Journal of Environmental Protection
Vol.4 No.3(2013), Article ID:28648,8 pages DOI:10.4236/jep.2013.43029
Comparative Study of Lead Removal by Extracts of Spinach, Coffee, and Tea
![]()
Chemistry Department, Dillard University, New Orleans, USA.
Email: *lagwaramgbo@dillard.edu
Received January 1st, 2013; revised February 1st, 2013; accepted February 27th, 2013
Keywords: Heavy Metals; Tea; Coffee; Lead Remediation; Water Contamination; Phytoremediation
ABSTRACT
The use of tea and coffee for the removal of heavy metal from aqueous lead solutions has been reported. However, those studies were limited to expended dry biomass of coffee and tea and the lead concentration in those studies range from 10 - 100 ppm of aqueous lead solution. This study compared the effectiveness of aqueous extracts of instant coffee (IC), coffee ground (CG), coffee bean (CB), Lipton tea (Tea), and spinach puree (SP) in removing lead from 1300 PPM of aqueous lead solution. After 24 hr of agitation at room temperature followed by centrifugation, the lead concentration (in ppm) in the liquid from each reaction tube was analyzed using EPA Method 6010 (Inductively Coupled PlasmaAtomic Emission Spectrometry (ICP-AES)). The results suggest that the order of lead removal was Spinach (99%) > Instant coffee (95%) > Tea (91%), >CG (62%) > CB (59%). In comparing the brewed versus the boiled extracts, the results demonstrated that temperature of the aqueous extract affected the lead removal potential of coffee and tea in decreasing order: IC (95%:79%), >Tea (91%:88%) > CG (62%:53%) > CB (59%:53%).
1. Introduction
Lead is a toxic contaminant with serious health implications to humans, animals, and plants. Lead has strong affinity for and complexes with many biomolecules and adversely affects the cardiovascular, nervous, gastrointestinal, reproductive, immune, renal, skeletal and muscular systems. In 1998, Johnson [1] reported that lead can cause cancer, birth defects, enzyme inhibition, and aberrations in DNA synthesis and mutation. Lead exposure to children has been associated with impaired cognitive skills [2], intellectual impairment [3], reduced mental IQ and quantitative skills [4], mild mental retardation and cardiovascular disease [5]. Chronic exposure of adults to lead can lead to neurological, reproductive system, and liver damage [6]; reduced cognitive function [7], and kidney disease [8]. Sadly, lead contamination from various sources is a global problem and represents a serious threat to soil, water, and air with a potential for propagation into the food chain. Thus, our global village is in need of innovative and cheap technology for the amelioration of lead contamination.
Presently, several technologies exist for the remediation of lead contaminated soil [9-11] and they include a) excavation and bury in landfills; b) capping; c) subsurface barriers; d) in-situ and ex-situ precipitation, solidification, stabilization; e) washing and f) biological treatment such as vermiremidiation [12], phytostabilization, phytodegradation or transformation, Phytochelation, and phytoextraction [13-16].
Although, few technologies such as electrodeposition and chemical precipitation exist for the remediation of lead contaminated water [17-19], they also have their limitations such as introduction of chemicals into the water and environment. Emerging research reports have shown that some substrates have the potential to remove lead from contaminated water [20-23].
However, the levels of contamination investigated were below 500 ppm and they used solid biosorbents such as waste solid tea leaves, coffee ground, or lignin. These adsorbents have the potential to increase the volume of solid waste in the water and environment. Thus, Agwaramgbo [24,25] reported the use of liquid extracts of edible plants for the exclusive removal of lead from contaminated water. It has been reported that plants including green tea, coffee, and green vegetables (spinach), contain polyphenols (tannins, catechins), gallic acid, and over one hundred other chemical compounds [26,27]. Tea and coffee also contain caffeine in addition to these other phytochemicals. These tannins or polyphenols are associated with the precipitation and removal of heavy metal from aqueous solutions [26-28]. The roasting process of coffee has a destructive effect on its constituent polyphenols (tannins) [28]. The research results presented here compare the lead removal abilities of the liquid extracts of spinach, brewed and boiled instant coffee, coffee ground, coffee bean, and Lipton tea.
