Green and Sustainable Chemistry
Vol.4 No.1(2014), Article ID:42861,4 pages DOI:10.4236/gsc.2014.41004
Oxone-Mediated Oxidative Esterification of Heterocyclic Aldehydes Using Indium(III) Triflate
Laboratory of Medicinal Chemistry, Faculty of Pharmacy, Takasaki University of Health and Welfare, Takasaki, Japan
Email: *mineno@takasaki-u.ac.jp
Copyright (c) 2014 Tomoko Mineno et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor-dance of the Creative Commons Attribution License all Copyrights (c) 2014 are reserved for SCIRP and the owner of the intellectual property Tomoko Mineno et al. All Copyright (c) 2014 are guarded by law and by SCIRP as a guardian.
Received January 17, 2014; revised February 4, 2014; accepted February 11, 2014
Keywords: Oxidative Esterification; Heterocyclic Aldehydes; Indium(III) Triflate
ABSTRACT
Recent investigations have shown the oxone-mediated oxidative methyl esterification of benzaldehyde derivatives using methanol. The reactions were accelerated in the presence of indium(III) triflate, a trivalent indium reagent, in many cases. Based on this method of methyl esterification of benzaldehyde derivatives, we further explored an application to heterocyclic aldehydes. The reactions were examined using methanol as well as other alcohols in order to establish a suitable range.
1. Introduction
Esters are one of the most fundamental of chemical components, and are generally prepared through condensation reactions between carboxylic acids and alcohols under either acidic or basic conditions. A number of methods are available for the condensation reactions. However, upon implementation of multi-steps synthesis, the intended chemical reactions may cause unwanted results when the carboxylate moiety is not protected and is left in unmasked free forms. In many situations, esters are the primary choice as convenient masking units of carboxylic acid functionality. In the field of chemotherapy, methyl esters, as well as ethyl esters, often serve as prodrugs in order to improve bioavailability. Esters are generally converted to their active carboxylate forms in the body via the function of esterase.
In a recent trend, the direct oxidative methyl esterification of aldehydes has received a great deal of interest in the field of organic synthesis. A couple of the esterifying transformations of aldehydes using metal-based reagents have been reported, including iridium [1-3], rhenium [4], ruthenium [5], rhodium [6], palladium [7,8], manganese [9], iron [10], copper [11], tin [12], and PDC [13,14]. However, the use of these heavy/transition metal oxidants could cause environmental concerns. As a consequence, esterification methodologies that combine catalytic amounts of fourth-row transition metal-based reagents, such as titanosilicate [15], vanadium pentoxide [16,17], iron salt, and zinc salt [18,19], have been introduced, although the oxidizing reagents are limited to the utilization of H2O2 in many cases.
Our previous report included an efficient method for high-yielding methyl esterification that is catalyzed by a trivalent indium reagent under mild reaction conditions [20]. The practical preparation of methyl esters can be carried out by treating carboxylic acids in methanol that contains a catalytic amount of trimethylsilyl chloride [21]. Our group has conducted research on trivalent indium reagents, and we have reported several methodologies for In(OTf)3 [22,23]. As part of our ongoing study, we have developed the oxone-mediated oxidative methyl esterification of benzaldehyde derivatives. The reactions were accelerated in the presence of In(OTf)3 in many cases, via the use of an effective oxidant, Oxone® monopersulfate compound (oxone), which is a triple salt of potassium composed of potassium peroxymonosulfate [24]. Based on this established methyl esterification of benzaldehyde derivatives [24,25], we further explored an application to heterocyclic aldehydes. The reactions were examined using methanol as well as other alcohols in order to elucidate a suitable range. Herein, we report the details of our study.
2. Results and Discussion
First, the three isomers of pyridinecarboxaldehydes were subjected to oxidative transformation (Table 1). The position of the aldehyde substituents was decisive for the formation of the corresponding methyl esters. Only 4- pyridinecarboxaldehyde yielded the expected ester product sufficiently (Table 1, entry 3). In contrast, 2-pyridinecarboxaldehyde seemed to be decomposed just after 1.5 h (Table 1, entry 1).
