Journal of Sustainable Bioenergy Systems
Vol.3 No.1(2013), Article ID:29075,7 pages DOI:10.4236/jsbs.2013.31004
Thermodynamic Analysis and Synthesis Gas Generation by Chemical-Looping Gasification of Biomass with Nature Hematite as Oxygen Carriers*
Key Laboratory of Renewable Energy and Gas Hydrate of Chinese Academy of Sciences, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou, China
Email: hefang@ms.giec.ac.cn
Received January 24, 2013; revised February 28, 2013; accepted March 8, 2013
Keywords: Thermodynamics; Synthesis Gas; Natural Hematite; Oxygen Carriers; Biomass Chemical Looping Gasification
ABSTRACT
Thermodynamic parameters of chemical reactions in the system were carried out through thermodynamic analysis. According to the Gibbs free energy minimization principle of the system, equilibrium composition of the reactions of chemical-looping gasification (CLG) of biomass with natural hematite (Fe2O3) as oxygen carrier were analyzed using commercial software of HSC Chemistry 5.1. The feasibility of the CLG of biomass with hematite was experimental verified in a lab-scale bubbling fluidized bed reactor using argon as fluidizing gas. It was indicated the experimental results were consistent with the theoretical analysis. The presence of oxygen carrier gave a significant effect on the biomass conversion and improved the synthesis gas yield obviously. It was observed that the gas content of CO and H2 was over 70% in CLG of biomass. The reduced hematite particles mainly existed in form of FeO. It was showed that the reduction of natural hematite with biomass proceeds in a stepwise manner from Fe2O3 ® Fe3O4 ® FeO. Reduction product of natural hematite can be restored the lattice oxygen by oxidation with air.
1. Introduction
Chemical-looping gasification (CLG) is a novel gasification technique that involves the use of oxygen carrier, which transfers oxygen from air to the fuel, which is partially oxidized into H2 and CO, avoiding the direct contact between them. Therefore, the air to fuel ratio is kept low to prevent the fuel from becoming fully oxidized to CO2 and H2O. [1]. A basic chemical-looping gasification system has two reactors, one for air and one for fuel, as is illustrated in Figure 1. On the contrast of traditional gasification technologies, CLG has several potential benefits as follows [2]. Firstly, the recycling of oxygen carrier can provide the oxygen needed for gasification, thus, it can save the cost for making pure oxygen. Secondly, the oxidation reaction of the metal oxide is very exothermic; however, the reduction reactions are endothermic. So, the heat for the endothermic reduction reactions is given by the circulating solids coming from the air reactor at higher temperature. And the same time, the oxygen carrier also can catalyze tar cracking, which reduced the content of tar in biomass gasification [3-5].
There are some works studying chemical looping gasification of solid fuels. He et al. [6] investigated the CLG of biomass with natural hematite as oxygen carriers in a bubbling fluidized bed reactor. It was found that the gasification efficiency and carbon conversion reached up to 75.8% and 94%, respectively. Cao et al. [7] tested in circulating fluidized bed reactor different oxygen carriers, which can capture that the concentration of CO2 enriched to 99%. B. Acharya et al. [8] put forward a set of system which produced hydrogen through biomass chemicallooping gasification. Efficiency of the system was as high as 87.49%, and the hydrogen concentration was found to be 71%, in addition, zero discharge of CO2 can realize.
The objective of this investigation was to explore the possibility of using natural hematite (Fe2O3) as the oxygen carrier in CLG of biomass. In the present work, the thermodynamics of biomass chemical looping gasification was analyzed, and the same time, CLG of biomass with natural hematite as oxygen carrier was experimen-
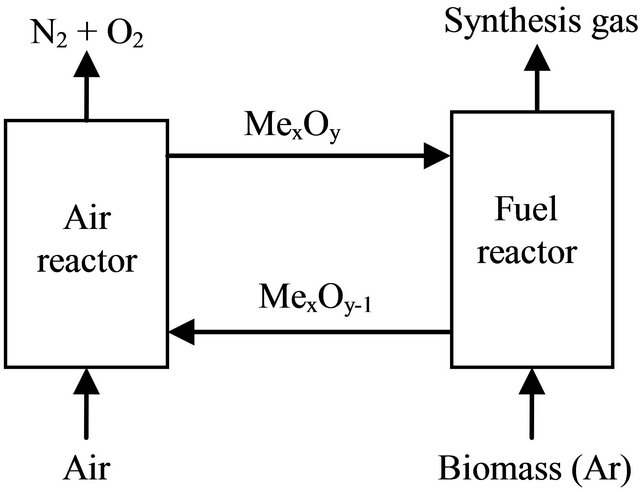
Figure 1. Chemical-looping gasification of biomass with oxygen carrier.
tally investigated in a lab-scale bubbling fluidized bed reactor using argon as fluidizing gas.
