Journal of Biophysical Chemistry
Vol. 3 No. 4 (2012) , Article ID: 25041 , 8 pages DOI:10.4236/jbpc.2012.34035
Tityus serrulatus venom and its toxins Ts1 and Ts5 increase cytosolic Ca2+ concentration in isolated vascular smooth muscle cells*
![]()
1Faculty of Ingá-UNINGÁ, Maringá, Brazil; *Corresponding Author: marioneto@uninga.br
2Laboratory of Pharmacology, Department of Physics and Chemistry, Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil
3Laboratory of Animal Toxins, Department of Physics and Chemistry, Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil
4Laboratory of Toxicology, Faculty of Pharmacy, Institute of Health Sciences, Federal University of Pará, Belém, Brazil
Received 3 July 2012; revised 14 August 2012; accepted 26 August 2012
Keywords: Ts1; Ts5; Na+ Channel; Ca2+ Channel; Tityus serrulatus
ABSTRACT
Voltage-gated Na+ channel (Nav channel) scorpion toxins are classified as (- and (-neurotoxins. Ts5 ((-neurotoxin) and Ts1 ((-neurotoxin) from Tityus serrulatus venom (TsV) interact with Nav channels, increasing Na+ influx and activateing voltage-dependent Ca2+ channels. This study aimed to investigate the effect of TsV, Ts1 and Ts5 on the cytosolic Ca2+ concentration ([Ca2+]C) in rat aortic smooth muscle cells. Toxins were isolated by ion exchange chromatography (Ts1) followed by RP-HPLC (Ts5). The rat aortic smooth muscle cells were isolated in Hanks buffer pH 7.4 and loaded with 5 (mol/L of Fura-2AM (45 minutes at 37˚C), in order to measure [Ca2+]C by fluorescence of Fura-2/AM (ratio 340/380 nm). The fluorescence was measured in one single cell (excitation: 340 and 380 nm; emission: 510 nm). TsV (100 and 500 (g/mL) and its toxins Ts1 and Ts5 (50 and 100 (g/mL each) led to a concentration-dependent increase in [Ca2+]C. Tetrodotoxin (1 (mol/L), a Nav channel blocker, and verapamil (1 (mol/L), a voltage-operated Ca2+ channel blocker, inhibited the increase in [Ca2+]C induced by TsV (500 (g/mL). In conclusion, TsV and its toxins induce a concentration-dependent increase in [Ca2+]C that probably occurs through interaction with Nav channels, thus inducing depolarization and consequent Ca2+ influx. This assumption is based on the fact that this effect is inhibited by tetrodotoxin and verapamil, showing a direct action of TsV toxins on aorta smooth muscle cells.
1. INTRODUCTION
Tityus serrulatus is the most dangerous scorpion species in Brazil, yielding a mortality rate of 1.04% among children [1]. Most of the symptoms and signs observed in the scorpion envenomation, such as fever, psychomotor agitation, salivation, lachrymation, increased gastrointestinal tract mobility, cardiac and respiratory arrhythmias, arterial hypertension followed by hypotension, cardiac failure, pulmonary edema and shock, is a conesquence of widespread peripheral nerve stimulation, culminating in a massive release of neurotransmitters [2-9].
Scorpion venoms are rich sources of different classes of peptides that represent useful tools for biological research. These peptides disrupt the normal function of excitable tissues found in muscles and nerves by interaction with voltage-gated Na+, K+, Ca2+ or Cl– channel [10-13]. Voltage-gated Na+ (Nav) channel neu rotoxins are the most important components of the scorpion venom, and they are the main agents responsible for the toxic effects of scorpion envenoming. Two types of toxins (α and β) that are active on the Nav channels have been described, and they are classified according to their specificity toward two different sites on the channel [12-14]. The α-neurotoxins bind to receptor site 3 retarding Nav channel inactivation and inducing a prolongation of the action potential depolarization phase. The β-neurotoxins bind to receptor site 4 and shift the voltage dependence of Nav channel activation to more negative potentials, giving rise to an increased tendency of the cell to fire spontaneously and repeatedly, independent of the membrane potential [13,14].
Scorpion neurotoxins (a and β) act on the Nav channel of nerve terminals, resulting in increased Na+ influx, which in turn causes depolarization of the cell membrane. Depolarization induces the opening of the voltage-activated calcium channel, allowing the entrance of Ca2+. This represents a key step in the regulation of a variety of cellular processes, such as the neurotransmitter release [7,15,16].
The venom of the Brazilian scorpion Tityus serrulatus Lutz & Mello (Buthidae family) contains both types of neurotoxins, represented by Ts5 (a-neurotoxin) and Ts1 (b-neurotoxin). Ts5 was first isolated and characterized by Arantes et al. (1994) [17] as a basic (pI = 8.0) toxic polypeptide (LD50 = 94 ± 7 μg/kg, i.v. in mice) that induces a prolongation of Nav channel inactivation. The amino acid sequence of the toxin Ts5 has also been determined. It has 64 amino acid residues and a calculated Mr of 7230 [18].
