World Journal of AIDS
Vol.07 No.01(2017), Article ID:74671,6 pages
10.4236/wja.2017.71004
When Does Drug Resistant TB Strike HIV/TB Patients?―A South India Experience
Suresh Shastri1, Sharath Burugina Nagaraja2*, Jaya Prasad Tripathy3, Anil Singarajipur1, Bharat Bhushan Rewari4
1State TB Office, Revised National TB Control Programme, Bangalore, India
2ESIC Medical College and PGIMSR, Bangalore, India
3The Union, South East Asia Office, New Delhi, India
4National AIDS Control Organization, New Delhi, India

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 28, 2016; Accepted: March 10, 2017; Published: March 13, 2017
ABSTRACT
Background: India is a high TB (tuberculosis) burden country. The advent of HIV (Human immunodeficiency virus) and DR-TB (drug resistant TB) has worsened the ongoing TB control efforts. A study was conducted to (a) to determine the duration for developing drug resistant TB after diagnosis of HIV (b) to ascertain the patients status after one year of DR-TB treatment in Karnataka, India. Methods: It is a retrospective cross-sectional study involving review of records and reports at ART (Anti-retroviral treatment) centres and DR-TB centres in Karnataka during the period 2013-2014. Results: The median time from being known as HIV positive to being diagnosed as DR-TB was 1168 days (IQR: 571 - 1955). At the end of 14 months, nearly 39% of patients had died and 49% of patients were on treatment. Conclusion: The National Health programmes should prioritize monitoring of the HIV/TB patients and develop appropriate novel strategies for community involvement.
Keywords:
HIV, DR-TB, Outcomes, Duration

1. Introduction
In India, tuberculosis (TB) still remains as a major public health problem and accounts for 25% of global TB burden. The advent of HIV in the last decade and the emergence of drug resistant tuberculosis have worsened the situation. The Revised National TB control Programme (RNTCP) and National AIDS Control Programme (NACP) are in tandem implementing the WHO (World Health Organization) recommended TB and HIV collaborative activities in the country since 2007 [1] . Nationally, about 5% of TB patients are found to HIV positive while it varies across the states. The states like Andhra Pradesh, Karnataka, Maharashtra, Tamil Nadu, Manipur and Nagaland are labeled as high HIV prevalent states and the prevalence of TB-HIV patients in these states varies from 10% - 30% of total TB patients [2] . The emergence of drug resistant TB is posing a threat to ongoing TB control efforts; according to the drug resistant surveys, the incidence of drug resistant TB is 2% - 5% among new cases and 14% - 17% among re-treatment cases [3] . The RNTCP is implementing programmatic management of drug resistant (PMDT) tuberculosis to tackle the emergence of drug resistant TB [4] . The PMDT programme was implemented in phased manner across the country and complete coverage was obtained in March 2012. The PMDT programme adopts different criteria for screening of drug resistant TB patients depending on the availability of resources. The RNTCP is moving forward to achieve universal access to TB care and according the “criteria C” of PMDT, all the HIV patients diagnosed with TB are screened for drug resistant TB [4] (Table 1). On diagnosis of drug resistant TB the programme ensures complete treatment of HIV/DR-TB (drug resistant tuberculosis).
There are enough evidences on the clinical and treatment outcomes of HIV/ DR-TB patients under different settings across the world [5] [6] [7] [8] . However, there is lack of information on the time taken to develop DR-TB after diagnosis of HIV; which depicts the effectiveness of health systems which includes timely diagnosis of HIV patients, monitoring the patients for development of tuberculosis, completion of anti-TB treatment and screening for DR-TB. We conducted this operational research study under programmatic settings (a) to determine the duration for developing drug resistant TB after diagnosis of HIV (b) to ascertain the patients status after one year of DR-TB treatment in Karnataka, India.