2. Materials & Methods
2.1. Preparation of Lead Nitrate Solution 1300 ppm
Using an analytical balance, 1.3 g of lead Nitrate from Fisher Scientific (L6200) was dissolved in enough deionized water (added incrementally) to give 1000 ml of solution. Then a stirring bar was dropped into the volumetric flask and the mixture was stirred until all the lead was completely dissolved. The solution continued to stir at room temperature until it was used.
2.2. Preparation of the Spinach Extract
Spinach (100 g) bought from a local market; was washed with deionized water and patted dry with kimwipes. The spinach was pureed in a regular kitchen blender using 200 ml of de-ionized water and filtered using a white cheese cloth bought from a local Wal-Mart store. The filtrate was put into four-50 ml centrifuge tubes and centrifuged at 3000 rpm for 10 minutes using a Thermo Centra CL2 bench-top centrifuge. Using disposable pipettes, three-25 ml portions of each resulting supernatant was carefully transferred into three-50 ml centrifuge tubes, respectively. The tubes were capped, labeled, and placed in a refrigerator until use.
2.3. Preparation of Brewed Coffee and Tea Extracts
Community Dark Rose Instant Coffee (IC), Coffee Ground (CG), Coffee Bean (CB); and Lipton Tea (Tea) were bought from a local market. Each beverage (45 g) was respectively brewed with 250 ml of deionized water using a coffee brewing machine. The temperature of each brewed aqueous extract was 50˚C - 60˚C. Each brewed extract was placed in an Erlenmeyer flask, covered, labeled (IC-B, CG-B, CB-B, Tea-B), and placed in a refrigerator until use.
2.4. Preparation of Heated Coffee and Tea Extracts
Respectively, 45 g of Community Dark Rose Instant Coffee (IC), Coffee Ground (CG), Coffee Bean (CB); and Lipton Tea (Tea) were placed in 500 ml beakers. Deionized water (250 ml) was added to each beaker and its content. The mixture was heated to a boil at 90˚C - 91˚C for five minutes. Each mixture was filtered, and the filtrate was centrifuged at 3000 rpm for ten minutes. The supernatant was transferred into three 50-ml centrifuge tubes. Each set of triplicate centrifuge tubes for instant coffee, coffee ground, coffee bean, and tea were labeled (IC-H, CG-H, CB-H, and Tea-H), and placed in a refrigerator until use.
2.5. Preparation of Control Lead Solution
Into three 50 ml centrifuge tubes was added 50 ml of the 1300 ppm of lead nitrate solution above to serves as control. Each tube was labeled as Pb Ctr.
3. Reaction of Spinach and Brewed Coffee and Tea Extracts
3.1. Reaction of 1300 ppm of Lead Solution with the Spinach Extract
Into each set of triplicate centrifuge tubes containing 25 ml of the spinach extract was added 25 ml of the 1300 ppm of the lead nitrate solution prepared above. The three tubes and their contents along with triplicate tubes containing the 1300 ppm lead solution were vortexed, tightly secured on the rack of a heavy duty Eberbach 6000 shaker, and agitated for 48 hours at room temperature.
3.2. Reaction of 1300 ppm of Lead Solution with the Brewed Coffee Extracts (IC-B, CG-B, and CB-B)
Following the same procedure used in the reaction of spinach extract with lead solution above, 25 ml of the lead nitrate solution (1300 ppm) prepared above was added to each of the three sets of triplicate centrifuge tubes containing 25 ml of the brewed instant coffee, coffee ground, and coffee bean extracts, respectively. The nine tubes and their contents were vortexed and agitated for 48 hours at room temperature on a heavy duty Eberbach 6000 shaker.
3.3. Reaction of 1300 ppm of Lead Solution with the Brewed Lipton Tea Extract (Tea-B)
Following the same procedure used in the reaction of spinach extract with lead solution above, 25 ml of the lead nitrate solution (1300 ppm) prepared above was added to each of the three triplicate centrifuge tubes containing 25 ml of the brewed Lipton tea. The three tubes and their contents were vortexed, and agitated for 48 hours at room temperature on a heavy duty Eberbach 6000 shaker.