Given the above results, we then focused on determining the limitations of alcohols, employing 4-pyridinecarboxaldehyde as the starting material. Thus, the reactions were attempted not only in methanol but also in other alcohols with elongated carbon chains, actually extending them one by one. Table 2 shows the results of the oxidative esterification using alcohols with longer carbon chains. Under the reaction conditions, the expected ethyl ester and propyl ester were prepared in a similar manner with no experimental differences (Table 2, entries 2 and 3). Moreover, the reactions using butanol and pentanol furnished the corresponding esters in high yields, despite requiring 65 h for completion (Table 2, entries 4 and 5). The reactions using heavier alcohols such as hexanol and heptanol, which are viscous liquids with boiling points of 156 and 176˚C, respectively, required high temperatures for reflux. However, neither produced any of the expected ester products, leaving blackened reaction mixtures (Table 2, entries 6 and 7).
The study was then resumed using 5-membered heterocyclic aldehydes under the same reaction processes. In the events shown in Table 3, the oxone-mediated oxidative methyl esterification of thiophenecarbaldehydes and furaldehydes resulted in moderate yields through the appropriate reaction temperatures.
3. Conclusion
In conclusion, heterocyclic aldehydes were subjected to oxone-mediated esterifying reactions. Reactions using methanol as well as other alcohols with longer carbon chains were attempted in order to elucidate a suitable range. In the case of 4-pyridinecarboxaldehyde, pentanol gave a good yield while hexanol and heptanol did not. Esterification was also conducted on 5-membered heterocyclic aldehydes, and methyl esters were formed in moderate yields. Further analysis of the method is now being conducted.
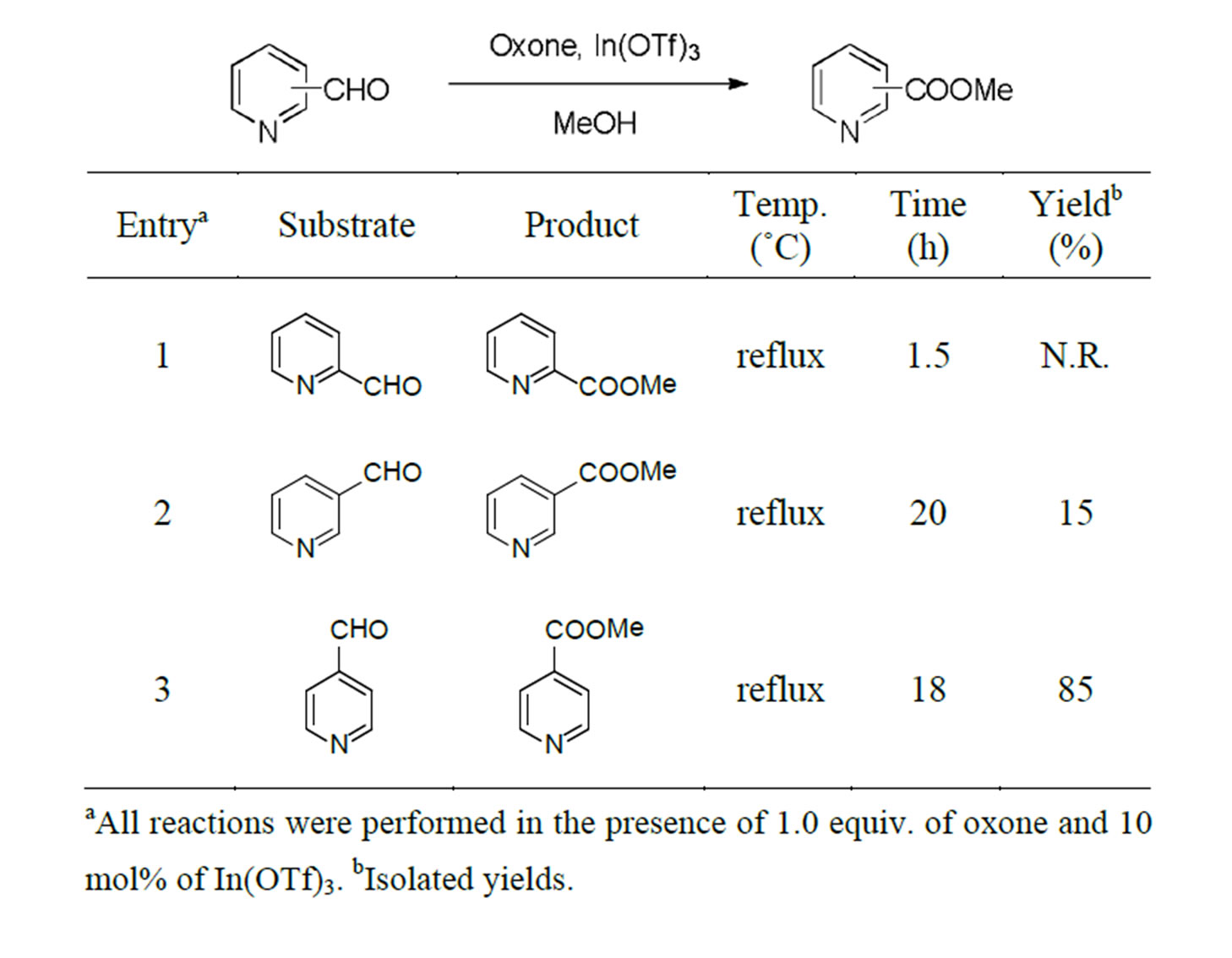
Table 1. Reactions of the three isomers of pyridinecarboxaldehydes.

Table 2. Reactions of 4-pyridinecarboxaldehyde in alcohols with elongated carbon chains.
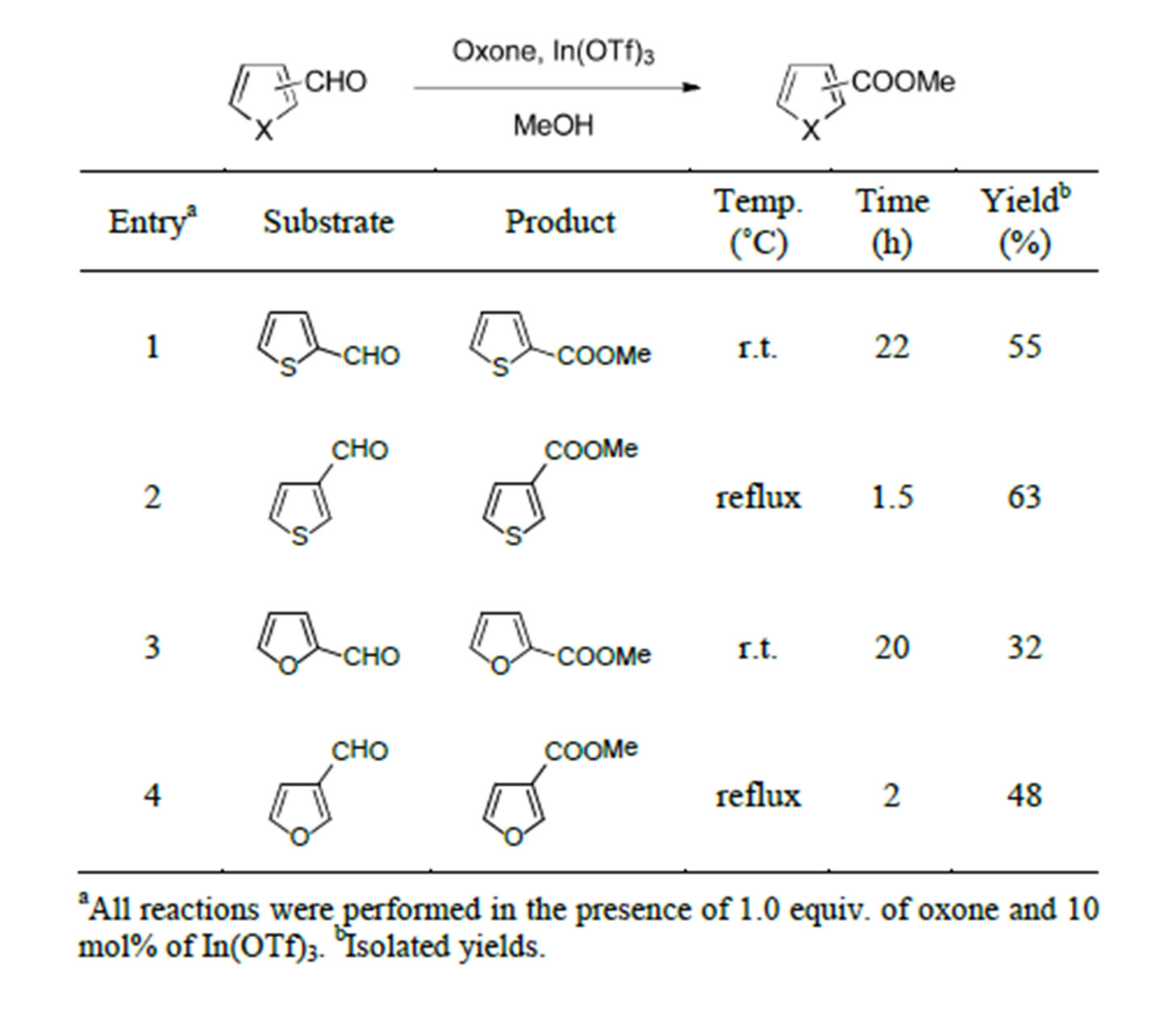
Table 3. Reaction of the 5-membered heterocyclic aldehydes.
4. Experimental
4.1. Materials and Instruments
All reagents were of analytical grade purchased commercially and used without further purification. All reactions were carried out under argon using magnetic stirring unless otherwise noted. 1H NMR and 13C NMR spectral data were recorded on a JEOL JMTC-500 spectrometer using TMS as the internal standard.