2. Thermodynamic Analysis of Chemical-Looping Gasfication
2.1. Chemical Reactions
Fe2O3 as oxygen carriers in the air reactor, the main reactions at 750˚C probably are:
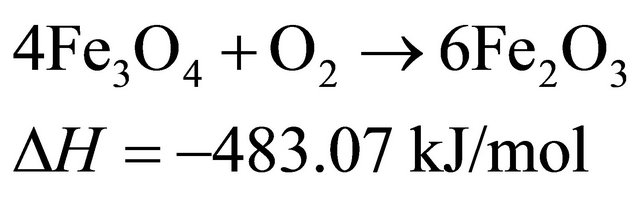 (1)
(1)
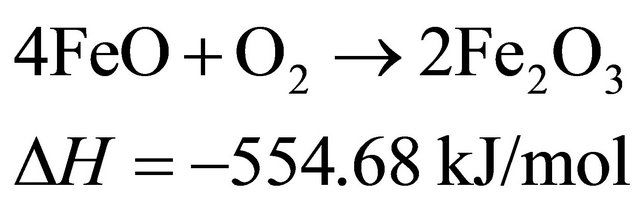 (2)
(2)
In the fuel reactor, the main reactions probably are: pyrolysis of biomass:
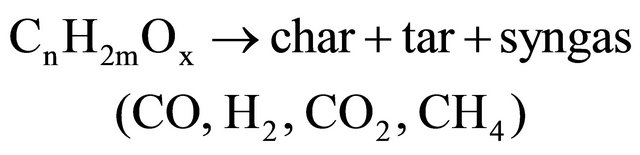 (3)
(3)
The reduction reactions of oxygen carrier particle with pyrolytic products of biomass at 650˚C [9]
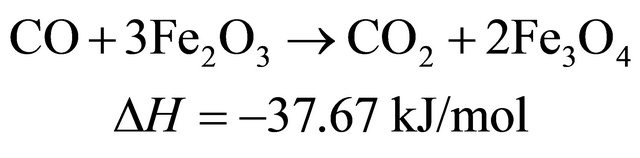 (4)
(4)
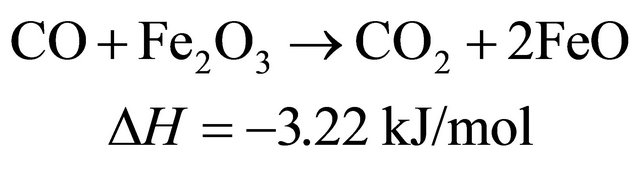 (5)
(5)
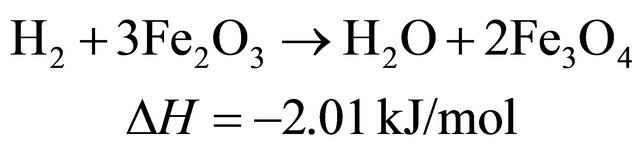 (6)
(6)
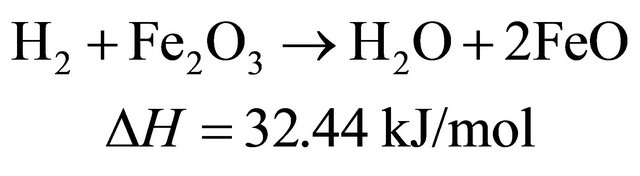 (7)
(7)
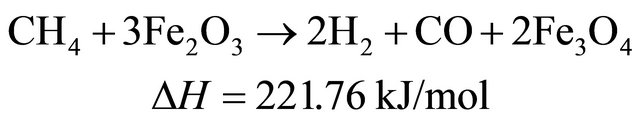 (8)
(8)
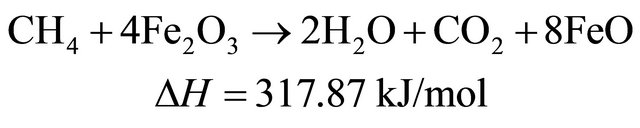 (9)
(9)
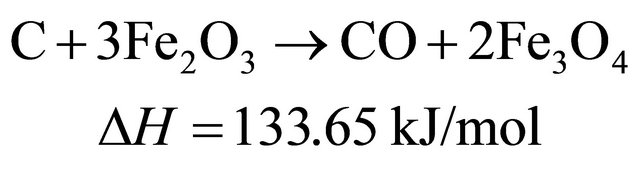 (10)
(10)
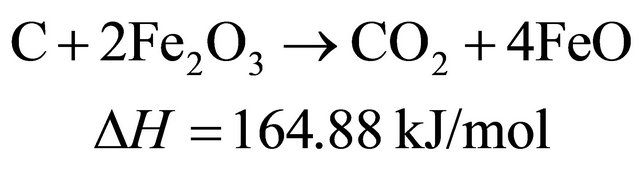 (11)
(11)
Therefore, in the presence of oxygen carriers, these reactions occur sequentially and simultaneously during biomass pyrolysis and gasification. The final products of biomass gasification are determined by the interaction of a couple of above mentioned reactions.