3.4. Reaction of 1300 ppm of Lead Solution with the Boiled Coffee Extracts (IC-H, CG-H, and CB-H)
Following the same procedure used in the reaction of brewed spinach extract with lead solution above, 25 ml of the lead nitrate solution (1300 ppm) prepared above was added to each of the three sets of the triplicate centrifuge tubes containing 25 ml of the boiled instant coffee (IC-H), coffee ground (CG-H), and coffee bean (CB-H)], respectively. The nine tubes and their contents were vortexed, and agitated for 48 hours at room temperature on a heavy duty Eberbach 6000 shaker.
3.5. Reaction of 1300 ppm of Lead Solution with the Boiled Lipton Tea Extract (Tea-H)
Following the same procedure used in the reaction of spinach extract with lead solution above, 25 ml of the lead nitrate solution (1300 ppm) prepared above was added to each of the three centrifuge tubes containing 25 ml of the boiled Lipton tea extract (Tea-H). The three tubes and their contents were vortexed, tightly secured on the rack of a heavy duty Eberbach 6000 shaker, and agitated for 48 hours at room temperature on a heavy duty Eberbach 6000 shaker.
3.6. Agitation of the Control Lead Solution
The three tubes containing the 1300 ppm lead solution prepared above were vortexed and agitated for 48 hours at room temperature on a heavy duty Eberbach 6000 shaker.
4. Sample Preparation and Analysis
4.1. Sample Preparation
After 48 hrs, the shaker was stopped and the tubes and their contents were centrifuged at 3000 rpm for ten minutes. The resulting clear supernatant in each tube was transferred into another labeled clean centrifuge tube. All the labeled centrifuge tubes with their liquid contents were sent to PACE Analytical Services, Inc for lead analysis.
4.2. Sample Analysis for Lead after Reaction
After the reaction period, the lead concentration (in ppm) in the liquid from each reaction tube was analyzed using EPA Method 6010 (Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES)).
4.3. Statistical Analysis
Use of descriptive statistics to interpret the data will be useful to determine if the extract isolation temperature is associated with the lead removing capacity of the extracts. Thus, Pearson correlation coefficient (r), percent variance (CV%) will be used to show if an association exists between the temperature of extract isolation and the lead removing capacity of the extract.
5. Results
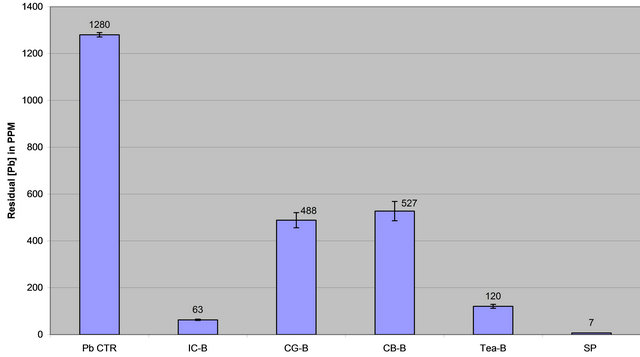
Data on Table 1 show residual lead in ppm in each reaction tube after contaminated water was treated with each extract for 48 hrs and the percent of lead removed relative to the control: Control (1280, 0%); IC-B (63, 95%, CG-B (488, 62%), CB-B (527, 59%), Tea-B (120, 91%, Spinach (7, 99%). Figures 1(a) and (b) show the amount of lead remaining in the reaction mixture after coffee, tea, and spinach extract treatment and the percent of lead removed, respectively.
6. Discussions
6.1. On Spinach Extract
The results on Table 1 and Figures 1(a) and (b) showed that spinach removed over 99% of the lead from contaminated water and served as a reference for the brewed and boiled coffee and tea extracts.
6.2. Comparison of Lead Removal by Brewed Coffee & Tea Extracts
Table 1 and Figures 1(a) and (b) clearly showed that spinach, instant coffee, and tea have unusually great ability to remove lead from contaminated water. However, ground coffee and coffee bean had a modest ability to remove lead from lead contaminated aqueous solution. The order of lead removal ability is: Spinach (99% > Instant Coffee (95%) > Tea (91%) > Coffee Ground (62%) > Coffee Bean (59%).
It is not surprising that instant coffee removed far more lead than ground coffee and coffee bean due to the fact that all the amount of instant coffee used solubilized in solution during brewing. Thus, instant coffee has more available lead-chelating or adsorbing agents in contact with the lead. However, in the case of ground coffee and coffee bean, only a fraction of the chelating chemical constituents of the solid biomass used solubilized in solution during brewing. Therefore, they had less amount
Table 1. Residual & removed lead in contaminated water after treatment with spinach and brewed coffee/tea extracts.