4.2. General Experimental Procedure
4-pyridinecarboxaldehyde (1 mmol) was dissolved in methanol (5 mL), then oxone (1 mmol) and In(OTf)3 (10 mol%) were added at room temperature. The reaction mixture was heated at reflux, and was monitored for completion by TLC. Upon cooling to room temperature, the reaction mixture was filtered. The filtrate was condensed using a rotary evaporator. Flush column chromatography on silica gel furnished the corresponding products, which were confirmed by spectroscopy.
4-Pyridinecarboxylic acid propyl ester: 1H NMR (500 MHz, MeOH-d4) δ = 8.73 (2H, dd, J = 4.5, 1.5 Hz), 7.93 (2H, dd, J = 4.5, 1.5 Hz), 4.32 (2H, t, J = 6.5 Hz), 1.81 (2H, sextet, J = 7.5 Hz), 1.04 (3H, t, J = 7.5 Hz); 13C NMR (125 MHz, MeOH-d4) δ = 166.1, 151.3, 139.8, 124.4, 68.5, 23.0, 10.7.
4-Pyridinecarboxylic acid pentyl ester: 1H NMR (500 MHz, MeOH-d4) δ = 8.73 (2H, dd, J = 5.0 and 2.0 Hz), 7.92 (2H, dd, J = 5.0 and 2.0 Hz), 4.36 (2H, t, J = 7.0 Hz), 1.79 (2H, quintet, J = 7.0 Hz), 1.47-1.38 (4H, m), 0.94 (3H, t, J = 7.0 Hz); 13C NMR (125 MHz, MeOH-d4) δ = 166.1, 151.3, 139.8, 124.4, 67.1, 29.4, 29.3, 23.4, 14.3.