2.2. Thermodynamic Analysis of Gasification Process
Effect of the temperature on the Gibbs free energy (∆rG) and chemical equilibrium constant (Logk) of reactions (4)-(11) was shown in Figure 2. In Figure 2, it found that the ∆rG is less than zero and the Logk is more than zero, which means that the reactions (4)-(11) can react in the thermodynamics. If dividing the reactions (4)-(11) into four groups, that is (4)-(5); (6)-(7); (8)-(9); (10)-(11), it can be seen that the former is less than the later for ∆rG, but the trend of Logk is opposite from the Figure 2, further, the trends of change increased with the increase of temperature. As we known, if the smaller ∆rG and the greater Logk, the reaction will be easier to occur. So, it can conclude that oxygen carriers were reduced gradually in the atmosphere of biomass pyrolysis. It meant the oxygen carriers changed as follows: Fe2O3 ® Fe3O4 ® FeO.
2.3. Chemical Equilibrium of Biomass Gasification Process
There were a number of reactions in the CLG according to the theory analysis. However, some reactions were predominant and the other reactions were secondary in the actual process. If the secondary reactions were ignored, it can help us a better understanding of the CLG. According to the principle of Gibbs free energy minimization, equilibrium components of biomass with Fe2O3
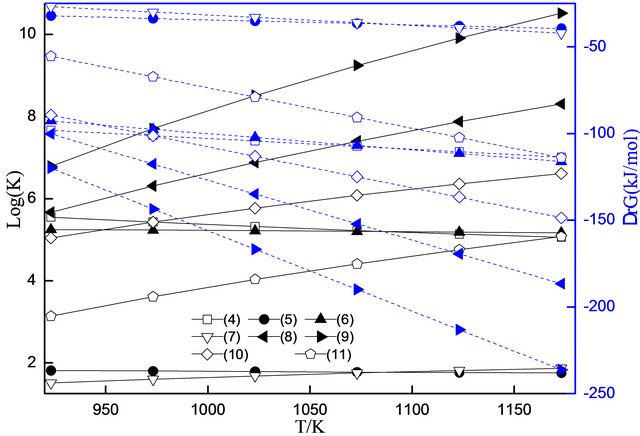
Figure 2. Effects of temperature on ∆rG and LogK of reaction (4)-(11).
were investigated through HSC Chemistry 5.1 software, which is a produced by Outokumpu company in Finland. The approximate formula of biomass is CH1.34O0.65, regardless the S and N.
In order to illustrate the influence of the biomass to Fe2O3 ratio on equilibrium composition, the equilibrium composition was calculated by changing Fe2O3 content at the same temperature. The result was shown in Figure 3. The temperature is set at 750˚C and the initial value of CH1.34O0.65 is 1kmol in the process. In Figure 3(a), the Fe2O3 content was gradually increased from 0.02 kmol to 6 kmol. As seen in figure, the content of incomplete oxidation products (CO + H2) gradually decreased and the content of complete oxidation products (CO2 + H2) gradually increased with the amount of Fe2O3 gradually increased, respectively. As the amount of Fe2O3 is nearly 5 kmol, the amount of H2 + CO is almost zero, and the content of H2O + CO2 is almost constantly. At the same time, the amount of carbon which produced by carbon deposit reaction decreased with increase the amount of Fe2O3, the carbon is almost disappeared as the Fe2O3 content near 1kmol. It can be observed the reduction products of Fe2O3 are mainly FeO and Fe3O4. As the amount of Fe2O3 is less than 3.5 kmol, the main reduction product is FeO, however, the main reduction product is Fe3O4 when the amount of Fe2O3 is more than 3.5 kmol. In this paper, it concerned to produce syngas which is mainly composed of H2 and CO. Thus, in order to obtain syngas instead of CO2 and water, it is necessary to keep the ratio of lattice oxygen to fuel as low as to prevent the fuel from being fully oxidized to CO2 and H2O. The effect of the amount of Fe2O3 from 0.02 to 1.2 kmol on the equilibrium composition is shown in detail in Figure 3(b). As shown in the figure, the amount of CO reaches maximum when the Fe2O3 content is about 0.2 kmol, and then which is declined gradually with the amount of Fe2O3 increasing. It is worth to note that the amount of CO2 is more than CO as the Fe2O3 content is more than 0.7 kmol, which means that biomass is completely oxidized due to the lattice oxygen increasing. So, it can produce more CO2 rather than CO. Therefore, the amount of Fe2O3 must be kept between 0.2 kmol and 0.7 kmol to obtain the syngas. The reduction product of Fe2O3 is mainly FeO, and almost nonexistent Fe3O4. At the same time, it can infer that carbon deposition was caused by material excessive from the Figure 3.