 (a)
(a)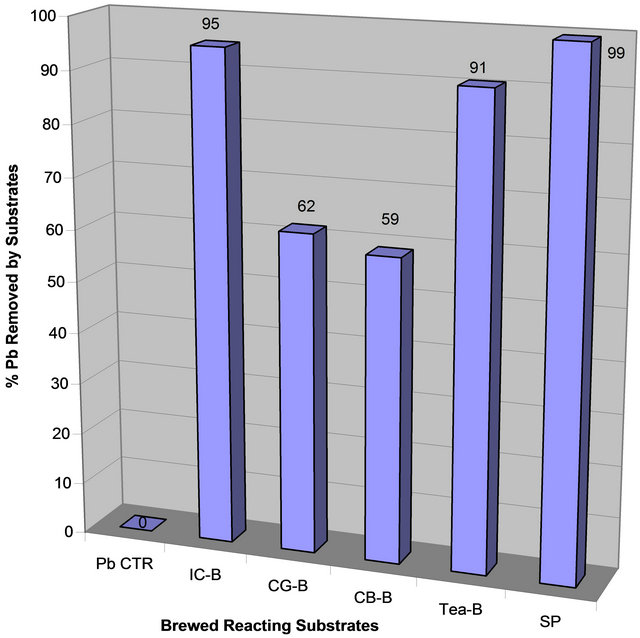 (b)
(b)
Figure 1. (a) Lead concentration remaining after contaminated water was treated with extracts of spinach and brewed coffee and tea; (b) Percent lead removed after contaminated water was treated with extracts of spinach and brewed coffee and tea.
of the lead chelating substances brewed extracts. It is worthy to note that even though equal amount of dry solid biomass of tea, ground coffee, and coffee bean was used, tea removed 40% - 50% more lead. It is possible to conclude that tea has more lead chelating agents per unit dry weight than coffee ground and coffee bean.
Figure 2 above showed that there is a strong association between plant type and their lead removal potential (R2 of 0.7721 for brewed extracts and 0.8787 for boiled extracts).
6.3. Comparison of Lead Removal by Boiled Coffee and Tea Extracts
The data on Table 2 and Figures 3(a) and (b) suggest that Tea extract removed more lead than instant coffee, while ground coffee and coffee beans removed equal amount of lead Thus, the order of lead removal is Tea (88%) > Instant Coffee (79%), Coffee Ground (53%) ≈ Coffee Bean (53%).
It is possible that instant coffee contains some volatile or semi-volatile lead chelating components that evaporated during heating.
6.4. Effect of Temperature on Lead Removal by Coffee and Tea: A Comparison of Brewed Versus Boiled Coffee & Tea Extracts
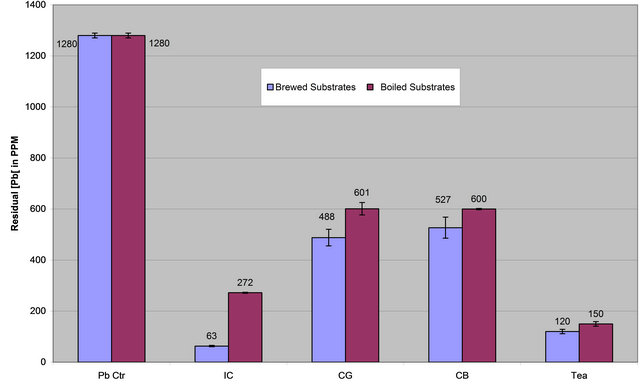
From Table 3 and Figures 4(a) and (b), it is apparent that lead removal by each extract is clearly affected by an

Figure 2. Line graph of data on Table 4: Comparison of lead removal of coffee and tea extracts isolated at 60˚C (brewed) & 90˚C (heated or boiled) R2 (brewed) = 0.7705, & R2 (heated) = 0.8787.