2. Methods
It is a retrospective cross-sectional study conducted in the south Indian state of India, Karnataka during the period 2013-2014. The state of Karnataka is implementing the joint TB/HIV collaborative activities since 2006; while the criteria for screening all HIV-TB patients for resistance to first line anti-TB drugs at
Table 1. Presumptive drug resistant TB case criteria’s in PMDT under RNTCP.
anti-retroviral treatment (ART) centers are being implemented from 2012 onwards. There are 61 ART centers in the state and the sputum samples of presumptive drug resistant TB cases are sent to accredited state culture and drug susceptibility testing (CDST) laboratory for diagnosis of DR-TB; the samples were subjected to solid culture testing to test susceptibility for Isoniazid, Rifampicin, Ethambutol and streptomycin. If positive for rifampicin with or without other drugs, they were initiated on DR-TB treatment as per PMDT guidelines; the treatment is for 24 months with 6 months of Intensive phase and 18 months of continuation phase. The line list of all the patients whose samples were positive for DR-TB was made by DR-TB coordinator in coordination with ART centre staff and they updated the documentation of HIV diagnosis, pre-ART and ART details. The date of diagnosis and prognosis of DR-TB treatment was collected from the records of DR-TB centre. The median time taken to develop drug resistance was taken as the cut off to classify early and late incidence. The freely available software Epidata analysis (V2.2.2.178) (Epidata association, Odense, Denmark) was used for the analysis. Since, this is an operational research study involving review of records and reports, ethics approval was not sought for the study. However, the permission for the same was obtained from the competent programme authorities.
3. Results
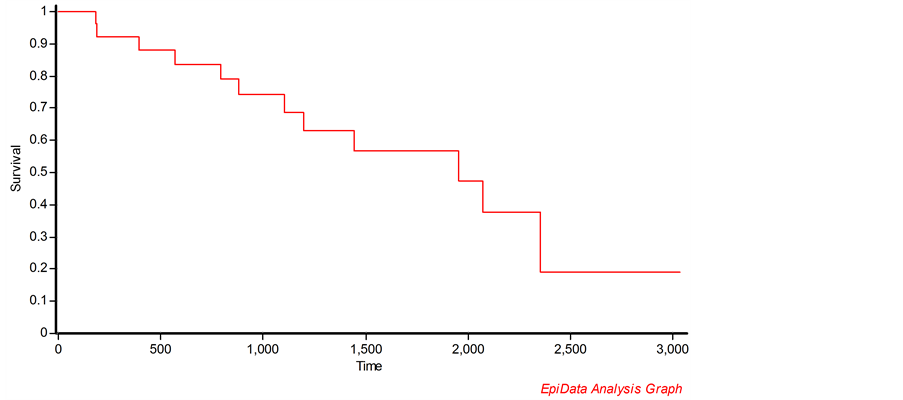
Of the 580 patients whose samples were tested at the laboratory 31 (5.3%) patients were tested positive for drug resistant TB and were initiated on treatment. All the patients had history of previous anti-TB treatment. The median time from being known as HIV positive to being diagnosed as DR-TB was 1168 days (IQR: 571 - 1955) (Table 2). The median age for males and females who developed the DR-TB was 35 years and 29.5 years respectively. The median base line CD4 count was 215 cells/mm3 (IQR: 155-351). Majority of them were initiated on tenofovir based regimen. Low CD4 count (<200 cells/mm3) was significantly associated with early incidence of DR-TB (Table 3). Nearly 55% and 36% of patients had culture conversion at the end of 6 months and 12 months treatment. At the end of 14 months, nearly 39% of patients had died and 49% of patients were on treatment. Figure 1 shows the survival probabilities of HIV/DR-TB patients in days.
Table 2. Timing of development of DR-TB during antiretroviral treatment, Karnataka, India.
Table 3. Characteristics of patients based on incidence of DR-TB during anti-retroviral treatment, Karnataka, India.
*p-value < 0.05 is found to be significant.
Figure 1. Kaplan-Meier plot showing the survival probability (in days) of HIV/DR-TB patients initiated on treatment in Karnataka, India.
4. Discussion
This is the first operational research conducted in the country that looked into the duration of time between onset of HIV diagnosis and DR-TB diagnosis. Broadly, among those persons who are HIV positive and later develops tuberculosis took nearly three years to develop drug resistant TB. It is well known that the addition of DR-TB treatment to ongoing ART treatment basically prolongs the survival rate and increases the cure rates. However, due to the longer duration of DR-TB treatment (18 - 24 months) the culture conversion rates are considered as intermediate outcomes and predictors of cure rates.
