Energy and Power Engineering
Vol.06 No.12(2014), Article ID:50897,6 pages
10.4236/epe.2014.612038
Dynamic Simulation of Solid Adsorption Solar Refrigerator System with AC/CH3OH as a Working Pair
Anan Pongtornkulpanich
Thermal Energy Research Unit, School of Renewable Energy Technology (SERT), Naresuan University, Phitsanulok, Thailand
Email: ananpo@nu.ac.th
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 August 2014; revised 2 September 2014; accepted 16 September 2014
ABSTRACT
Solid adsorption system, one of alternative refrigeration systems, is utilized to provide cold for refrigerator or air-conditioner and can be operated by assistance of solar heat. System performance study through computer usage to develop simulation program and simulate behaviors of system operation can give designed system which suits for user’s need. Also, the present study aims to develop dynamic simulation program of solid adsorption refrigeration system operated by solar assistance to simulate behaviors of system operation and its performance. Flat plate collectror is utilized to provide thermal energy for system’s adsorber and activated carbon/methanol is used to be a suitable working pair. Simulation procedure starts with various solar radiation intensities as input energy on solar collector and water is used as collector working fluid. Behavior of system operation can be considered to be 4 steps as isosteric heating, isobaric desorption, isosteric cooling and isobaric adsorption, respectively. This research studies the effect of varying solar radiation intensity on temperature, pressure of adsorber, adsorption ratio at each steps of system operated ranging from 6:00 am (the first day) to 6:00 am (the next day) and system performance which is defined as coefficient of performance, COP. In addition, the simulation result shows monthly average COP of 0.43 compared to a result of another previous research work under the same operating condition and the percentage error is 7.5%.
Keywords:
Lumped Parameter Dynamic Simulation Program, Solid Adsorption Refrigeration System, Solar Energy, Working Pair

1. Introduction
Energy provision for refrigeration system and air-conditioner plays a major role in world cooling market. Referring International Institute of Refrigeration, it is estimated that 15% of electricity produced in a whole world is utilized for refrigerating and air conditioning systems. Currently, world demand of air conditioner trends to be continuously increased with estimated rate of 17%. Almost air conditioners has been used as type of vapor compression system which provides several disadvantages of consuming huge amount primary energy and CFC refrigerant caused for ozone depletion. The development in refrigeration system assisted by alternative energy such as solar heat and waste heat has been happened. Especially, hot climate region such as Thailand, there have been utilization of solar driven refrigeration systems for industrial, transportation and residential sectors. Examples of refrigeration technologies, which can be assisted by solar heat, are absorption, adsorption, evaporative ejector etc. From comparative performance study of thermal refrigeration technologies, absorption type yields the highest system performance ranging from 0.7 - 0.8 while system has high complex and can generate crystallization problem of salt solution or working pair at high generator temperature and concentration. While the ejector type seems more interesting for using single refrigerant, the system has a difficulty of ejector design to obtain high ejector and system performance. Adsorption refrigeration system is one of attractive system because the system provides a little amount of components and non-complex configuration. Additionally, Activated carbon (AC)/CH3OH is commonly used as working pair because of its cheap cost and availability. There are no moving parts resulting in lower maintenance cost. Refrigerating efficiency can be defined as coefficient of performance, COP which varies as performance characteristics of the system. In the present study, dynamic simulation program has been developed to study the effect of various parameters on COP and utilized to simulate behavior of system operation, which makes understanding in operating system and obtains suitable results for design system having efficient system performance.
2. Dynamic Simulation Program for Solar Adsorption Refrigeration System
2.1. Lumped Parameter Dynamic Modeling for Adsorber
Mathematical model of lumped parameter has been developed to simulate refrigeration system performance of several types of adsorption cycle such as single and double bed which consists of conservation Equations and correlation equation between temperature and pressure of adsorption process or isosteric equation. The model is used to simulate behavior of system in each step of operating time which obtained results resembles with experimental result and can simulate system performance as well. For lumped parameter model, the change in various parameters only depends on time. Mathematical expression in the model is appeared in form of ordinary differential equation [1] .