 (a)
(a)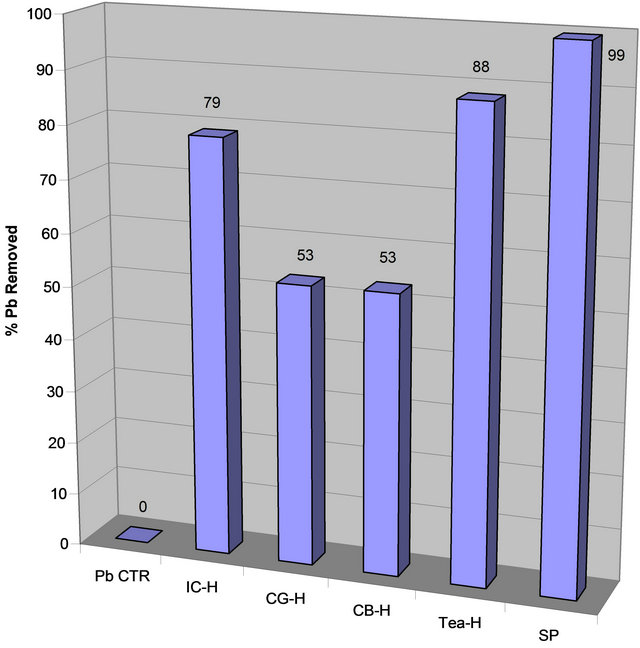 (b)
(b)
Figure 3. (a) Lead concentration remaining after contaminated water was treated with extracts of spinach and boiled coffee and tea; (b) Percent lead removed after contaminated water was treated with extracts of spinach and boiled coffee and tea.
 (a)
(a)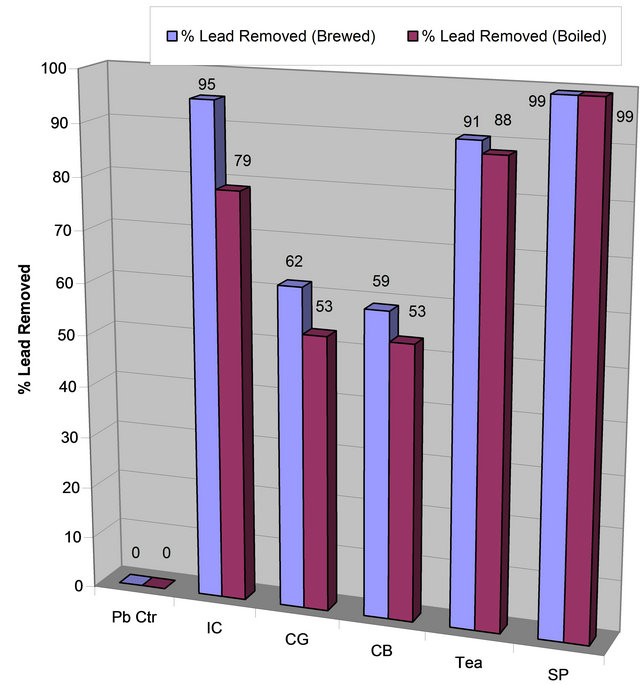 (b)
(b)
Figure 4. (a) Lead concentration remaining after contaminated water was treated with extracts of brewed & boiled coffee and tea (IC-B, IC-H; CG-B, CG-H; CB-B, CB-H; Tea-B, Tea-H); (b) Percent lead removed after contaminated water was treated with brewed and boiled coffee and tea extracts (IC-B, IC-H; CG-B, CG-H; CB-B, CB-H; Tea-B, Tea-H).
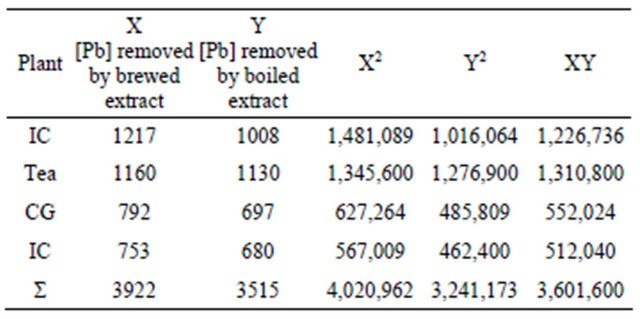
increase in temperature of extraction. The data in Table 3 suggest that heating the extracts from a normal brewing temperature of 60˚C to a boiling temperature of 90˚C effectively decreases the lead removal potential of all extracts reported in this paper.. Such a decrease was very steep in the case of instant coffee. The brewed extract from instant coffee removed 95% of the lead from the contaminated aqueous solution while the corresponding boiled extract (IC-H), removed 79% of the lead. Coffee Ground and Coffee Bean had a small but noticeable reduction lead removal as the temperature increased from 60˚C to 90˚C, a reduction of 9% and 6%, respectively (CG-B, 62% vs CG-H, 53%; CB-B, 59% vs CB-H, 53%). The data in Table 4 has a Pearson correlation coefficient of 0.949 which suggests that there is a strong association between increase in temperature of extract isolation and decreased capacity of lead removal by the extract. Also, the data may demonstrate that tea contains more lead-
Table 2. Residual & removed lead in contaminated water after treatment with extracts of spinach and boiled coffee and tea.