According to the analysis of the thermodynamic theories, it can obtain the synthesis gas which the main component is CO and H2 through the ratio of fuel to lattice oxygen is kept low level. Meanwhile, the main reduction product of Fe2O3 is FeO.
In the next work, CLG of biomass with natural hematite as oxygen carrier was experimentally studied in a lab-scale bubbling fluidized bed reactor using argon as
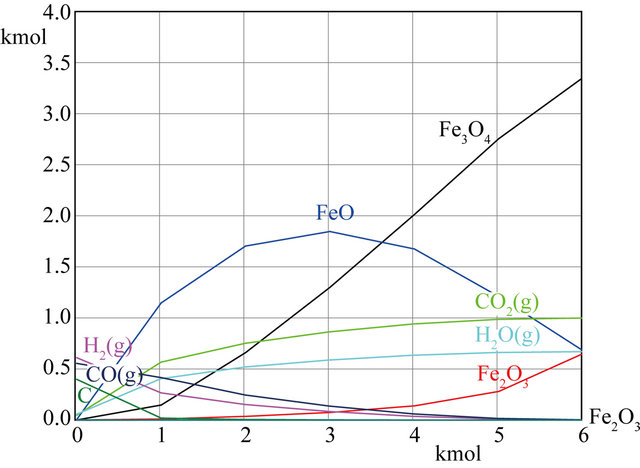 (a)
(a)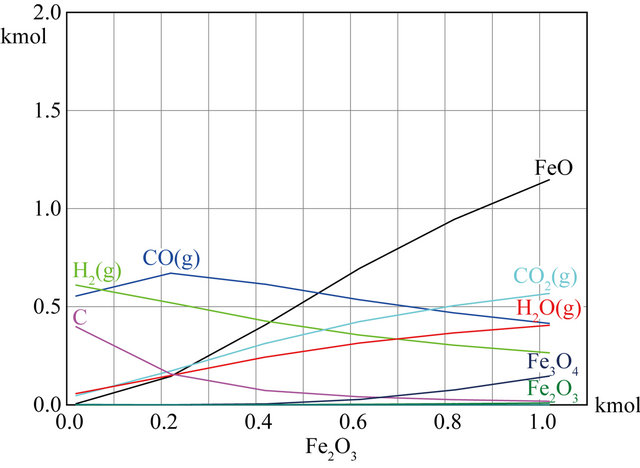 (b)
(b)
Figure 3. Effects of Fe2O3 on equilibrium composition at 750˚C.
fluidized gas.
3. Experimental
3.1. Raw Materials
The sawdust of pine with particle sizes between 250 - 425 μm was dried in the oven which kept 105˚C. The dry-basis proximate analysis and ultimate analysis of the sawdust are showed in Table 1. The hematite with particle sizes between 180 - 250 μm which was supplied by Guangdong Iron & Steel Group Co. Ltd. The elements composition analysis of the hematite is presented in Table 2. According to the Fe3+ fraction, it is calculated that the Fe2O3 content is about 81.66% in the hematite.
3.2. Laboratory Setup
The experiments were conducted with a bubbling fluidized bed reactor of quartz placed in a transparent furnace, as is illustrated in Figure 4. The reactor is in length of 1000 mm and an inner diameter of 60 mm. The bed tem-
Table 1. Proximate and ultimate analysis of pine.
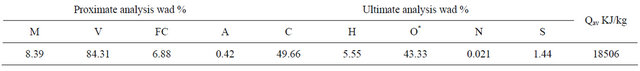
*: by difference.
Table 2. Elements composition analysis of iron ore (%).