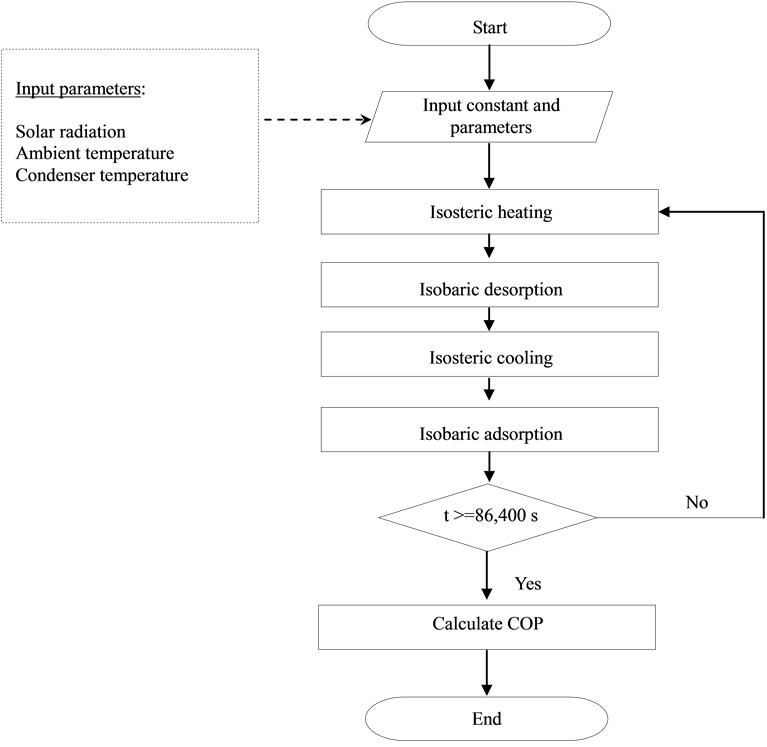
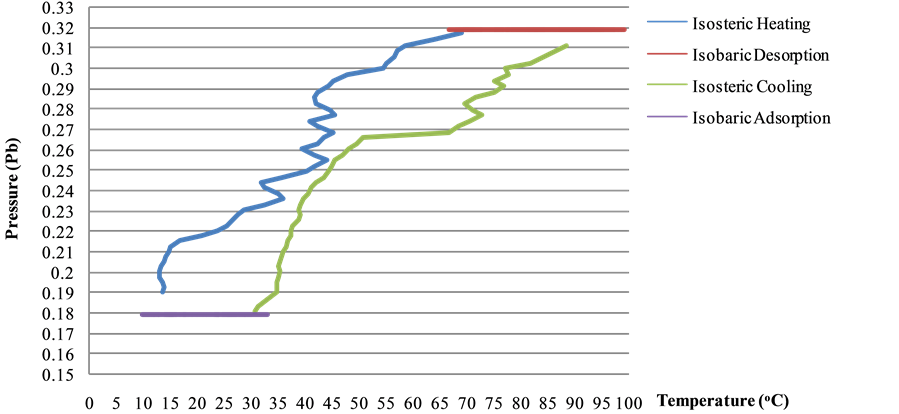
Based on fundamental concept of adsorption refrigeration system [2] [3] , the following system operation can be considered to be four steps as shown in Figure 1.
2.1.1. Heating and Desorption Process
As shown in Figure 1, when solar energy incidents on adsorber of refrigeration system, temperature and pressure
Figure 1. P-T-X equilibrium chart of adsorption refrigeration system.
within adsorber increase. Pressure increases until Equaling to condenser pressure and valve, which is connected between adsorber and condenser, is opened. Water vapor in adsorber flows into condenser for condensing. Desorption process releases energy to remove interaction force between methanol molecule acting on porous wall of activated carbon. This energy depends on desorption rate from activated carbon.