The major toxic component from T. serrulatus venom (TsV) is a long-chain b-neurotoxin Ts1 [19, 20], also called TsTX-I, toxin VII or toxin-g (updated nomenclature by Cologna et al., 2009 [21]), and it has an intravenous LD50 of 76 ± 9 μg/kg, compared to 375 ± 45 μg/kg for TsV [22]. Ts1 plays a major role in T. serrulatus envenoming, accounting for approximately 16% of the soluble venom, while Ts5 represents only 2% of TsV. In rats, they induce intense catecholamine release, with concomitant increase in the mean arterial pressure [7].
Despite the fact that catecholamines and acetylcholine mediate most of the toxicological actions of scorpion venoms, other neurotransmitters are also involved in these actions [23-30]. Vasconcelos et al. (2005) [7] reported that the toxicity of Ts5 is not only related to its ability to release catecholamines, but modulation of the release of other neurotransmitters may be involved. The Ts5 causes a reduction in 3H-GABA and 3H-DA uptake in a Ca2+-dependent manner, not directly affecting GABA transporters, but, it involves Nav channels as a conesquence of depolarization [15].
Thus, both α- and β-neurotoxins may modulate the neurotransmitter release via a Ca2+-dependent mechanism, although these toxins act on the Nav channel, allowing increased Na+ influx. This in turn causes membrane depolarization, leading to the activation of voltage-dependent Ca2+ channels.
Most of the actions of the scorpion toxins are indirect and due to neurotransmitter release, but Teixeira Jr. et al. (2001) [31] have reported a possible direct action of TsV on the cardiac muscle. The venom-induced positive inotropic effects are not modified by pretreatment with metoprolol, or chemical sympathetic denervation with 6-OH-dopamine. These results demonstrate that the inotropic effects of the venom on the rat heart are not dependent on neurotransmitter release. The increase in the contractile force appears to be a direct effect of the venom on the cardiomyocyte [31]. Cardiac voltage-gated Na+ channels are also targets of scorpion toxins [32]. It has been demonstrated in earlier studies that vascular smooth muscle contraction is induced by Na+ channel activators (like Nav channel scorpions neurotoxins), veratridine and batrachotoxin [33].
Bearing in this background in mind and considering that the direct action of neurotoxins from scorpion venoms on vascular muscle cells are not yet fully understood, the objective of this study was to investigate the effect of TsV and its purified toxins Ts5 and Ts1 on the cytosolic Ca2+ concentration ([Ca2+]c) in isolated rat aorta smooth muscle cells, as well as the mechanism involved in this effect.
2. MATERIAL AND METHODS
2.1. Animals
Male Wistar rats (180 - 200 g) were obtained from the animal facility of the Campus of Ribeirão Preto, University of São Paulo (USP), and maintained under standard laboratory conditions of 12 h dark/light cycle, at a temperature of 21˚C. The rats were housed in polystyrene cages (4 rats/cage, 50 × 35 × 15 cm) containing stainless steel coverlids and wooden shavings as bedding. The animals were anesthetized and euthanized by decapitation on the day of the experiment and a segment of the thoracic aorta (15 mm) was isolated and maintained in Hanks solution.
All the experimental procedures were conducted according the Institutional Ethics Commitee of the University of São Paulo-Brazil (Protocol Nr. 03. 1.1069.53.0).
2.2. Ca2+ Measurements
The aortic smooth muscle cells were isolated on the day of the experiment and loaded with 5 mmol/L Fura-2AM for 45 minutes, in order to measure [Ca2+]c by fluorescence of Fura-2AM (ratio 340/380 nm). Experimental protocols were developed using the venom (TsV) and its toxins (Ts1 and Ts5) in the presence or in the absence of a sodium channel blocker (tetrodotoxin) and a calcium channel blocker (Verapamil) added 10 minutes prior to stimulation with the venom or toxins. Data are expressed as the difference between the basal and final fluorescence intensities (D%IF), which reflects the increase in cytosolic Ca2+ concentration ([Ca2+]c) obtained for each protocol.
Briefly, the aortas were dissected and longitudinally opened, and smooth muscle cells were isolated by enzyme digestion with collagenase in Hanks solution. The resulting cell solution was centrifuged at 200xg and suspended in Hanks solution. The cells were placed on glass coverslips and kept in a humidified 37˚C incubator gassed with 5% CO2 for 4 h. Then, the viability of the cells at this stage was determined by exclusion of 0.4% trypan blue dye, being invariably larger than 95%. The cells were then incubated with 5 mmol/L Fura-2AM in 1 mg/mL bovine soroalbumin (BSA) for 45 minutes, at 37˚C, and then washed for 20 minutes. Dishes containing Fura-2AM loaded cells were placed in a temperature regulated (37˚C) chamber mounted on the Nikon inverted microscope. Each fluorescence measurement was performed in one isolated cell, in the window of a dual wavelength spectrofluorometer (Deltaram, Photon Technology Intl), at excitation wavelengths of 340 and 380 nm and emission wavelength of 510 nm. Fura-2 fluorescent signals originated from the cells were collected and stored using a software package from Photon Technology International (Felix). Results represent the D%IF of the F/F0 ratio and are expressed in percentage. The F values represent the final fluorescence, after pharmacological stimulation, and F0 represents the basal fluorescence [34].