Table 3. Residual & removed lead in contaminated water after treatment with brewed and boiled coffee and tea extracts.
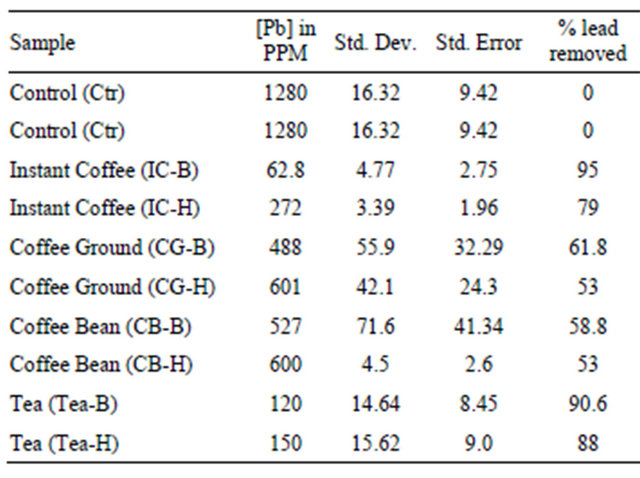
Table 4. Comparison of lead removed from contaminated water by brewed and boiled coffee and tea extracts the Pearson correlation coefficient R = 0.949, R2 = 0.9006, & CV = 90.06%.
Equation 1: Pearson Correlation Coefficient Equation

Substituting the values obtained from the Table 4 above into the equation, gave Pearson’s correlation coefficient of 0.949, with R2 of 0.9001 and percent variance of 90.01%.
precipitating phytochemicals such as tannins and polyphenols than coffee. Furthermore, it has been reported that the roasting process of coffee has a destructive effect on its chemical constituents due to volatilization, decomposition, and denaturation as the temperature is increased [27,28]. Thus, the order of lead removal by extracts isolated at 60˚C is Instant Coffee (95%) > Tea (91%) > Coffee Ground (62%) > Coffee Bean (59%) compared to the lead removal by extracts isolated at 90˚C; Tea (88%) > Instant Coffee (79%) > Coffee Ground (53%) ≈ Coffee Bean (53%). It is worthy to note that instant coffee has most of its phytochemical contents extracted into the solution whereas not all the chemicals are extracted into the solution in the case of tea, coffee ground, and coffee bean.
7. Conclusions
Extracts of spinach, instant coffee and tea effectively removed over 90% of the lead in contaminated aqueous solutions. Among the brewed extracts investigated, instant coffee had the highest lead-removal ability followed by tea. However, upon increasing the extract isolation temperature from 60˚C to 90˚C, each ex tract showed a reduction in lead-removing potential, particularly instant coffee. This finding is in agreement with the Pearson correlation coefficient of 0.949, suggesting that increasing the temperature reduces the lead removing potential of the extract. These results demonstrate that instant coffee and tea extracts could provide a convenient technique for lead removal from contaminated waters. Furthermore, the results suggest 1) that the amount of biologically active components of these extracts that contribute to lead removal varies from one extract to another; 2) that elevated temperatures above 60˚C decrease the extracts’ lead-removing potential. Such reduction may likely be in part, due to volatility, decomposition or denaturing of some of those chemical and biological functional groups in the extracts that play important role in lead complexation, precipitation, and adsorption [27,28].