Step 1 - 2 shows heat transferring to working pair within adsorber from heat source with temperature Th which pressure and temperature at evaporator condition (Pe, T1) increase to be pressure and temperature at condenser (Pc, T2). Adsorbed substance has constant concentration (x1) and no phase change. Heat applying in this step is called isosteric heating. Law of conservation energy for interval of 1 - 2 (isosteric heating) can be as Equation (1)
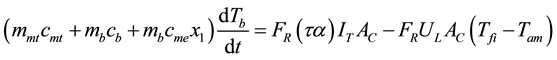 (1)
(1)
For step 2 - 3, heat still applies adsorb layer until temperature increases continuously from T2 to T3 with maintaining at constant condenser pressure. Adsorbed substance is evaporated from surface of adsorb substance. This process is called desorption process. Concentration of adsorbed substance is decreased from x1 to x2. Heat involving in this process is called isobaric heating which adsorbed substance vapor flows to condenser and condenses to be liquid flowing in bottle. Law of conservation energy for interval 2 - 3 (Isobaric desorption) is as Equation (2)
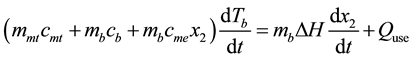 (2)
(2)
2.1.2. Cooling and Adsorption Process
After releasing heat at adsorber, temperature and pressure within adsorber is decreased. When pressure decreases to be evaporator pressure, valve, which is connected between adsorber and evaporator, is opened. Methanol vapor in evaporator flows to adsorber and is adsorbed into porous of activated carbon. Adsorber pressure is lower than evaporator pressure. This results in methanol vapor flowing continuously to adsorber.
Step 3 - 4 shows releasing heat from adsorber to decrease temperature of layer of adsorb substance and pressure of system from (Pc, T3) to (Pe, Te) with constant concentration of adsorbed substance, x2. Law of conservation energy for the interval of isosteric cooling (3 - 4) is as Equation (3)
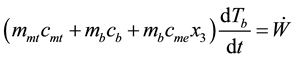 (3)
(3)
For step 4 - 1, valve at evaporator is opened and adsorbed substance flows from bottle to adsorber to exchange heat with air inside adsorber until evaporization occurance. The vapor flows in adsorber and is adsorbed at surface of adsorbent. This process is called adsorption process. In this process, heat is released continuously from adsorbent layer until temperature decreases from T4 to T1 at constant evapoarator pressure, Pe. This step is called is isobaric cooling which concentration of adsorbed substance is increased from x2 to x1. When system approaches to adsorption Equilibrium or system has the same pressure, this process is terminated. Law of conservation energy for interval of isobaric adsorption (3 - 4) can be as Equation (4)
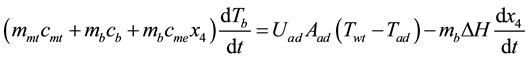 (4)
(4)
2.2. Thermodynamic and Kinetic of Adsorption Cycle
Adsorption process involves with three main parameters: pressure (kPa), temperature (K) and concentration of adsorbent (x, kgrefrigerant/kgadsorbent). When experiment data is plotted between log or lnP and −1/T at constant W, P-T-X Equilibrium chart is obtained to be used for design the system, as shown in Figure 1 [4] [5] .
2.3. Condenser Model
Adsorbed substance, which is separated from adsorbent, is condensed to be liquid at condenser. Heat is removed from adsorbed substance using cooling water flowing through adsorber. Mathematical expression can be given as
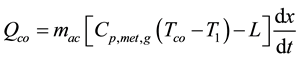 (5)
(5)
2.4. Evaporator Model
At evaporator, adsorbed substance is evaporated, and the vapor is adsorbed with adsorbent at adsorber. Mathematical Equation can be as Equation (6)
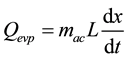 (6)
(6)
2.5. Refrigeration System Performance
This is expressed to be coefficient of performance which can be defined as the ratio of refrigerating effect to energy input to the system as Equation (7)
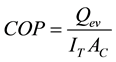 (7)
(7)
3. Simulation Program
Lumped parameter dynamic simulation program of solar adsorption refrigeration system with AC/CH3OH as a working fluid to simulate behavior of system in each step of operating time and system performance has been developed. Flow chart of the simulation is appeared as Figure 2. The program starts from inputing solar radiation, ambient temperature and condenser temperature to determine pressure and temperature of adsorber column for isosteric heating process, isobaric desorption, isosteric cooling and isobaric adsorption, respectively. Dynamic calculation will be continued until time is Equal and more than 86,400 s. After that, refrigeration system efficiency or COP is determined [6] -[11] .
