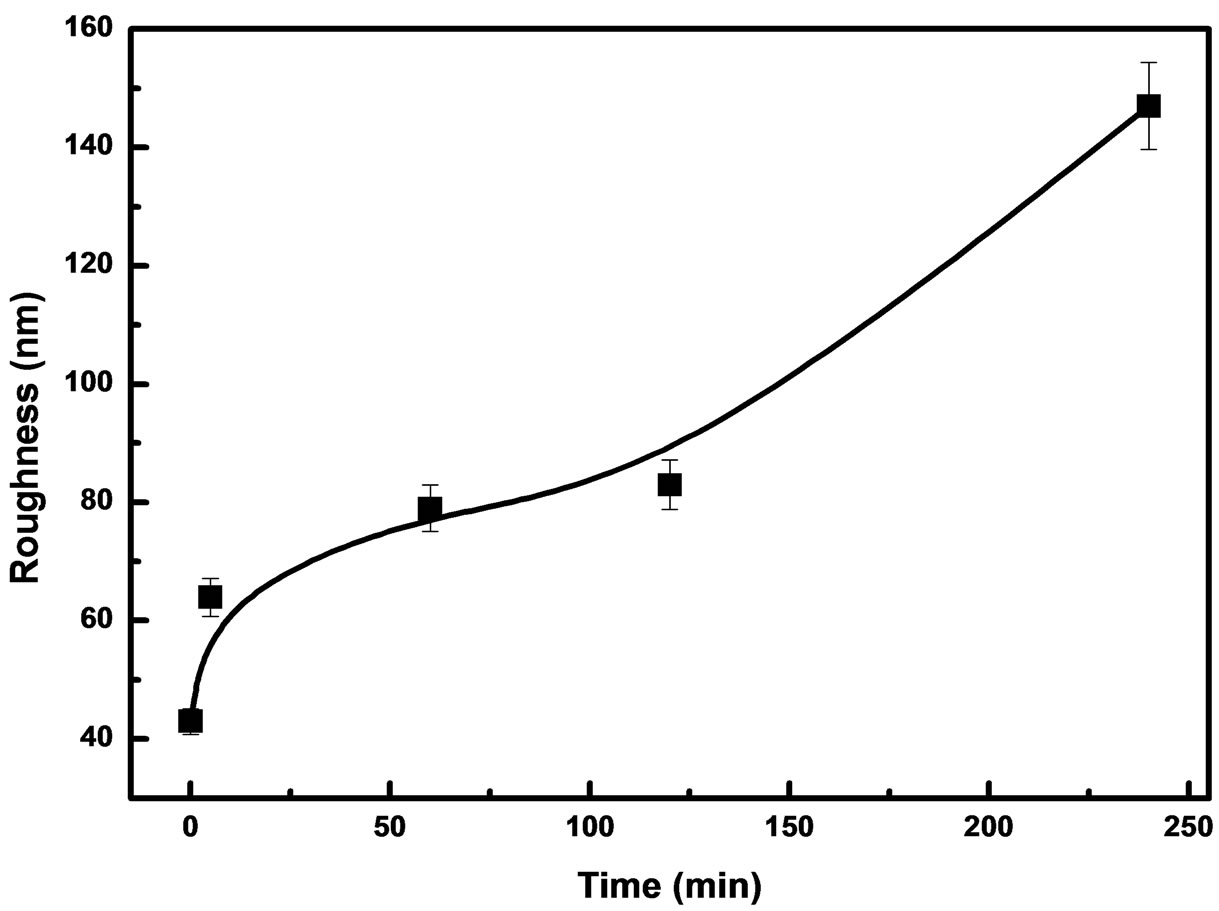
Figure 4. Surface roughnesses for biofilms of C. freundii as a function of the contact time.