8. Acknowledgements
The authors thank Dr. Phyllis Dawkins, Dillard University’s CTLAT, Andrew Mellon Foundation, People’s Environmental Center, and Dillard University School of Science, Technology, Engineering, and Mathematics.
REFERENCES
- F. M. Johnson, “The Genetic Effect of Environmental lead,” Mutation Research, Vol. 410, No. 2, 1998, pp. 123- 140. doi:10.1016/S1383-5742(97)00032-X
- S. Tong, “Lead Exposure and Cognitive Development: Persistence and a Dynamic Pattern,” Journal of Pediatrics and Child Health, Vol. 34, No. 2, 1998, pp. 114-118. doi:10.1046/J.1440-1754.1998.00187.x
- R. L. Canfield, C. R. Henderson Jr. , D. A. Cory-Slechta, C. Cox, T. A. Jusko and B. P. Lanphear, “Intellectual Impairment in Children with Blood Lead Concentrations Below 10 mg per Deciliter,” The New England Journal of Medicine, Vol. 348, 2003, pp. 1517-1526. doi:10.1056/NEJMoa022848
- M. L. Miranda, K. Dohyeong, M. A. Galeano, C. J. Paul, A. P. Hull and S. P. Morgan, “The Relationship between Early Childhood Blood Lead Levels and Performance on End-Of-Grade Tests,” Environmental Health Perspectives, Vol. 115, No. 8, 2007, pp. 1242-1247. doi:10.1289/ehp.9994
- L. J. Fewtrell, A. Pruss-Ustun, P. Landrigan and J. L. Ayuso-Mateos, “Estimating the Global Burden of Disease of Mild Mental Retardation and Cardiovascular Diseases from Environmental Lead Exposure,” Environmental Research, Vol. 94, No. 2, 2004, pp. 120-133. doi:10.1016/S0013-9351(03)00132-4
- H. Needleman, C. McFarland, R. Ness, S. Fienberg and M. Tobin, “Bone Lead Levels in Adjudicated Delinquents. A Case Control Study,” Neurotoxicology and Teratology, Vol. 24, No. 6, 2003, pp. 711-717. doi:10.1016/50892-0362(02)00269
- R. A. Shih, H. Hu, M. G. Weisskopf and B. S. Schwartz, “Cumulative Lead Dose and Cognitive Function in Adults. A Review of Studies that Measured Both Blood Lead and Bone Lead,” Environmental Health Perspectives, Vol. 115, No. 3, 2007, pp. 483-492. doi:0.1289/ehp.9786
- J. L. Lin, D. T. Lin-Tan, K. H. Hsu and C. C. Yu, “Environmental Lead Exposure and Progression of Chronic Renal Diseases in Patients without Diabetes,” New England Journal of Medicine, Vol. 348, 2003, pp. 277-286.
- M. I. Lone, Z.-L. He, P. J. Stopffella and X.-E. Yang, “Phytoremediationof Heavymetal Polluted Soils and Water: Progresses and Perspectives,” Journal of Zhejiang University Science B, Vol. 9, No. 3, 2008, pp. 210-220. doi:10.1631/jzus.B0710633
- R. L. Chaney, M. Malik, Y. M. Li, S. L. Brown, E. P. Brewer, A. J. Scott and A. J. M. Baker, “Phytoremediation of Soil Metals,” Current Opinion in Biotechnology, Vol. 8, No. 3, 1997, pp. 279-284. doi:10.1016/S0958-1669(97)80004-3
- D. E. Salt, M. Blaylock, P. B. A. N. kumar, V. Dushenkov, B. D. Ensley, L. Chet and L. Raskin, “Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants,” Biotechnology, Vol. 13, No. 5, 1995, pp. 468-474. doi:10.1038/nbt0595-468
- R. K. Sinha, S. Agarwal, K. Chauhan, V. Chandran and B. K. Soni, “Vermiculture Technology: Reviving the Dreams of Sir Charles Darwin for Scientific Use of Earthworms in Sustainable Development Programs,” Technology and Investment, Vol. 1, No. 3, 2010, pp. 155-172. doi:10.4236/ti.2010.13019
- P. B. A. N. Kumar, V. Dushenkov, H. Motto and I. Raskin, “Phytoextraction: The Use of Plants to Remove Heavy Metal from Soils,” Environmental Science & Technology, Vol. 29, No. 5, 1995, pp. 1232-1238. doi:10.1021/es00005a014
- M. M. Lasat, “Phytoextraction of Toxic Metals: A Review of Biological Mechanisms,” Journal of Environmental Quality, Vol. 31, No. 1, 2002, pp. 109-120.