Our study findings have following programmatic implications. First, HIV/TB patients develops DR-TB within a short span of three years which calls for action to have daily TB regimen for TB patients and intensified monitoring of such highly vulnerable population. Though, the National TB/HIV policy guidelines recommends to have daily TB regimen among HIV/TB patients, the RNTCP is yet to make it operational owing to the non-availability of TB drugs for daily regimen [9] . The ART treatment is self administered while TB treatment requires direct observation; the fact that HIV-TB patients are still stigmatized in the society cannot be undermined and the programme should address this issue by providing special incentives for directly observed treatment (DOT) providers there by encouraging the quality of DOT in these vulnerable patients. Second, the culture conversion rate is over half at six months and over one third at twelve months; nearly half of patients were on treatment at the end of twelve months while 40% had died and rest were lost to follow-up. The high mortality rate in the first year of treatment highlights the need for newer strategies and diagnostics that could detect DR-TB even before the symptoms becomes more explicit in HIV/TB patients. Loss to follow up may be owed to the difficulty in swallowing good number of pills from ART and DR-TB treatment with persistent motivation; reduction in number of pills by developing combination pills may increase compliance to treatment.
The strength of the study is that it includes the data from all the ART centres from a high HIV prevalent state and thus reflects the ground reality of programme implementation. The limitations includes the individual data from 549 patients are not available to compare the characteristics of those with and without DR-TB. However, this should not be a hindrance to interpretation of the existing results.
5. Conclusion
To conclude, though less proportion of HIV/TB patients developed drug resistant TB the disease strikes within three years. The programme should prioritize monitoring of the HIV/TB patients and develop appropriate strategies for community involvement. Further research is needed to ascertain the facts in these areas.
Conflict of Interest
We declare no conflict of interest.
Authorship Statement
SS, SBN and BR developed the idea. SS, SBN, JPT, AS collected the data and analyzed the data. The draft manuscript was approved by all the authors.
Cite this paper
Shastri, S., Nagaraja, S.B., Tripathy, J.P., Singarajipur, A. and Rewari, B.B. (2017) When Does Drug Resistant TB Strike HIV/TB Patients?―A South India Experience. World Journal of AIDS, 7, 34-39. https://doi.org/10.4236/wja.2017.71004
References
- 1. National AIDS Control Organization and Central TB Division, Government of India. National Framework for Joint HIV/TB Collaborative Activities. October 2009.
- 2. Central TB Division, Ministry of Health and Family Welfare, Government of India. Annual status report, TB India 2014.
- 3. Ramachandran, R., Nalini, S., Chandrasekar, V., Dave, P.V., Sanghvi, A.S., Wares, F., et al. (2009) Surveillance of Drug-Resistant Tuberculosis in the State of Gujarat, India. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union against Tuberculosis and Lung Disease [Internet], 13, 1154-1160.
http://www.ncbi.nlm.nih.gov/pubmed/19723407 - 4. World Health Organization (2011) Guidelines for the Programmatic Management of Drug Resistant TB.
- 5. Llaro, K., Bonilla, C., Sebastian, J., Bayona, J., Lygizos, M., Anger, H., et al. (2012) HIV-Positive Patients Treated for Multidrug-Resistant Tuberculosis: Clinical outcomes in the HAART Era. The International Journal of Tuberculosis and Lung Disease, 16, 348-354.
- 6. Gandhi, N.R., Shah, N.S., Andrews, J.R., Vella, V., Moll, A.P., Scott, M., et al. (2010) HIV Coinfection in Multidrug- and Extensively Drug-Resistant Tuberculosis Results in High Early Mortality. American Journal of Respiratory and Critical Care Medicine [Internet], 181, 80-86.
http://www.ncbi.nlm.nih.gov/pubmed/19833824 - 7. Isaakidis, P., Paryani, R., Khan, S., Mansoor, H. and Manglani, M. (2013) Poor Outcomes in a Cohort of HIV-Infected Adolescents Undergoing Treatment for Multidrug-Resistant Tuberculosis in Mumbai, India. PLoS One, 8, Article ID: e68869.
- 8. Isaakidis, P., Cox, H.S., Varghese, B., Montaldo, C., Da Silva, E., Mansoor, H., et al. (2011) Ambulatory Multi-Drug Resistant Tuberculosis Treatment Outcomes in a Cohort of HIV-Infected Patients in a Slum Setting in Mumbai, India. PloS One [Internet], 6, Article ID: e28066.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3228724&tool=pmcentrez&rendertype=abstract - 9. Li, J., Munsiff, S.S., Driver, C.R. and Sackoff, J. (2005) Relapse and Acquired Rifampin Resistance in HIV-Infected Patients with Tuberculosis Treated with Rifampin- or Rifabutin-Based Regimens in New York City, 1997-2000. Clinical Infectious Diseases, 41, 83-91.