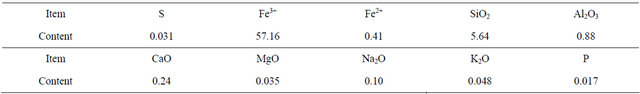
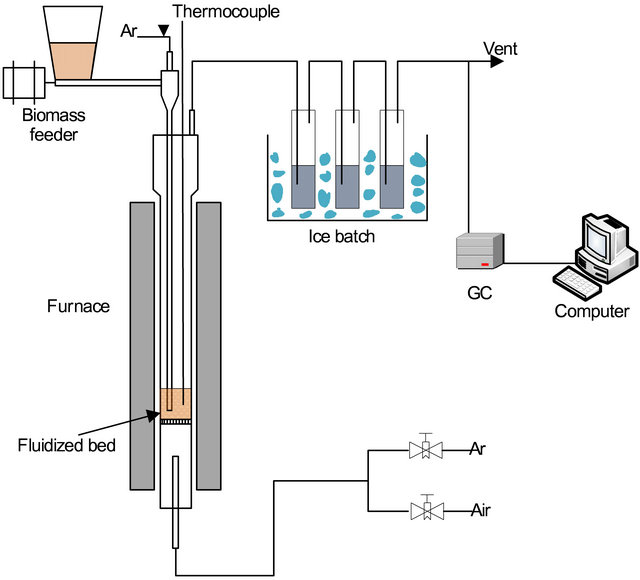
Figure 4. Schematic layout of the laboratory setup.
perature was measured 5 mm above the porous quartz plate using a K-type thermocouple. Oxygen carrier particles were placed on the porous plate and were then heated in air flow to set temperature. During the reducing period, the fluidizing gas was argon (800 L/hr), which was introduced from the bottom of the reactor. When the temperature comes to the set value, biomass was continuously fed (by a screw feeder) in a hopper at the top of the reactor. At the same time, argon (200 L/hr) was introduced from the top of the hopper. Therefore, the biomass sample was pushed by argon flow into the fluidized bed through a drop tube. The flue gases are passed through the cold trap to collect solid particles, water, and tar then introduced into the sampling bag. The composition of the gas products is measured using a gas chromatograph (SHIMADZUA Gas Chromatograph, GC-20B). All the oxygen carrier samples were performed with an X-Ray Diffraction (X’Pert PRO XRD).
4. Results and Discussion
4.1. Effect of the Presence of Hematite Particles on the Biomass Gasification
Based on the thermodynamic analysis, the mass of oxygen carriers and biomass material were set to 150 g and 44 g in a single test, respectively. The ratio of lattice oxygen to fuel is 0.42.
The effect of the presence of hematite particles on the CLG of biomass was investigated at temperatures of 750˚C. Figure 5 shows the comparison of the gas yields
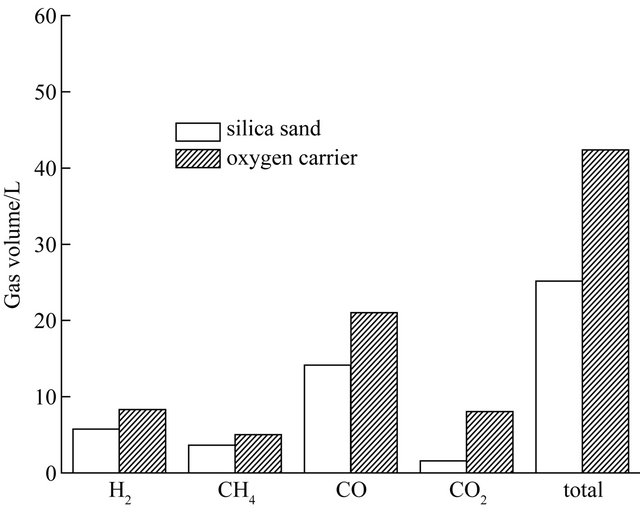
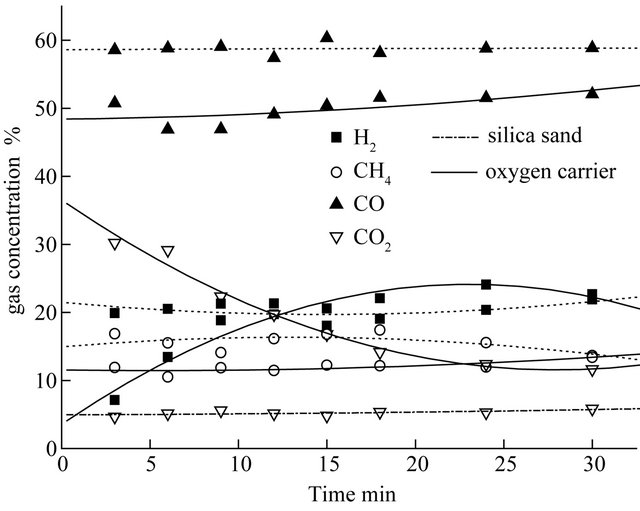
Figure 5. The effect of oxygen carrier on CLG of biomass at 750˚C.