4. Simulation Results
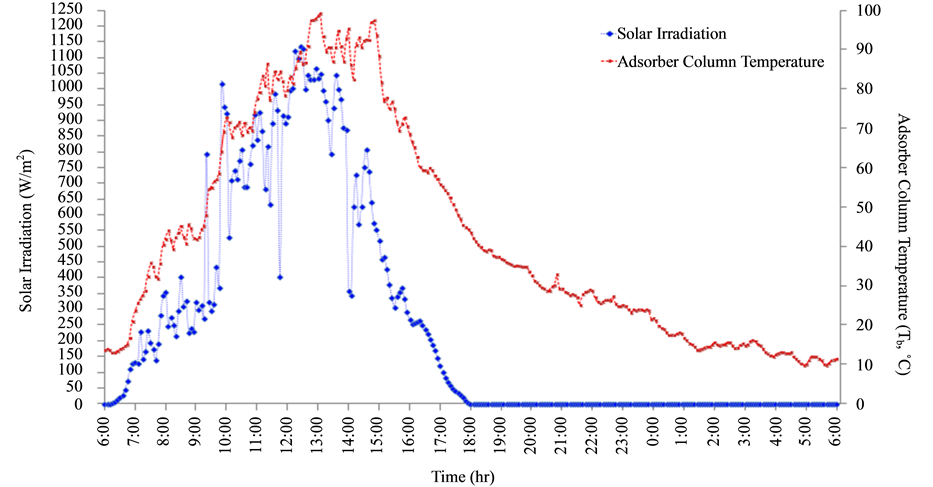
As shown in Figure 3, adsorber column temperature and solar radiation intensity are plotted with operating time. Simulation result shows that system operation of a typical day can be considered in 4 steps of simulation. In the first step, isosteric heating, heat from flat plate collector is supplied and collected at adsorber column. As solar radiation intensity increases, temperature of adsorber column increases.
The increase of temperature approaches to boiling point temperature of the pair, 65˚C at 09:55 am. Methanol starts to evaporate and continue to step 2. At this step, isobaric desorption, methanol vapor leaves from adsorber to be condensed at condenser. After that, refrigerant flows to evaporator to be evaporated and yields refrigerating effect there. At evening, solar radiation intensity is depleted until temperature of adsorber column is lower than 65˚C about 16:10 pm which approaches to isosteric cooling. That is temperature and pressure of adsorber column is decreased continuously by circulating cooling water at adsorber while the amount of refrigerant is constant. When there has not been heat from solar radiation applying the system, temperature of adsorber decreases about 30˚C at 20:10 pm. Adsorbent within adsorber adsorbs methanol vapor from evaporator again. This process is isobaric adsorption. The amount of refrigerant, which is expressed in term of adsorption ratio (xb), slowly increases at the next morning day. The cycle is continued by starting at isosteric heating when the system receieves solar radiation. Figure 4 shows P-T-X Equilibrium chart which is obtained from simulation program as compared to theoretical P-T-X chart.
5. Model Validation [12] - [15]
In order to check the accuracy of the developed simulation program, comparison of simulated result and previous research result (Kiratti 2009) has been performed. Table 1 shows the comparison of COP obtained from simulation program and that of previous research and percentage error. The result shows that simulated COP is almost the same previous research value with low percentage error of 7.5. This means that the result obtained
Figure 2. Flow chart of the simulation.
Figure 3. Relationship between solar radiation intensity (It), adsorber column temperature (Tb) and time on a typical day.
Figure 4. P-T-X equilibrium chart obtained from simulation program of solar adsorption refrigeration system.
Table 1. Comparison between simulated COP and previous research result and percentage error under the same operating condition.
*Source: Kiratti (2009).
with presented model is in acceptable agreement with the previous one.
Dynamic simulation program of adsorption cooling system consists of many system components modeling such as flat plate collector, adsorber, condenser and evaporator. Lumped parameter concept has been used to write this dynamic simulation program to study operation behavior of solar adsorption cooling system and to study variation of solar radiation intensity affecting temperature pressure and adsorption ratio to compare to theoretically operational behavior. Additionally, from performance comparative study, it is found that the system performance, COP, which is obtained from program, is almost similar to COP value from previous research work with the percentage error of 7.5%, as shown in Table 1.