2.3. Venom Extraction and Ts1 and Ts5 Isolation
2.3.1. Soluble Venom (TsV)
Lyophilized TsV was purchased from Phoneutria Biotechnology and Services (Belo Horizonte-MG, Brazil) and stored at –20˚C. The venom was dissolved in 0.9% (w/v) NaCl and centrifuged at 11,270 × g at 27 ˚C, for 5 minutes. TsV quantification was based on total proteins.
2.3.2. Protein Determination
Determination of total proteins was carried out according to the method described by Bradford [35], using commercially acquired kits (Bio-Rad Protein assay, Bioagency®), and confirmed by the method of absorbance at 205/280 nm [36]. BSA was used as standard.
2.3.3. Ion Exchange Chromatography
TsV was fractionated at 5˚C by ion exchange chromatography as previously described [22]. The soluble extract from the crude venom (400 mg) was chromatographed on a 2.5 × 63.0 cm carboxymethyl cellulose-52 column (Whatman), which had been equilibrated and initially eluted with 0.01 mol/L ammonium bicarbonate buffer pH 7.8 up to 468 mL effluent, when a convex concentration gradient was started from 0.01 mol/L to 1.00 mol/L buffer. The flow rate was 26.7 mL/h. The resulting pools designated I-XIII were then lyophilized until salt-free, followed by determination of the buffer concentration of each fraction by measurement of the corresponding conductivity. Pure Ts1 (pool X-III) was obtained in this single chromatographic step. The recovery was calculated in terms of the absorbance at 280 nm.
2.3.4. Reverse-Phase Liquid Chromatography
RP-HPLC of the lyophilized pool XI was performed in a Shimadzu HPLC system, using a C-18 reverse-phase analytical column Shim Pack CLC-ODS (M) 5 μm, 0.46 × 25.0 cm (Shimadzu Instruments Corp., Tokyo, Japan) at room temperature. The column had been previously equilibrated with buffer A (5% acetonetrile + 0.1% trifluoroacetic acid). Elution was performed with a linear gradient of Buffer B (60% acetonitrile in 0.1% trifluoroacetic acid) at a flow rate of 1.0 mL/min. Absorbance was monitored at 214 nm. The resulting highly purified toxin was previously named TsTX-V [17]. The RP-HPLC of Ts1 was carried out under the same conditions.
2.4. Drugs and Solutions
Albumin bovine fraction V, fatty acid-free, Albumin bovine fraction V, Dimethyl sulfoxide (Grade I) DM-SO, Poly-L-lysine solution, tetrodotoxin and verapamil were obtained from Sigma Chem. Co. (St Louis, MO-USA). Fura-2AM was purchased from Molecular Probes (Eugene, Oregon, USA).
The Hanks solution was as follows (in mmol/L): 145.0 NaCl, 1.6 CaCl2, 5.0 KCl, 1.0 MgCl2, 0.5 Na-H2PO4, 10.0 dextrose and 10.0 HEPES (pH 7.4).
2.5. Statistical Analysis
The effect on the cytosolic Ca2+ concentration ([Ca2+]c ) elicited by TsV or its toxins (Ts1 and Ts5) was represented by the percentage of the D of fluorescence intensity of each protocol in relation to the positive control (100%) KCl 60 mmol/L. Analysis of the data and plotting of the figures were performed with the aid of the software GraphPad PRISMTM, Version 3.02 (2000) (San Diego, CA, USA). Data are expressed as mean ±SEM. In each set of experiments, n indicates the number of cells studied. Differences between mean values were assessed by one-way analysis of variance (ANOVA) followed by a Newman-Keuls post-hoc test, and values of P < 0.05 were considered significant.
3. RESULTS
The effects of TsV, Ts1 and Ts5 in increasing [Ca2+]c in isolated rat aorta smooth muscle cells were evaluated and compared to the depolarizing effect of KCl 60 mmol/L (positive control: 100%).
TsV (100 and 500 mg/mL) increased the [Ca2+]c in 49.6% ± 2.6% and 103.7% ± 5.2%, respectively, when compared with the positive control (100%), showing a concentration-dependent effect (Figures 1 and 2). The lower TsV concentration induced an increase in [Ca2+]c that was different from those induced by the control (P < 0.001; n =
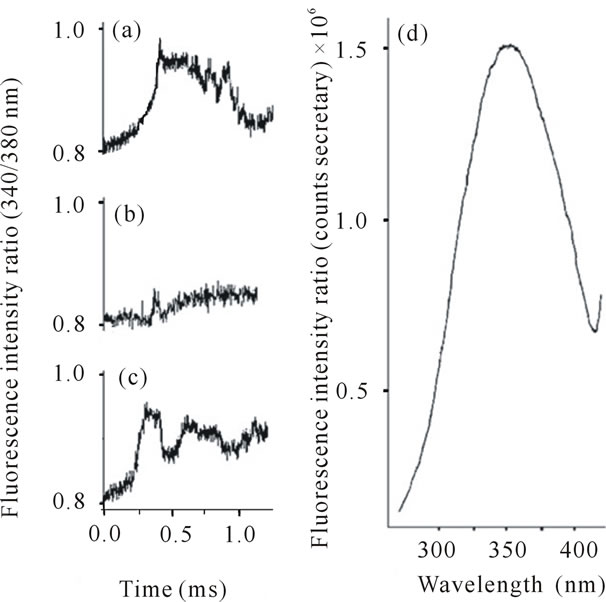
Figure 1. Representative records of assays performed with vascular smooth muscle cells of rat aorta. Ratio of fluorescence intensity (340/380 nm) after perfusion of the cells with: ((a) KCl 60 mmol/L (b)) TsV 100 µg/mL and (c)) TsV 500 µg/mL. (d)) Representative fluorescence profile of cell loaded with the probe Fura-2AM (counts/sec).