- M. Sihl, M. A. Hajabbasi and H. Shareatmadari, “Heavy Metal Extraction Potential of Sunflower (Helianthus annuus) and Canola Brassica Napus,” Caspian Journal of Environmental Sciences, Vol. 3, No. 1, 2005, pp. 35-42.
- R. D. Reeves, “Tropical Hyper-Accumulators of Metals and Their Potential for Phytoextraction,” Plant Soil, Vol. 249, No. 1, 2003, pp. 57-65. doi:10.1023/A:1022572517197
- R. D. Armstrong, M. Todd, J. W. Atkinson and K. Scott, “Selective Electrodeposition of Metals from Simulated Waste Solutions,” Journal of Applied Electrochemistry, Vol. 26, No. 4, 1996, pp. 379-384. doi:0.1007/BF00251322
- T. R. Harper and N. W. Kinham, “Removal of Arsenic from Wastewater Using Chemical Precipitation Methods,” Water Environment Research, Vol. 64, No. 3, 1992, pp. 200-203. doi:10.2175/WER.64.3.2
- T. K. Radicic and S. Raicevic, “In Situ Lead Stabilization Using Natural and Synthetic Apatite,” Chemical Industry & Chemical Engineering Quarterly, Vol. 14, No. 4, 2008, pp. 269-271. doi:10.2298/CICEQ0804269K
- T. W. Tee and Abd R. M. Khan, “Removal of Lead, Cadmium, and Zinc by Waste Tea Leaves,” Environmental Technology Letters, Vol. 9, No. 11, 1988, pp. 123- 1232. doi:10.1080/09593338809384685
- S. S. Ahluwalia and D. Goyal, “Removal of Heavy Metal by Waste Tea Leaves from Aqueous Solutions,” Engineering in Life Sciences, Vol. 5, No. 2, 2005, pp. 158-162. doi:0.1002/elsc.200420066
- T. Tokimoto, N. Kawasaki, T. Nakamura, J. Akutagawa and S. Tanada, “Removal of Lead Ions in Drinking Water by Coffee Grounds as Vegetable Biomas,” Journal of Colloid and interface Science, Vol. 281, No. 1, 2005, pp. 56-61. doi:10.1016/j.jcis.2004.08.083
- B. MW. P. K. Amarasinghe and R. A. Williams, “Tea Waste as a Low Cost Adsorbent for the Removal of Cu and Pb from Wastewater,” Chemical Engineering Journal, Vol. 132, No. 1-3, 2007, pp. 299-309. doi:10.1016/j.cej.2007.01.016
- L. Agwaramgbo, E. Agwaramgbo, C. Mercadel, S. Edwards and E. Buckles, “lead Remediation of Contaminated Water by Charcoal, LA Red Clay, Spinach, and Ustard Green,” Journal of Environmental Protection, Vol. 2, No. 9, 2011, pp. 1240-1244. doi:10.4236/jep.2011.29142
- L. Agwaramgbo, C. Thomas, C. Grays, J. Small and T. Young, “An Evaluation of Edible Plant Extracts for the Phytoremediarion of Lead Contaminated Water,” Journal of Environmental Protection, Vol. 3, No. 8, 2012, pp. 722-730. doi:10.4236/jep.2012.38086
- M. McDonald, I. Mila and A. Scalbert, “Precipitation of Metal Ions by Plant Polyphenols: Optimal Conditions and Origin of Precipitation,” Journal of Agricultural and Food Chemistry, Vol. 44, No. 2, 1996, pp. 599-606. doi:10.1021/jf950459q
- V. S. P. Chaturvedula and I. Prakash, “The Aroma, Taste, Color and Bioactive Constituents of Tea,” Journal of Medicinal Plants Research, Vol. 5, No. 11, 2011, pp. 2110- 2124.
- J. W. England, “Coffee and Tea as Precipitants for Poisons,” Sanitarian, Vol. 49, No. 392, 1902, pp. 524-526.
NOTES
*Corresponding author.