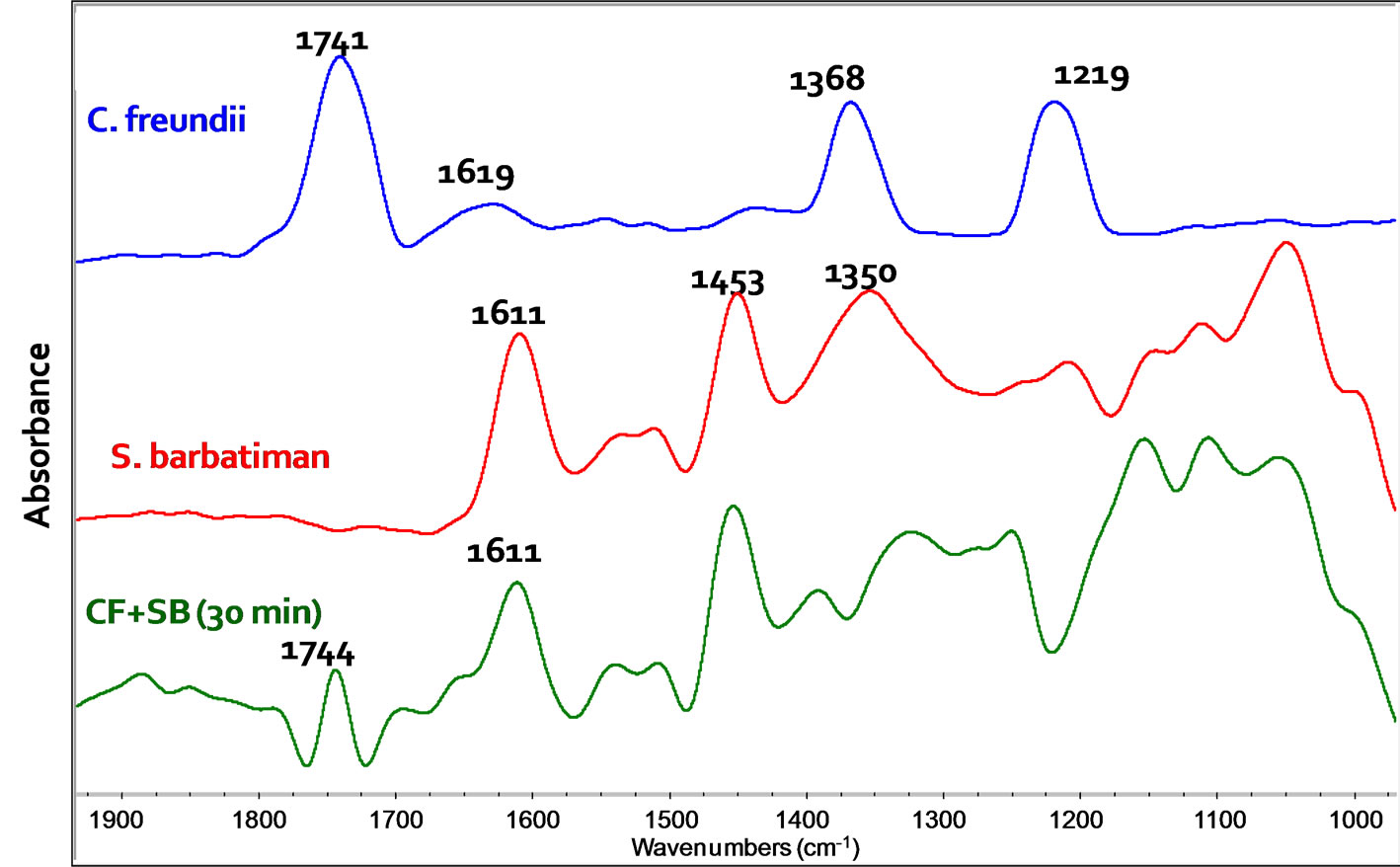
Figure 5. FT-IR spectra of C. freundii, extract of S. barbatiman and C. freundii dispersed in S. barbatiman extract after 30 min.
4. Conclusion
The antimicrobial activity of S. barbatiman against C. freundii was demonstrated by susceptibility tests. FT-IR spectroscopy and AFM results suggested that S. barbatiman causes damages to the cellular wall of C. freundii bacteria and then leads them to death. Further studies are necessary to clear this point; however, possible therapeutic uses of the S. barbatiman against C. freundii should be explored in which FT-IR spectroscopy and AFM analyses can play an important role.