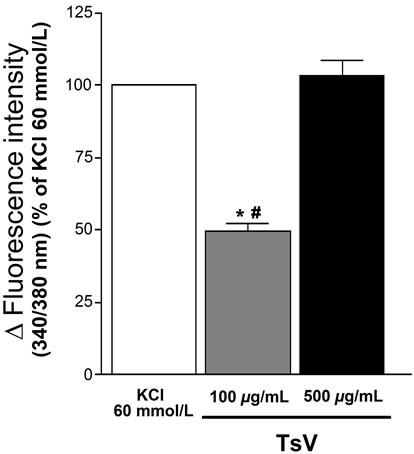
Figure 2. Effect of the TsV on Ca2+ cytosolic concentration ([Ca2+]c) in rat aortic smooth muscle cells. Bars represent [Ca2+]c stimulated with TsV 100 and 500 mg/mL. # P < 0.001: TsV 100 mg/mL vs KCl 60 mmol/L (n = 3, ANOVA); *P < 0.001: TsV 100 mg/mL vs TsV 500 mg/mL (n = 3, ANOVA).
3) and the higher TsV concentration (P < 0.001; n = 3).
In order to investigate the Na+-dependence of the Ca2+ influx elicited by TsV, the rat aortic smooth muscle cells were pre-incubated with tetrodotoxin (TTX), a Nav channel blocker, or verapamil, a voltage-operated Ca2+ channel blocker. After incubation for 10 minutes, the cells were stimulated with 500 mg/mL of TsV, and the increase in [Ca2+]c was determined and compared with the control (100%). TsV at 500 mg/mL was selected because at this concentration its effect on the [Ca2+]c was similar to that of the control, more precisely 103.7% ± 5.2%. When the cells were incubated with TTX (1 mmol/L), the increase in [Ca2+]C elicited by TsV (500 mg/mL) was reduced to 15.7% ± 3.1% (P < 0.001). However, when the cells were incubated with verapamil (1 mmol/L), the increase in [Ca2+]c elicited by TsV (500 mg/mL) was reduced to 47% ± 5.8% (P < 0.01) (Figure 3). Cells pre-incubation with TTX was more effective in reducing the effect of TsV on the [Ca2+]c than pre-incubation with verapamil (P < 0.05).
The effects of Ts1 and Ts5 (50 and 100 mg/mL of each) on the increase in [Ca2+]c was investigated and compared with the control (100%) (Figure 4). Ts1, 50 mg/mL and 100 mg/mL, increased the [Ca2+]c in 43.9% ± 3.1% and 121.8% ± 8.9%, respectively (Figure 5). The effect of the higher Ts1 concentration was different from that of lower concentration (P < 0.001; n = 3), as observed with the TsV. Ts5 exhibited a similar concentration-dependent effect. The concentrations of 50 mg/mL and 100 mg/mL increased the [Ca2+]c in 52.6% ± 8.3% and 79.5% ± 6.1%, respectively (Figure 5). However, the effect of Ts1 (100 mg/mL) was greater than that of Ts5 at the same concentration.
4. DISCUSSION
Scorpion venoms are extremely rich on bioactive peptides (toxins) that often carry diverse functions and are presumably needed to achieve synergistic effects for rapid prey immobilizing and consequent defense. The
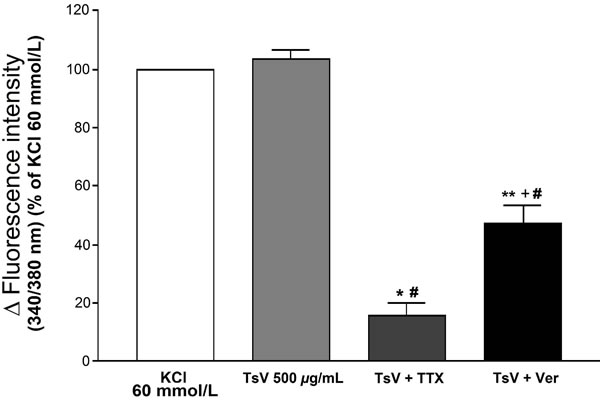
Figure 3. Effects of tetrodotoxin (TTX) and verapamil (Ver) on the effect elicited with TsV on Ca2+ cytosolic concentration ([Ca2+]c) in rat aortic smooth muscle cells. Bars represent [Ca2+]c stimulated with TsV 500 mg/mL, TsV (500 mg/mL) pre-incubated with TTX (1mmol/L), and TsV (500 mg/mL) pre-incubated with verapamil (1mmol/L). #P < 0.001: each group vs KCl 60 mmol/L (n = 3, ANOVA); *P < 0.001: TsV 500 mg/mL vs TsV + TTX (n = 3, ANOVA); **P < 0.01: TsV 500 mg/mL vs TsV + Ver (n = 3, ANOVA); +P < 0.05: TsV + TTX vs TsV + Ver (n = 3, ANOVA).