of each gaseous component and the sum of the yields of H2, CH4, CO and CO2 in the process of biomass CLG with those of blank tests during testing period of 30 min. In the blank tests, the hematite particles were replaced by silica sand. Therefore, the process taking place in the blank tests is only pyrolysis rather than CLG of biomass. As shown in Figure 5, the volume yields of each gaseous constituent were obviously enlarged in the presence of hematite particles. The total gas yields were increased from 25.17 L up to 42.39 L at 750˚C. The yields of the generated gases (H2, CH4, CO, and CO2) obtained with hematite particles were apparently larger than with silica sand, which suggest that the lattice oxygen in the hematite particles was used as the gasifying agent during biomass gasification. The main components of gaseous product are CO and H2 which reached about 70% amount of the total volume of the gaseous product. The promoter action of lattice oxygen to carbon conversion was more obvious than to hydrogen conversion. So, the carbon can convert into carbon dioxide and carbon monoxide more completely, it can cause the volume of gaseous product which contained carbon element going up obviously, especially the volume of carbon dioxide. Figure 5 also illustrates gas concentrations (on the basis of argon free) of biomass gasification with oxygen carrier and pyrolysis with silica sand during 30 min at 750˚C. Clearly, it was found that the concentrations of H2, CH4, CO and CO2 were kept stable when biomass was pyrolyzed with inert silica sand. Whereas, there were significant changes for the gaseous concentration of biomass with hematite particles as reaction time increasing, especially the concentration of CO2 and H2. The CO and CH4 concentrations of biomass with hematite particles were both lower than those of biomass with silica sand. H2 concentration increased with the reaction time going on in the CLG of biomass probably owing to a part of biomass was totally oxidized into CO2 and H2O which subsequently accelerated the hydrogen generation reactions taking place rightward. In addition, in the initial stage of biomass gasification with hematite particles, the CO2 fraction was more than 30% in the generated gas due to the easily availability of active lattice oxygen in the oxygen carrier particles. As the reactions proceeded, the concentration of CO2 decreased gradually to 10% approximately. These results may be contributed to the amount of valid hematite particles decreasing with the reactions proceeding. So, portion of biomass taken out by fluidized gas after only pyrolytic reaction, which can cause the proportion of pyrolysis gas increasing in the gaseous products. Through the contrast test, it experimental verified the feasibility of biomass chemical-looping gasification with hematite particles as oxygen carriers.
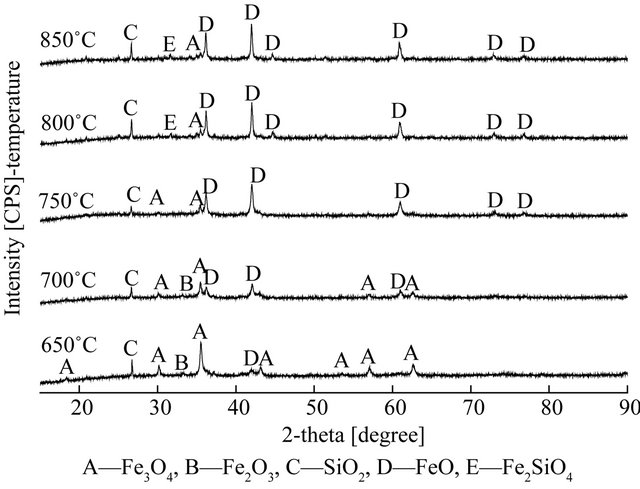
4.2. XRD Analysis of Oxygen Carriers
Figure 6 shows the XRD patterns of fresh oxygen carrier, the oxidized oxygen carrier with the air, and the reduced oxygen carrier of the fuel reactor under different temperature, respectively. In Figure 6(b), it can be seen that the mainly existed in form of Fe2O3 and SiO2 for fresh oxygen carriers. Comparison to fresh oxygen carrier, there was no chemical changes occurred in the regeneration of reacted oxygen carrier, and Fe2O3 was found the major phase with minor amounts of Fe3O4. The forms of oxygen carriers after the reduction reaction changed as temperature rising, as seen in Figure 6(a), at lower temperature (650˚C), the variation of oxygen carriers was from Fe2O3 to Fe3O4, the main existence form of reduced oxygen carriers was FeO at higher temperature (≥750˚C).