6. Conclusion
Lumped parameter dynamic simulation program for solar adsorption refrigeration system using AC/CH3OH as working pair has been developed to simulate behavior of system operation at any time. Effect of various parameters, solar radiation, adsorber column temperature, adsorption ratio and pressure, on COP is studied. Dynamic model of the system is validated. The result shows that simulated COP value equals to 0.43 and is almost the same previous research value with percentage error of 7.5. It can be concluded that the dynamic simulation program can be used to simulate various parameters of the system with good validity.
References
- Tungad, K. (2009) Molding and Operation of Composite Adsorbent in Adsorption Cooling System. Master Thesis. http://tdc.thailis.or.th/tdc/basic.php
- Saengtienchai, K. (2009) Analysis of 1-D adsorption Cooling System. Master Thesis. http://tdc.thailis.or.th/tdc/basic.php
- Kumluang, T. (2007) Dynamic Modelling of Solar Adsorption Cooling System by Using Zeolite/Water as Working Pair. Master Thesis. http://tdc.thailis.or.th/tdc/basic.php
- Kiatsiriroej, T. (2006) Case Study on Unsteady Adsorption Cooling System. http://tdc.thailis.or.th/tdc/basic.php
- Peerapong Tumutok (2005) Development of Heating and Cooling Adsorption System with Solar-Assisted by Using AC-Methanol as Working Pair. http://tdc.thailis.or.th/tdc/basic.php
- Solar Energy Technology (2010) Division of Solar Energy. www.dede.go.th/dede/.../usr/.../solarenergytechnologypaper.pdf
- Pongtornkulpanich, A. and Sukchai, S. (2009) Technology Development and Research on Solar Cooling Systems. 16th REGWA Conference “Use of Renewable Energy Sources and Hydrogen Technology”, 5-7 November 2009, FH-Stral- sund-University of Applied Sciences, Germany.
- Dieng, A.O. and Wang. R.Z. (2001) Literature Review on Solar Adsorption Technologies for Ice-Making and Airconditioning Purposes and Recent Developments in Solar Technology. Renewable and Sustainable Energy Reviews, 5, 313-342. http://dx.doi.org/10.1016/S1364-0321(01)00004-1
- Pongtornkulpanich, A., Thepa, S., Amornkitbamrung, M. and Butcher, C. (2008) Experience with Fully Operational Solar-Driven 10-Ton LiBr/H2O Single-Effect Absorption Cooling System in Thailand. Renewable Energy, 33, 943- 949. http://dx.doi.org/10.1016/j.renene.2007.09.022
- Anyanwu, E.E. (2003) Review of Solid Adsorption Solar Refrigerator I: An Overview of the Refrigeration Cycle. Energy Conversion and Management, 44, 301-312. http://dx.doi.org/10.1016/S0196-8904(02)00038-9
- Anyanwu, E.E. and Zekwe, C.I. (2003) Design, Construction and Test Run of a Solid Adsorption Solar Refrigerator Using Activated Carbon/Methanol, as Adsorbent/Adsorbate Pair. Energy Conversion and Management, 44, 2879-2892. http://dx.doi.org/10.1016/S0196-8904(03)00072-4
- Anyanwu, E.E., Oteh, U.U. and Ogueke, N.V. (2001) Simulation of a Solid Adsorption Solar Refrigerator Using Activated Carbon/Methanol Adsorbent/Refrigerant Pair. Energy Conversion and Management, 42, 899-915. http://dx.doi.org/10.1016/S0196-8904(00)00091-1
- Sumathy, K. and Li, Z.F. (1999) Experiments with Solar-Powered Adsorption Ice-Maker. Renewable Energy, 16, 704- 707. http://dx.doi.org/10.1016/S0960-1481(98)00256-0
- Wang, L.W., Wang, R.Z. and Oliveira, R.G. (2009) A Review on Adsorption Working Pairs for Refrigeration. Renewable and Sustainable Energy Reviews, 13, 518-534. http://dx.doi.org/10.1016/j.rser.2007.12.002
- Li Y. and Sumathy, K. (2004) Modeling and Simulation of a Solar Powered Two Bed Adsorption Air Condition System. Energy Conversion and Management, 45, 2761-2775. http://dx.doi.org/10.1016/j.enconman.2003.12.004