Figure 4. Representative records of assays performed with vascular smo oth muscle cells of rat aorta. Ratio of fluorescence intensity (340/380 nm) after perfusion of the cells with: (a) KCl 60 mmol/L (b) Ts1 50 µg/ mL and (c) Ts5 50 µg/mL.

Figure 5. Effect of the toxins Ts1 and Ts5 on Ca2+ cytosolic concentration ([Ca2+]c) in rat aortic smooth muscle cells. Bars represent [Ca2+]c stimulated with Ts1 50 and 100 mg/mL, and Ts5 50 and 100 mg/mL. #P < 0.05: group vs KCl 60 mmol/L (n = 3, ANOVA); *P < 0.001: Ts1 50 mg/mL vs Ts1 100 mg/mL (n = 3, ANOVA); **P < 0.01: Ts1 100 mg/mL vs Ts5 100 mg/mL (n = 3, ANOVA); +P < 0.01: Ts5 50 mg/mL vs Ts5 100 mg/mL (n = 3, ANOVA).
major cause of death observed in victims stung by scorpions of the Buthidae family has been mainly attributed to cardiovascular toxicity resulting from the massive release of catecholamines from adrenal and noradrenergic nerve terminals, together with complications arising from the onset of pulmonary edema and respiratory arrest [2-4,7-9,16]. In addition to the role of catecholamines and others neurotransmitters, a direct action of scorpion toxins on the cardiovascular system has been repeatedly reported [29,37,38].
The synergistic actions of aand b-toxins as well as the K+ channel toxins are responsible for the lethality of the Tityus serrulatus venom (TsV). The aand b-toxins are capable of exerting two distinct effects on the gating mechanism of Na+ currents in nerves and muscles: a toxins block voltage-dependent inactivation of Na+ channels and b toxins shift the voltage dependent activation of these channels to a more negative membrane potential. The a-toxins induce a prolongation of the repolarization phase of the action potential while b-toxins promote a repetitive firing after a unique stimulation [13, 14].
The main goal of this study was to show for the first time the effect of Tityus serrulatus scorpion venom and its toxins Ts1 and Ts5 on the cytosolic Ca2+ concentration ([Ca2+]c) in isolated rat aorta smooth muscle cells. The scorpion venom and its toxins increased the fluorescence intensity related to the increase in [Ca2+]c. Consequently, the contractile effect on the vascular smooth muscle cells was increased in a concentration-dependent manner.
The TsV (100 and 500 mg/mL) and toxins (50 and 100 mg/mL) concentrations were chosen on the basis of their respective effects. The higher TsV and Ts1 concentrations (500 and 100 mg/mL, respectively) induced effects similar to those observed with the positive control (KCl 60 mmol/L), which produced the maximum contractile effect on aorta smooth muscle cells. The lower concentrations were important to demonstrate that the venom and toxin effects are concentration-dependent and allowed a comparative analysis of the effectiveness of TsV, Ts1 and Ts5.
Our results show that TsV and its toxins Ts1 and Ts5 cause an increase in [Ca2+]c in rat aortic smooth muscle cells. We suggest that the contractile effect induced by the scorpion venom and its toxins are due to the Na+ influx through voltage-gated Na+ channels (Nav), which induces membrane depolarization and results in activation of voltage-dependent Ca2+ channels. This is supported by the fact that the change in fluorescence intensity induced TsV was powerfully reduced by tetrodotoxin (TTX), a Na+ channel blocker. Since both neurotoxins have been obtained from the venom, the effect elicited by TsV is, at least in part, dependent on the presence of Ts1 and Ts5. The contribution of Ts1 to the venom effect is probably greater than the one of Ts5, because it is present in a larger proportion in the venom and causes a larger increase in the [Ca2+]c at the concentration of 100 mg/mL.
To confirm the hypothesis of extracellular Ca2+ influx via voltage-dependent Ca2+ channels, we incubated the cells with verapamil, a selective Ca2+-L channel blocker. Since the increase in [Ca2+]c induced by TsV is partially inhibited by verapamil, we suggest that voltage-operated Ca2+ channels sensitive to verapamil are indirectly activated by the scorpion venom. However, it is possible that activation of voltage-operated Ca2+ channel is not the only mechanism involved in the [Ca2+]c increase caused by TsV, since the block observed in the presence of verapamil was only partial. Based on these experimental evidences, the increase in [Ca2+]c that is no inhibited by verapamil could be explained by activation of mechanisms that release Ca2+ from intracellular organelles such as the sarcoplasmic reticulum, CIRC (Ca2+-induced release of Ca2+), and Ca+2 released via IP3 and ryanodine receptors [39].
Grolleau et al. [40] reported that other targets such as high voltage activated calcium channels and non capacitive calcium entry (NCCE) may be involved in the neurotoxicity of toxin VII, a β-neurotoxin from TsV. These findings have generated many interesting questions relating envenoming physiopathology relationships. NCCE is thought to be an important pathway for calcium influx into many cellular types including excitable as well as nonexcitable cells such as fibroblasts, vascular smooth muscle cells and neurosecretory neurons [40-42].