However, the variation of hematite particles as oxygen carriers was from Fe2O3 to Fe3O4 in the CLC of biomass [10-12].
The elements content of Fe in hematite particles was analyzed as shown in Figure 7, it found that the ferric
 (a)
(a)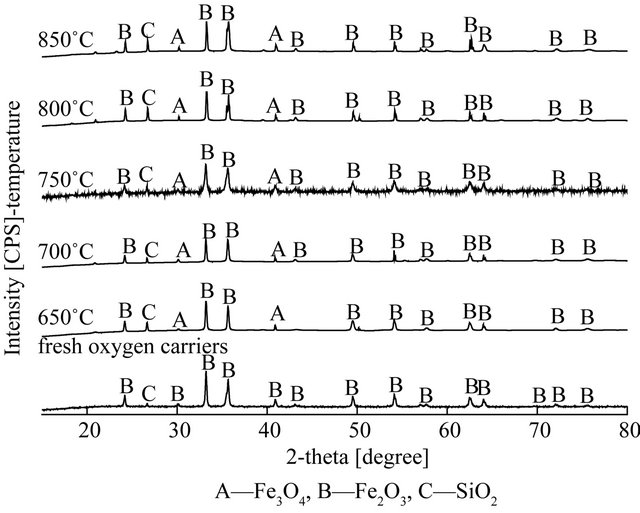 (b)
(b)
Figure 6. XRD patterns of fresh and reacted hematite particles at different temperatures. (a) Reduction; (b) Oxidization.
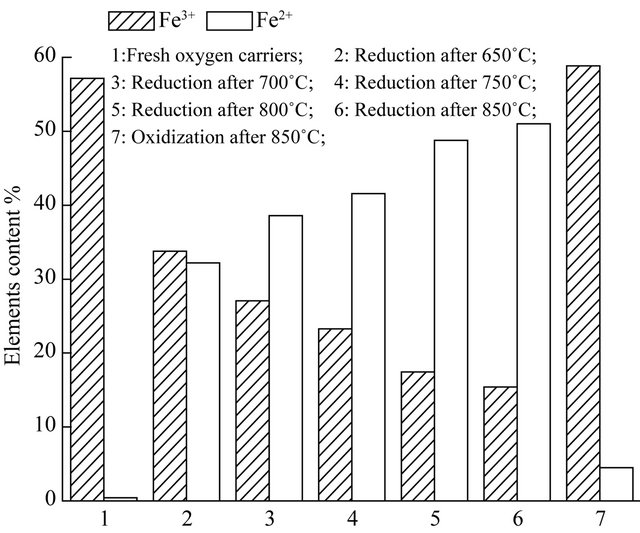
Figure 7. Elements composition analysis of hematite particles.
iron was reduced to ferrous iron in the CLG of biomass. The tendency of conversion was more and more obviously with increase of temperature. The capacity of lattice oxygen in oxygen carriers was recovered by air oxidization, which was affected slightly on the reduction temperature. So, it is feasible that the hematite particles can be used circularly as oxygen carriers in the CLG of biomass, which have higher oxygen transfer ability.
According to the analysis of existence form of reduced oxygen carriers, it found that hematite particles undergone the process of biomass gasification as oxygen carriers, and the mainly existence form of hematite particles were FeO after reduction as the temperature higher than 750˚C. Further, the reduction of hematite particles with biomass proceeded in a stepwise manner from Fe2O3 ® Fe3O4 ® FeO. So, the main reactions were (2), (5), (7), (9), and (11) in the CLG of biomass with the hematite particles as oxygen carriers.
5. Conclusion
The process in CLG of biomass mixed with natural hematite as oxygen carriers was analyzed using the theory of thermodynamics, and then, the verification test was studied in a bubbling fluidized bed reactor with argon as fluidizing gas. It was found that the theory analysis was coincidence with the results of experiment. Consequently, it is feasibility natural hematite as oxygen carriers in CLG of biomass. It can obtain synthesis gas which mainly included CO and H2 by limiting the ratio of lattice oxygen to fuel between 0.2 and 0.7. The presence of oxygen carriers could obviously affect the process of biomass thermal conversion, which can significantly increase gas yield and carbon conversion rate. The main components of gaseous product were CO and H2 which reached about 70% amount of the total volume of the gaseous product. The concentration of CO was the highest and the concentration of CH4 was the lowest during the biomass gasification. In addition, the concentrations of H2, CO, and CH4 in the product gas slowly increased with the reaction proceeding, and the CO2 concentration showed an opposite trend. XRD analysis showed that the iron element in the reduced hematite particles mainly existed in form of FeO, with minor formation of Fe3O4. Further, the lattice oxygen released, corresponding to the transformation Fe2O3 ® Fe3O4 ® FeO, provided the oxygen element needed in biomass gasification. The capacity of lattice oxygen in reduced oxygen carriers can be recovered through air oxidization.