REFERENCES
- N. Yamamoto, Y. Obora and Y. Ishii, “Iridium-Catalyzed Oxidative Methyl Esterification of Primary Alcohols and Diols with Methanol,” Journal of Organic Chemistry, Vol. 76, No. 8, 2011, pp. 2937-2941. http://dx.doi.org/10.1021/jo2003264
- S. Kiyooka, Y. Wada, M. Ueno, T. Yokoyama and R. Yokoyama, “[IrCl(cod)]2-Catalyzed Direct Oxidative Esterification of Aldehydes with Alcohols,” Tetrahedron, Vol. 63, No. 51, 2007, pp. 12695-12701. http://dx.doi.org/10.1016/j.tet.2007.10.003
- S. Kiyooka, M. Ueno and E. Ishii, “Iridium-Catalyzed Oxidative Esterification Reaction of Aliphatic Aldehydes and Olefinic Alcohols with Pre-Coordination of the Double Bond of Alcohols to Iiridium,” Tetrahedron Letters, Vol. 46, No. 27, 2005, pp. 4639-4642. http://dx.doi.org/10.1016/j.tetlet.2005.04.093
- J. H. Espenson, Z. Zhu and T. H. Zauche, “Bromide Ions and Methyltrioxorhenium as Cocatalysts for Hydrogen Peroxide Oxidations and Brominations,” Journal of Organic Chemistry, Vol. 64, No. 4, 1999, pp. 1191-1196. http://dx.doi.org/10.1021/jo9817164
- S. Murahashi, T. Naota, K. Ito, Y. Maeda and H. Taki, “Ruthenium-Catalyzed Oxidative Transformation of Alcohols and Aldehydes to Esters and Lactones,” Journal of Organic Chemistry, Vol. 52, No. 19, 1987, pp. 4319-4327. http://dx.doi.org/10.1021/jo00228a032
- R. Grigg, T. R. B. Mitchell and S. Sutthivaiyakit, “Oxidation of Alcohols by Transition Metal Complexes. IV. The Rhodium-Catalyzed Synthesis of Esters from Aldehydes and Alcohols,” Tetrahedron, Vol. 37, No. 24, 1981, pp. 4313-4319. http://dx.doi.org/10.1016/0040-4020(81)85027-2
- R. Lerebours and C. Wolf, “Chemoselective Nucleophilic Arylation and Single-Step Oxidative Esterification of Aldehydes Using Siloxanes and a Palladium-Phosphinous Acid as a Reaction Switch,” Journal of the American Chemical Society, Vol. 128, No. 40, 2006, pp. 13052- 13053. http://dx.doi.org/10.1021/ja063476c
- L.-L. Wei, L.-M. Wei, W.-B. Pan and M.-J. Wu, “Palladium-Catalyzed Esterification-Hydroarylation Reactions of 2-Alkynylbenzaldehydes with Aryl Iodides in Methanol,” Synlett, No. 9, 2004, pp. 1497-1502. http://dx.doi.org/10.1055/s-2004-829056
- R. K. Sharma and C. Sharma, “Efficient Oxidative Methyl Esterification of Aldehydes by Silica-Supported Manganese Complex: Clean and Recyclable Catalyst,” Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, Vol. 48, No. 2, 2011, pp. 155-163. http://dx.doi.org/10.1080/10601325.2011.537531
- R. S. Reddy, J. N. Rosa, L. F. Veiros, S. Caddick and P. M. P. Gois, “NHC/Iron Cooperative Catalysis: Aerobic Oxidative Esterification of Aldehydes with Phenols,” Organic & Biomolecular Chemistry, Vol. 9, No. 9, 2011, pp. 3126-3129. http://dx.doi.org/10.1039/c1ob05151b
- W.-J. Yoo and C.-J. Li, “Copper-Catalyzed Oxidative Esterification of Alcohols with Aldehydes Activated by Lewis Acids,” Tetrahedron Letters, Vol. 48, No. 6, 2007, pp. 1033-1035. http://dx.doi.org/10.1016/j.tetlet.2006.11.169
- G. Qian, R. Zhao, D. Ji, G. Lu, Y. Qi and J. Suo, “Facile Oxidation of Aldehydes to Esters Using S·SnO2/SBA-1- H2O2,” Chemistry Letters, Vol. 33, No. 7, 2004, pp. 834- 835. http://dx.doi.org/10.1246/cl.2004.834