Most of the effects of scorpion toxins are indirect and due to the release of adrenergic and cholinergic neurotransmitters. However, the results obtained in this work show that TsV and the toxins Ts1 and Ts5 are also able to interact directly with Nav channel in vascular smooth muscle cells (VSMC) and increase [Ca2+]c, which causes contraction of the blood vessel. The presence and function of Nav channel in VSMC has been shown [43-45].
Clinically, these results could be related with many symptoms promoted by scorpion venom, such as respiretory paralysis, altered consciousness, convulsions [46] and cardiovascular abnormalities related to arterial hypertension, in human envenomation [2].
5. CONCLUSION
In conclusion, TsV and its toxins Ts1 and Ts5 produce a direct effect on rat aorta smooth muscle cells, inducing a concentration-dependent increase in [Ca2+]c, probably by activation of Nav channels sensitive to tetrodotoxin. Consequently, there is particular extracellular Ca2+ influx, at least in part via Ca2+ channels sensitive to verapamil.
6. ACKNOWLEDGEMENTS
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
- Brazilian Ministry of Health. Secretaria de Vigilância em Saúde (MS/SUS). (2010) Situação epidemiológica das zoonoses de interesse para a saúde pública. Boletim Eletrônico Epidemiológico, 2, 19.
- Freire-Maia, L. (1995) Peripheral effects of Tityus serrulatus scorpion venom. Journal of Toxicology, Toxin reviews, 14, 423-435.
- Ismail, M. (1995) The scorpion envenoming syndrome. Toxicon, 33, 825-858. doi:10.1016/0041-0101(95)00005-7
- Cupo, P., Azevedo-Marques, M.M., Hering, S. (2003). Escorpionismo. In: Cardoso, J.L.C., França, F.O.S., Wen, F.H., Málaque, C.M.S., Haddad Jr, V., Eds., Animais Peçonhentos do Brasil: Biologia, Clínica e Terapêutica dos Acidentes, Sarvier, São Paulo, 198-208.
- Fukuhara, Y.D.M., Dellalibera-Joviliano, R., Cunha, F.Q.C., Reis, M.L. and Donadi, E.A. (2004) The kinin system in the envenomation caused by the Tityus serrulatus scorpion sting. Toxicology and Applied Pharmacology, 196, 390-395. doi:10.1016/j.taap.2003.12.026
- Diaz, P., Chowell, G., Ceja, G., D’Auria, T.C., Lloyd, R.C. and Castillo-Chavez, C. (2005) Pediatric electrocardiograph abnormalities following Centruroides limpidus tecomanus scorpion envenomation. Toxicon, 45, 27-31. doi:10.1016/j.toxicon.2004.09.008
- Vasconcelos, F., Lanchote, V.L., Bendhack, L.M., Giglio, R.J., Sampaio, S.V. and Arantes, E.C. (2005) Effects of voltage-gated Na+ channel toxins from Tityus serrulatus venom on rat arterial blood pressure and plasma catecholamines. Comparative Biochemistry and Physiology C, Comparative Pharmacology and Toxicology, 141, 85-92. doi:10.1016/j.cca.2005.05.012
- Cusinato, D.A.C., Souza, A.M., Vasconcelos, F., Guimarães, L.F.L., Leite, F.P., Gregório, Z.M.O., Giglio, J.R. and Arantes, E.C. (2010) Assessment of biochemical and hematological parameters in rats injected with Tityus serrulatus scorpion venom. Toxicon, 56, 1477-1486. doi:10.1016/j.toxicon.2010.09.003
- Bahloul, M., Chaari, A., Dammak, H., Samet, M., Chtara, K., Chelly, H., Ben Hamida, C., Kallel, H. and Bouaziz, M. (2011) Pulmonary edema following scorpion envenomation: Mechanisms, clinical manifestations, diagnosis and treatment. International Journal of Cardiology. doi:10.1016/j.ijcard.2011.10.013
- Olamendi-Portugal, T., García, B.I., López-González, I., Van Der Walt, J., Dyason, K., Ulens, C., Tytgat, J., Felix, R., Darszon, A. and Possani, L.D. (2002) Two new scorpion toxins that target voltage-gated Ca2+ and Na+ channels. Biochemical and Biophysical Research Communication, 299, 562-568. doi:10.1016/S0006-291X(02)02706-7
- de la Vega, R.C.R. and Possani, L.D. (2005) Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure-function relationships and evolution. Toxicon, 46, 831-844. doi:10.1016/j.toxicon.2005.09.006
- Catterall, W.A., Cestèle, S., Yarov-Yarovoy, V., Yu, F.H., Konoki, K. and Scheuer, T. (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon, 49, 124-141. doi:10.1016/j.toxicon.2006.09.022
- Bosmans, F. and Tytgat, J. (2007) Voltage-gated sodium channel modulation by scorpion a-toxins. Toxicon, 49, 142-158.