6. Acknowledgements
The financial support of National Natural Science Foundation of China (51076154) is gratefully acknowledged. This work was also supported by Science and Technology Project of Guangdong (2010B010900047), “12th Five Years” National Science and Technology Support Program (2011BAD15B05).
REFERENCES
- J. Bolhar-Nordenkampf, T. Proll, P. Kolbitsch, et al., “Performance of a NiO-Based Oxygen Carrier for Chemical Looping Combustion and Reforming in a 120 kW Unit,” Energy Procedia, Vol. 1, No. 1, 2009, pp. 19-25. doi:10.1016/j.egypro.2009.01.005
- L. F. De Diego, M. Ortiz, F. Garcia-Labiano, et al., “Synthesis Gas Generation by Chemical-Looping Reforming Using a Nibased Oxygen Carrier,” Energy Procedia, Vol. 1, No. 1, 2009, pp. 3-10. doi:10.1016/j.egypro.2009.01.003
- K. Tomishige, T. Kimura, J. Nishikawa, et al., “Promoting Effect of the Interaction between Ni and CeO2 on Steam Gasification of Biomass,” Catalysis Communications, Vol. 8, No. 2, 2007, pp. 161-166.
- T. Nordgreen, T. Liliedahl and K. Sjostrom, “Metallic Iron as a Tar Breakdown Catalyst Related to Atmospheric, Fluidized Bed Gasification of Biomass,” Fuel, Vol. 85, No. 5-6, 2006, pp. 689-694. doi:10.1016/j.fuel.2005.08.026
- C. Courson, E. Makaga, C. Petit, et al., “Development of Ni Catalysts for Gas Production from Biomass Gasification Reactivity in Steam and Dry Reforming,” Catalysis Today, Vol. 63, No. 2, 2000, pp. 427-437. doi:10.1016/S0920-5861(00)00488-0
- F. He, Z. Huang, H. B. Li, et al., “Biomass Direct Chemical Looping Conversion in a Fluidized Bed Reactor with Natural Hematite as an Oxygen Carrier,” Proceedings of the 2011 Asia-Pacific Power and Energy Engineering Conference, Wuhan, 25-28 March 2011.
- Y. Cao and W.-P. Pan, “Investigation of Chemical Looping Combustion by Solid Fuels. 1. Process Analysis,” Energy & Fuels, Vol. 20, No. 5, 2006, pp. 1836-1844. doi:10.1021/ef050228d
- B. Acharya, A. Dutta and P. Basu, “Chemical-Looping Gasification of Biomass for Hydrogen-Enriched Gas Production with In-Process Carbon Dioxide Capture,” Energy Fuel, Vol. 23, No. 10, 2009, pp. 5077-5083.
- A. Abad, J. Adanez, F. Garcia-Labiano, et al., “Mapping of the Range of Operational Conditions for Cu-, Fe-, and Ni-Based Oxygen Carriers in Chemical-Looping Combustion,” Chemical Engineering Science, Vol. 62, No. 1-2, 2007, pp. 533-549. doi:10.1016/j.ces.2006.09.019
- L. H. Shen, J. H. Wu, J. Xiao, et al., “Chemical-Looping Combustion of Biomass in a 10 kWth React or with Iron Oxide as an Oxygen Carrier,” Energy and Fuels, Vol. 23, No. 5, 2009, pp. 2498-2505. doi:10.1021/ef900033n
- H. Leion, E. Jerndal, B. M. Steenari, et al., “Solid Fuels in Chemical-Looping Combustion Using Oxide Scale and Unprocessed Iron Ore as Oxygen Carriers,” Fuel, Vol. 88, No. 10, 2009, pp. 1945-1954. doi:10.1016/j.fuel.2009.03.033
- A. Rubel, K. L. Liu, J. Neathery, et al., “Oxygen Carriers for Chemical Looping Combustion of Solid Fuels,” Fuel, Vol. 88, No. 5, 2009, pp. 876-884. doi:10.1016/j.fuel.2008.11.006
NOTES
*Project supported by Natural Science Foundation of China (No 51076154), Guangdong Provincial Science & Technology Research Foundation (2010B010900047) and “12th Five Years” National Science and Technology Support Program (2011BAD15B05).