- P. J. Garegg, L. Olsson and S. Oscarson, “Synthesis of Methyl (Ethyl 2-O-acyl-3,4-di-O-benzyl-1-thio-β-Dglucopyranosid)uronates and Evaluation of Their Use as Reactive β-Selective Glucuronic Acid Donors,” Journal of Organic Chemistry, Vol. 60, No. 7, 1995, pp. 2200- 2204. http://dx.doi.org/10.1021/jo00112a046
- B. O’Connor and G. Just, “A New Method for the Conversion of Aldehydes to Methyl Esters Using Pyridinium Dichromate and Methanol in Dimethylformamide,” Tetrahedron Letters, Vol. 28, No. 28, 1987, pp. 3235-3236. http://dx.doi.org/10.1016/S0040-4039(00)95480-7
- S. P. Chavan, S. W. Dantale, C. A. Govande, M. S. Venkatraman and C. Praveen, “Titanosilicate (TS-1) Catalyzed Oxidation of Aromatic Aldehydes to Esters,” Synlett, No. 2, 2002, pp. 267-268. http://dx.doi.org/10.1055/s-2002-19744
- R. Gopinath and B. K. Patel, “A Catalytic Oxidative Esterification of Aldehydes Using V2O5-H2O2,” Organic Letters, Vol. 2, No. 5, 2000, pp. 577-579. http://dx.doi.org/10.1021/ol990383+
- R. Gopinath, B. Barkakaty, B. Talukdar and B. K. Patel, “Peroxovanadium-Catalyzed Oxidative Esterification of Aldehydes,” Journal of Organic Chemistry, Vol. 68, No. 7, 2003, pp. 2944-2947. http://dx.doi.org/10.1021/jo0266902
- X.-F. Wu and C. Darcel, “Iron-Catalyzed One-Pot Oxidative Esterification of Aldehydes,” European Journal of Organic Chemistry, Vol. 2009, No. 8, 2009, pp. 1144- 1147. http://dx.doi.org/10.1002/ejoc.200801176
- X.-F. Wu, “Zinc-Catalyzed Oxidative Esterification of Aromatic Aldehydes,” Tetrahedron Letters, Vol. 53, No. 26, 2012, pp. 3397-3399. http://dx.doi.org/10.1016/j.tetlet.2012.04.111
- T. Mineno and H. Kansui, “High Yielding Methyl Esterification Catalyzed by Indium(III) Chloride,” Chemical & Pharmaceutical Bulletin, Vol. 54, No. 6, 2006, pp. 918- 919. http://dx.doi.org/10.1248/cpb.54.918
- T. Mineno and M. J. Miller, “Stereoselective Total Synthesis of Racemic BCX-1812 (RWJ-270201) for the Development of Neuraminidase Inhibitors as Anti-Influenza Agents,” Journal of Organic Chemistry, Vol. 68, No. 17, 2003, pp. 6591-6596. http://dx.doi.org/10.1021/jo034316b
- T. Mineno, “A Fast and Practical Approach to Tetrahydropyranylation and Depyranylation of Alcohols Using Indium Triflate,” Tetrahedron Letters, Vol. 43, No. 44, 2002, pp. 7975-7978. http://dx.doi.org/10.1016/S0040-4039(02)01864-6
- T. Mineno, N. Nikaido and H. Kansui, “One-Step Transformation of Tetrahydropyranyl Ethers Using Indium(III) Triflate as the Catalyst,” Chemical & Pharmaceutical Bulletin, Vol. 57, No. 10, 2009, pp. 1167-1170. http://dx.doi.org/10.1248/cpb.57.1167
- T. Mineno, M. Sakai, A. Ubukata, K. Nakahara, H. Yoshimitsu and H. Kansui, “The Effect of Indium Triflate(III) in Oxone-Mediated Oxidative Methyl Esterification of Aldehydes,” Chemical & Pharmaceutical Bulletin, Vol. 61, No. 8, 2013, pp. 870-887. http://dx.doi.org/10.1248/cpb.c13-00072
- B. R. Travis, M. Silvakumar, G. O. Hollist and B. Borhan, “Facile Oxidation of Aldehydes to Acids and Esters with Oxone,” Organic Letters, Vol. 5, No. 7, 2003, pp. 1031- 1034. http://dx.doi.org/10.1021/ol0340078
NOTES
*Corresponding author.