- Schiavon, E., Pedraza-Escalona, M., Gurrola, G.O., Olamendi-Portugal, T., Corzo, G., Wanke, E. and Possani, L.D. (2012) Negative-shift activation, current reduction and resurgent currents induced by b-toxins from Centruroides scorpions in sodium channels. Toxicon, 59, 283-293. doi:10.1016/j.toxicon.2011.12.003
- Cecchini, A.L., Vasconcelos, F., Amara, S.G., José Roberto Giglio, J.R. and Arantes, E.C. (2006) Effects of Tityus serrulatus scorpion venom and its toxin TsTX-V on neurotransmitter uptake in vitro. Toxicology and Applied Pharmacology, 217, 196-203. doi:10.1016/j.taap.2006.09.003
- Gwee, M.C., Nirthanan, S., Khoo, H.E., Gopalakrishnakone. P., Kini, R.M. and Cheah, L.S. (2002) Autonomic effects of some scorpion venoms and toxins. Clinical and Experimental Pharmacology & Physiology, 29, 795-801. doi:10.1046/j.1440-1681.2002.03726.x
- Arantes, E.C., Riccioppo Neto, F., Sampaio, S.V., Vieira, C.A. and Giglio, J.R. (1994) Isolation and characterization of TsTX-V, a new neurotoxin from Tityus serrulatus scorpion venom which delays the inactivation of Na+ channels. Biochimica et Biophysica Acta, 1199, 69-75. doi:10.1016/0304-4165(94)90098-1
- Marangoni, S., Toyama, M.H., Arantes, E.C., Giglio, J.R., Da Silva, C.A., Carneiro, E.M., Gonçalves, A.A. and Oliveira, B. (1995) Amino acid sequence of TsTx-V, an a-toxin from Tityus serrulatus scorpion venom, and its effect on K+ permeability of b-cells from isolated rat islets of Langerhans. Biochimica et Biophysica Acta, 1243, 309-314. doi:10.1016/0304-4165(94)00142-K
- Jonas, P., Vogel, W., Arantes, E.C. and Giglio, J.R. (1986) Toxin gamma of the scorpion Tityus serrulatus modifies both activation and inactivation of sodium permeability of nerve membrane. Pflügers Archiv, 407, 92-99. doi:10.1007/BF00580727
- Pinheiro, C.B., Marangoni, S., Toyama, M.H. and Polikarpov, I. (2003) Structural analysis of Tityus serrulatus Ts1 neurotoxin at atomic resolution: insights into interactions with Na+ channels. Acta Crystallographica: Section D, Biological Crystallography, 59, 405-415. doi:10.1107/S090744490202111X
- Cologna, C.T., Marcussi, S., Giglio, J.R., Soares, A.M. and Arantes, E.C. (2009) Tityus serrulatus scorpion venom and toxins: An overview. Protein Peptides Letters, 16, 920-932. doi:10.2174/092986609788923329
- Arantes, E.C., Prado, W.A., Sampaio, S.V. and Giglio, J.R. (1989) A simplified procedure for the frationalization of Tityus serrulatus venom: Isolation and partial characterization of TsTX-IV, a new neurotoxin. Toxicon, 8, 907-916. doi:10.1016/0041-0101(89)90102-5
- Zoccal, K.F., Bitencourt. C.S., Secatto, A., Sorgi, C.A., Bordon, K.C., Sampaio, S.V., Arantes, E.C. and Faccioli, L.H. (2011) Tityus serrulatus venom and toxins Ts1, Ts2 and Ts6 induce macrophage activation and production of immune mediators. Toxicon, 57, 1101-1118. doi:10.1016/j.toxicon.2011.04.017
- Akaike, H., Shin, M.C., Kubo, C. and Akaike, N. (2009) Effects of scorpion toxin on excitatory and inhibitory presynaptic terminals. Toxicology, 264, 198-204. doi:10.1016/j.tox.2009.08.010
- Soualmia, H., Eurin, J. and Djeridane, Y. (2009) Scorpion toxin of Androctonus australis garzonii induces neuropeptide Y release via bradykinin stimulation in rat atria and kidneys. Peptides, 30, 1553-1556. doi:10.1016/j.peptides.2009.04.022
- Liu, T., Bai, Z.T., Pang, X.Y., Chai, Z.F., Jiang, F. and Ji, Y.H. (2007) Degranulation of mast cells and histamine release involved in rat pain-related behaviors and edema induced by scorpion Buthus martensi Karch venom. European Journal of Pharmacology, 57, 546-556.