5. Acknowledgements
This work was supported by CNPq and Capes (Brazil). We thank M. Fátima Rodrigues Souza and Elpídio de Souza Filho for discussion about S. Barbatiman.
REFERENCES
- K. Ishida, S. Rozental, J. C. P. De Mello and C. V. Nakamura, “Activity of tannins from Stryphnodendron adstringenson on Cryptococcus neoformans: Effects on Growth, Capsule Size and Pigmentation,” Annals of Clinical Microbiology and Antimicrobials, Vol. 8, 2009, pp. 1-10.
- D. L. Nelson and M. M. Cox, “Lehninger Principles of Biochemistry,” 4th Edition, 2004. http://bcs.whfreeman.com/lehninger/default.asp
- D. Voet and J. G. Voet, “Biochemistry,” 4th Edition, 2011. http://www.wiley.com/WileyCDA/WileyTitle/productCd-HEP001782,descCd-INSTRUCTOR.html
- Y. Cong, J. Wang, Z. Chen, K. Xiong, Q. Xu and F. Hu, “Characterization of Swarming Motility in Citrobacter freundii,” PFEMS Microbiology Letters, Vol. 317, No. 2, 2011, pp. 160-171. http://dx.doi.org/10.1111/j.1574-6968.2011.02225.x
- A. Kumar, H. Shaaban, K. Koduru, S. Abo, I. Sidhom and G. Guron, “Citrobacter freundii-Induced Cold Agglutinin Hemolysisi,” Annals of Hematology, Vol. 90, No. 7, 2011, pp. 855-856. http://dx.doi.org/10.1007/s00277-010-1096-9
- J. G. Whalen, T. W. Mully and J. C. English, “Spontaneous Citrobacter freundii Infection in an Immunocompetent Patient,” Archives of Dermatology, Vol. 143, No. 1, 2007, pp. 124-125.
- S. B. Ferreira, J. D. Palmeira, J. H. Souza, J. M. Almeida, M. C. P. Figueiredo, A. S. Pequeno, T. A. Arruda, R. M. P. Antunes and R. M. R. Catão, “Evaluation of the Antimicrobial Activity in Vitro of the Hydroalcoholic Extract Stryphnodendron adstringens against of Staphylococcus aureus Strains,” RevistaBrasileira de AnálisesClínicas, Vol. 42, 2010, pp. 27-31. http://www.sbac.org.br/pt/pdfs/rbac/rbac_42_01/rbac_42_01_06.pdf
- K. Sahu, H. Bansal, C. Mukherjee, M. Sharma and P. K. Gupta, “Atomic Force Microscopic Study on Morphological Alterations Induced by Photodynamic Action of Toluidine Blue O in Staphylococcus aureus and Escherichia coli,” Journal of Photochemistry and Photobiology B: Biology, Vol. 96, No. 1, 2009, pp. 9-16. http://dx.doi.org/10.1016/j.jphotobiol.2009.03.008
- H. M. Al-Qadiri, N. I. Al-Alami, M. A. Al-Holy and B. A. Rasco, “Using Fourier Transform Infrared (FT-IR) Absorbance Spectroscopy and Multivariate Analysis to Study the Effect of Chlorine-Induced Bacterial Injury in Water,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 19, 2008, pp. 8992-8997. http://dx.doi.org/10.1021/jf801604p
- P. D. Nichols, J. M. Henson, J. B. Guckert, D. E. Nivens and D. C. White, “Fourier Transform-Infrared Spectroscopic Methods for Microbial Ecology: Analysis of Bacteria, Bacteria-Polymer Mixtures and Biofilms,” Journal of Microbiological Methods, Vol. 4, No. 2, 1985, pp. 79- 94. http://dx.doi.org/10.1016/0167-7012(85)90023-5
- P. Das, S. Mukherjee and R. Sen, “Antimicrobial Potential of a Lipopeptide Biosurfactant Derived from a Marine Bacillus circulans,” Journal of Applied Microbiology, Vol. 104, No. 6, 2008, pp. 1675-1684. http://dx.doi.org/10.1111/j.1365-2672.2007.03701.x
NOTES
*Corresponding author.