- Conceição, I.M., Jurkiewicz, A., Fonseca, D.R., Opperman, A.R., Freitas, T.A., Lebrun, I. and Garcez-do-Carmo, L. (2005) Selective release of ATP from sympathetic nerves of rat vas deferens by the toxin TsTX-I from Brazilian scorpion Tityus serrulatus. British Journal of Pharmacology, 144, 519-527. doi:10.1038/sj.bjp.0706062
- Teixeira, C.E., de Oliveira, J.F., Baracat, J.S., Priviero, F.B.M., Okuyama, C.E., Rodrigues Netto, N. Jr., Fregonesi, A., Antunes, E. and De Nucci, G. (2004) Nitric oxide release from human corpus cavernosum induced by a purified scorpion toxin. Urology, 63, 184-189. doi:10.1016/S0090-4295(03)00785-4
- Weisel-Eichler, A. and Libersat, F. (2004) Venom effects on monoaminergic systems. Journal of Comparative Physiology A, 190, 683-690. doi:10.1007/s00359-004-0526-3
- Fernandes, V.M., Romano-Silva, M.A., Gomes, D.A., Prado, M.A., Santos, T.M. and Gomez, M.V. (2004) Dopamine release evoked by beta scorpion toxin, tityus gamma, in prefrontal cortical slices is mediated by intracellular calcium stores. Cell Molecular Neurobiology, 24, 757-767. doi:10.1007/s10571-004-6917-8
- Teixeira Jr., A.L., Fontoura, B.F., Freire-Maia, L., Machado, C.R.S., Camargos, E.R.S. and Teixeira, M.M. (2001) Evidence for a direct action of Tityus serrulatus scorpion venom on the cardiac muscle. Toxicon, 39, 703-709. doi:10.1016/S0041-0101(00)00200-2
- Sun, H.Y., Zhou, Z.N. and Ji, Y.H. (2005) The role of voltage-gated Na+ channels in excitation-contraction coupling of rat heart determined by BmK I, an alpha-like scorpion neurotoxin. Toxicology in Vitro, 19, 183-190. doi:10.1016/j.tiv.2004.07.005
- Shinjoh, M., Nakaki, T., Otsuka, Y., Sasakawa, N. and Kato, R. (1991) Vascular smooth muscle contraction induced by Na+ channel activators, veratridine and batrachotoxin. European Journal of Pharmacology, 205, 199-202. doi:10.1016/0014-2999(91)90820-G
- Neto, M.A., Lunardi, C.N., Rodrigues, G.J. and Bendhack, L.M. (2011) Vasodilatation induced by forskolin involves cyclic GMP production. Journal of Biophysical Chemistry, 2, 373-379. doi:10.4236/jbpc.2011.24042
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3
- Scopes, R.K. (1974) Measurement of protein by spectrophotometry at 205 nm. Analytical Biochemistry, 59, 277-282. doi:10.1016/0003-2697(74)90034-7
- Ouanes-Besbes, L., El Atrous, S., Nouira, S., Aubrey, N., Carayon, A., El Ayeb, M. and Abroug, F. (2005) Direct vs. mediated effects of scorpion venom: an experimental study of the effects of a second challenge with scorpion venom. Intensive Care Medicine, 31, 441-446. doi:10.1007/s00134-005-2555-y
- Drumond Y.A., Couto, A.,S., Moraes-Santos, T., Almeida, A.P. and Freire-Maia, L. (1995) Effects of toxin Ts-gamma and tityustoxin purified from Tityus serrulatus scorpion venom on isolated rat atria. Comparative Biochemistry and Physiology. Part C, Comparative Pharmacology, Toxicology and Endocrinology, 111, 183-190.
- Shannon, T.R., Ginsburg, K.S. and Bers, D.M. (2000) Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophysical Journal, 78, 334-343. doi:10.1016/S0006-3495(00)76596-9
- Grolleau, F., Stankiewicz, M., Kielbasiewicz, E., Martin-Eauclaire, M.-F., Lavialle, C., De Vente, J. and Lapied, B. (2006) Indirect activation of neuronal noncapacitative Ca2+ entry is the final step involved in the neurotoxic effect of Tityus serrulatus scorpion β-toxin. European Journal of Neuroscience, 23, 1465-1478. doi:10.1111/j.1460-9568.2006.04667.x
- Wicher, D., Messutat, S., Lavialle, C. and Lapied, B. (2004) A new regulation of non-capacitative calcium entry in insect pacemaker neurosecretory neurons. Involvement of arachidonic acid, NO-guanylyl cyclase/cGMP, and cAMP. Journal of Biological Chemistry, 279, 50410-50419. doi:10.1074/jbc.M405800200
- Moneer, Z. and Taylor, C.W. (2002) Reciprocal regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells: Only the latter operates during receptor activation. The Biochemical Journal, 362, 13-21.
- Cox, R.H., Zhou, Z. and Tulenko, T.N. (1998) Voltagegated sodium channels in human aortic smooth muscle cells. Journal of Vascular Research, 35, 310-317.
- Platoshyn, O., Remillard, C.V., Fantozzi, I., Sison, T. and Yuan, J.X.-J. (2005) Identification of functional voltagegated Na+ channels in cultured human pulmonary artery smooth muscle cells. Pflügers Archiv European Journal of Physiology, 451, 380-387. doi:10.1007/s00424-005-1478-3
- Meguro, K., Iida, H., Takano, H., Morita, T., Sata, M., Nagai, R. and Nakajima, T. (2009) Function and role of voltage-gated sodium channel NaV1.7 expressed in aortic smooth muscle cells. American Journal of Physiology, Heart and Circulatory Physiology, 296, H211-219. doi:10.1152/ajpheart.00960.2008
- Zhu, S. and Gao B. (2006) Molecular characterization of a possible progenitor sodium channel toxin from the Old World scorpion Mesobuthus martensii. FEBS Letters, 580, 5979-5987. doi:10.1016/j.febslet.2006.09.071
NOTES
*Conflict of Interest: There are no conflicts of interest with regards to this manuscript.

